Functionalized Graphene from Electrochemical Exfoliation of Graphite toward Improving Lubrication Function of Base Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
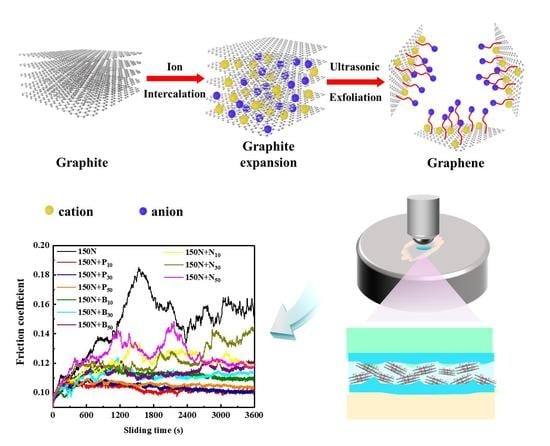
2.2. Electrochemical Exfoliation of Graphene
- (a)
- Three ILs, [BMIm][PF6], [BMIm][BF4], and [BMIm][NTf2], were mixed with acetonitrile solvents. Electrolytes of different concentrations were prepared with volume ratios of IL and acetonitrile solvents at 1:10, 1:30, and 1:50, respectively. The molecular structures of the three ILs s are shown in Figure 1. Then, electrochemical exfoliation was carried out in the traditional two-electrode system, using graphite rods as cathodes and anodes, keeping the two electrodes parallel and 4 cm apart from each other, and connecting a direct-current (DC) power supply.
- (b)
- A DC voltage of 15 V was applied at both ends of the electrode. The electrolyte was colorless and transparent at first, but after 30 min, the anode graphite gradually decomposed and peeled off into the electrolyte, the color of the solution began to change, the particles separated from the graphite matrix appeared at the bottom, and the solution blackened completely after 2 h of exfoliation.
- (c)
- In 50 mL electrolyte, NMP of 50 mL was added to the exfoliated suspension and a voltage of 15 V was applied at both ends for 2 h. Then the supernatant was centrifuged at 12,000 rpm, after ultrasound for 1 h. The collected products were washed with anhydrous ethanol 3 times and then dried in a vacuum drying box at 60 °C for 36 h. The products are shown in Table 1.
2.3. Tribological Characterization
3. Results and Discussions
3.1. Chemical and Structural Characterization of the Products
3.2. Mechanism Analysis of the Electrochemical Exfoliation of Graphene from IL Organic Solutions
3.3. Mechanism Analysis of the Electrochemical Exfoliation of Graphene from the IL Organic Solutions
3.4. Tribological Performance of the Exfoliated Graphene
3.5. Lubrication Mechanisms of Exfoliated Graphene
4. Conclusions
- (a)
- The modification density was proportional to the concentration of the ILs in the organic electrolyte. There were obvious differences in the modified graphene exfoliated in the different ILs in organic solvents, which may have been due to the different size of anions used.
- (b)
- The hybrid oils containing modified graphene exfoliated with [BMIm][PF6] and [BMIm][BF4] ILs possessed high dispersion stability. The hybrid oil remained stable after 14 days.
- (c)
- In contrast, the P10 sample had the best antifriction and wear resistance, and the friction coefficient and wear rate were reduced by 32% and 39%, respectively.
