1. Introduction
Lubricating oil has a significant impact on the working efficiency and service life of mechanical equipment, since lubricants can reduce the friction and wear between moving parts, as well as the energy loss in a mechanical movement. With the rapid development of nanomaterials, researchers have recently conducted extensive studies on the application of nanomaterials as lubricant additives. It has been found that nanomaterials with unique characteristics (e.g., small dimensions, large surface area, and high surface activity) can improve the friction-reducing and anti-wear abilities of lubricating oil [
1,
2,
3,
4]. Among various nanomaterials, nano-copper with low shear strength and grain boundary slip effect might be a promising multifunctional lubricant additive thanks to its synergistic friction reduction, anti-wear, and self-repairing abilities [
5,
6,
7,
8]. This new nanoadditive can perform excellently, providing more reliable support for the smooth operation of mechanical equipment. Considering energy and environmental perspectives, lubricating oil can effectively reduce energy loss and improve the energy efficiency of mechanical equipment, thereby relieving energy pressure to a certain extent. In addition, reducing friction and wear also helps to extend the service life of mechanical equipment and reduce resource consumption and waste generation. Therefore, studying nanomaterials as lubricant additives holds significance for the machinery industry. Nanoscale particles are dispersed in a conventional fluid medium (e.g., water, oil, or glycol) to form a homogeneous and stable fluid medium known as a nanofluid, in which the lubricating oil is used as a solvent known as a nanolubricant. In future developments, we can anticipate further optimization and broader application of nanolubricants to meet mechanical equipment’s increasingly stringent performance and environmental requirements. This advancement promises new possibilities for industrial production. Consequently, in-depth investigation and application of nanolubricants possess significant scientific and practical value.
Viscosity is a pivotal property of lubricating oil, acting as a crucial indicator of lubricating oil fluidity and internal friction. Viscosity values that are either too high or too low can detrimentally affect the lubricant’s performance: increased viscosity may lead to heightened frictional forces during fluid lubrication, while reduced viscosity can decrease the lubricant’s load-bearing capacity [
9,
10]. Breki et al. [
11] integrated Einstein’s viscosity equation with dynamic lubrication theory, elucidating the relationship between the friction coefficient and the solid-phase volume fraction, underscoring the significance of viscosity in fluid lubrication. This underscores the importance of investigating the influence of nanoadditives on the viscosity of nanolubricants. However, the existing literature presents divergent results and conclusions concerning the impact of nanoadditives on lubricant viscosity [
12,
13,
14,
15,
16,
17]. Various experimental data show that the addition of nanomaterials may cause complex changes in lubricating oil viscosity, and the specific effect may depend on the type of nanoparticles, mass fraction, and operating conditions. For example, Ma et al. [
18] demonstrated that introducing ZnO nanoparticles enhances the viscosity of SAE50 lubricant, likely due to augmented resistance to lubricant flow induced by nanoparticle agglomeration under van der Waals forces. Hemmat et al. [
19] found that Al
2O
3 nanoadditives elevate the viscosity of 10W40 lubricant at 55 °C by 132%, noting that nanolubricant viscosity initially increases and then diminishes with rising temperature, attributed to the augmented shear thermal effect. They [
20] further found that MgO nanoparticles reduce the viscosity of 5W30 lubricant, with the spherical nanoparticles functioning as roller balls between fluid layers. Mustafa et al. [
21] observed that at low concentrations of TiO
2–CuO NPs, the mobility between nano-lubricating oil is