- (d)
- P10 had a thinner layer, which could better enter the friction interface, to form a tribo-film, accompanied by the disorder transformation of its structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, H.; Liu, Y.; Li, J. Duplex DNA/graphene oxide biointerface: From fundamental understanding to specific enzymatic effects. Adv. Funct. Mater. 2012, 22, 3083–3088. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in photocatalysis: A review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Du, S.; Sun, J.; Wu, P. Preparation, characterization and lubrication performances of graphene oxide-TiO2 nanofluid in rolling strips. Carbon 2018, 140, 338–351. [Google Scholar] [CrossRef]
- Chouhan, A.; Mungse, H.P.; Khatri, O.P. Surface chemistry of graphene and graphene oxide: A versatile route for their dispersion and tribological applications. Adv. Colloid Interface Sci. 2020, 283, 102215. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Li, J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, T.; Yue, Q.; Liu, H.; Ma, S.; Cai, M.; Zhou, F. Graphene oxide/brush-like polysaccharide copolymer nanohybrids as eco-friendly additives for water-based lubrication. Tribol. Int. 2021, 157, 106895. [Google Scholar] [CrossRef]
- Xue, S.; Cen, Y.; Yang, H.; Honda, T.; Nakanishi, Y.; Zhang, L.; Zhang, B.; Zeng, X. The enhanced lubrication of water-based cutting fluid by functionalized GO. Tribol. Lett. 2020, 68, 93. [Google Scholar] [CrossRef]
- Paul, G.; Hirani, H.; Kuila, T.; Murmu, N.C. Nanolubricants dispersed with graphene and its derivatives: An assessment and review of the tribological performance. Nanoscale 2019, 11, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 66–669. [Google Scholar] [CrossRef]
- Yang, W.; Chen, G.; Shi, Z.; Liu, C.C.; Zhang, L.; Xie, G.; Cheng, M.; Wang, D.; Yang, R.; Shi, D.; et al. Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nat. Mater. 2013, 12, 792–797. [Google Scholar] [CrossRef]
- Sun, K.; Fan, X.; Zhang, W.; Xue, P.; Diao, D. Contact-focusing electron flow induced nanosized graphene sheet formation in amorphous carbon films for fast low-friction. Carbon 2019, 149, 45–54. [Google Scholar] [CrossRef]
- Liu, Y.; Song, A.; Xu, Z.; Zong, R.; Zhang, J.; Yang, W.; Wang, R.; Hu, Y.; Luo, J.; Ma, T. Interlayer friction and superlubricity in single-crystalline contact enabled by two-dimensional flake-wrapped atomic force microscope tips. ACS Nano 2018, 12, 7638–7646. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir-blodgett assembly of graphite oxide single layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; He, Y.; Luo, J. In situ green synthesis of the new sandwichlike nanostructure of Mn3O4/graphene as lubricant additives. ACS Appl. Mater. Interfaces 2019, 11, 36931–36938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mao, J.; Li, Y.; He, Y.; Luo, J. Friction-induced nano-structural evolution of graphene as a lubrication additive. Appl. Surf. Sci. 2018, 434, 21–27. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Wang, Y.; Zhou, J.; Song, L. Separating nano graphene oxide from the residual strong-acid filtrate of the modified hummers method with alkaline solution. Appl. Surf. Sci. 2015, 329, 83–86. [Google Scholar] [CrossRef]
- Gebreegziabher, G.G.; Asemahegne, A.S.; Ayele, D.W.; Dhakshnamoorthy, M.; Kumar, A. One-step synthesis and characterization of reduced graphene oxide using chemical exfoliation method. Mater. Today Chem. 2019, 12, 233–239. [Google Scholar] [CrossRef]
- Chen, X.; Dubois, M.; Radescu, S.; Rawal, A.; Zhao, C. Liquid-phase exfoliation of F-diamane-like nanosheets. Carbon 2021, 175, 124–130. [Google Scholar] [CrossRef]
- Lund, S.; Kauppila, J.; Sirkiä, S.; Palosaari, J.; Eklund, O.; Latonen, R.-M.; Smått, J.-H.; Peltonen, J.; Lindfors, T. Fast high-shear exfoliation of natural flake graphite with temperature control and high yield. Carbon 2021, 174, 123–131. [Google Scholar] [CrossRef]
- Najafabadi, A.T.; Gyenge, E. High-yield graphene production by electrochemical exfoliation of graphite: Novel ionic liquid (IL)–acetonitrile electrolyte with low IL content. Carbon 2014, 71, 58–69. [Google Scholar] [CrossRef]
- Liu, W.W.; Aziz, A. Review on the effects of electrochemical exfoliation parameters on the yield of graphene oxide. ACS Omega 2022, 7, 33719–33731. [Google Scholar] [CrossRef]
- Ejigu, A.; Kinloch, I.A.; Dryfe, R.A. Single stage simultaneous electrochemical exfoliation and functionalization of graphene. ACS Appl. Mater. Int. 2017, 9, 710–721. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Cooper, A.J.; Dryfe, R.A.; Kinloch, I.A. How to get between the sheets: A review of recent works on the electrochemical exfoliation of graphene materials from bulk graphite. Nanoscale 2015, 7, 6944–6956. [Google Scholar] [CrossRef]
- Liu, J.; Poh, C.K.; Zhan, D.; Lai, L.; Lim, S.H.; Wang, L.; Liu, X.; Gopal Sahoo, N.; Li, C.; Shen, Z.; et al. Improved synthesis of graphene flakes from the multiple electrochemical exfoliation of graphite rod. Nano Energy 2013, 2, 377–386. [Google Scholar] [CrossRef]
- Cooper, A.J.; Wilson, N.R.; Kinloch, I.A.; Dryfe, R.A.W. Single stage electrochemical exfoliation method for the production of few-layer graphene via intercalation of tetraalkylammonium cations. Carbon 2014, 66, 340–350. [Google Scholar] [CrossRef]
- Singh, V.V.; Gupta, G.; Batra, A.; Nigam, A.K.; Boopathi, M.; Gutch, P.K.; Tripathi, B.K.; Srivastava, A.; Samuel, M.; Agarwal, G.S.; et al. Greener electrochemical synthesis of high quality graphene nanosheets directly from pencil and its SPR sensing application. Adv. Funct. Mater. 2012, 22, 2352–2362. [Google Scholar] [CrossRef]
- Hou, K.; Wang, J.; Yang, Z.; Ma, L.; Wang, Z.; Yang, S. One-pot synthesis of reduced graphene oxide/molybdenum disulfide heterostructures with intrinsic incommensurateness for enhanced lubricating properties. Carbon 2017, 115, 83–94. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Hai, G.; Li, J.; Zhu, H.; Sang, T.P.; Yang, J. Na2CO3 and graphene nanocomposites toward efficient lubrication. Carbon 2021, 177, 138–150. [Google Scholar] [CrossRef]
- Ye, X.; Ma, L.; Yang, Z.; Wang, J.; Wang, H.; Yang, S. Covalent functionalization of fluorinated graphene and subsequent application as water-based lubricant additive. ACS Appl. Mater. Inter. 2016, 8, 7483–7488. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, X.; Zhang, C.; Zhang, J.; Luo, J.; Zhang, J. Modified graphene as novel lubricating additive with high dispersion stability in oil. Friction 2020, 9, 143–154. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.-S.; Zeng, X. Correlation between molecular structure and interfacial properties of edge or basal plane modified graphene oxide. ACS Appl. Nano Mater. 2018, 1, 2763–2773. [Google Scholar] [CrossRef]
- Song, H.; Wang, Z.; Yang, J.; Jia, X.; Zhang, Z. Facile synthesis of copper/polydopamine functionalized graphene oxide nanocomposites with enhanced tribological performance. Chem. Eng. J. 2017, 324, 51–62. [Google Scholar] [CrossRef]
- Sanes, J.; Avilés, M.-D.; Saurín, N.; Espinosa, T.; Carrión, F.-J.; Bermúdez, M.-D. Synergy between graphene and ionic liquid lubricant additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Mo, Y.; Bai, P.; Wei, Q.; Jin, L.; You, S.; Wang, M.; Li, L.; Chen, X.; et al. Synergistic lubricating effect of graphene/ionic liquid composite material used as an additive. Friction 2020, 9, 1568–1579. [Google Scholar] [CrossRef]
- Mao, J.; Zhao, J.; Wang, W.; He, Y.; Luo, J. Influence of the micromorphology of reduced graphene oxide sheets on lubrication properties as a lubrication additive. Tribol. Int. 2018, 119, 614–621. [Google Scholar] [CrossRef]
















| ILs | 1:10 | 1:30 | 1:50 |
|---|---|---|---|
| [BMIm][PF6] | P10 | P30 | P50 |
| [BMIm][BF4] | B10 | B30 | B50 |
| [BMIm][NTf2] | N10 | N30 | N50 |
| Samples | C% | N% | O% | F% |
|---|---|---|---|---|
| graphite | 92.34 | — | 7.66 | — |
| P10 | 84.28 | 8.23 | 3.25 | 3.45 |
| P30 | 85.91 | 7.65 | 3.49 | 2.38 |
| P50 | 87.61 | 7.02 | 3.72 | 1.36 |
| B10 | 85.31 | 7.18 | 4.06 | 2.58 |
| B30 | 86.5 | 6.33 | 4.24 | 1.99 |
| B50 | 88.38 | 5.41 | 4.55 | 1.29 |
| N10 | 74.42 | 7.42 | 11.12 | 4.24 |
| N30 | 76.14 | 7.05 | 10.46 | 3.35 |
| N50 | 78.16 | 6.57 | 9.58 | 2.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, X.; Zhang, W.; Zhao, Z.; Fan, X. Functionalized Graphene from Electrochemical Exfoliation of Graphite toward Improving Lubrication Function of Base Oil. Lubricants 2023, 11, 166. https://doi.org/10.3390/lubricants11040166
Zhang C, Zhang X, Zhang W, Zhao Z, Fan X. Functionalized Graphene from Electrochemical Exfoliation of Graphite toward Improving Lubrication Function of Base Oil. Lubricants. 2023; 11(4):166. https://doi.org/10.3390/lubricants11040166
Chicago/Turabian StyleZhang, Chunfeng, Xiaojun Zhang, Wei Zhang, Zhuang Zhao, and Xiaoqiang Fan. 2023. "Functionalized Graphene from Electrochemical Exfoliation of Graphite toward Improving Lubrication Function of Base Oil" Lubricants 11, no. 4: 166. https://doi.org/10.3390/lubricants11040166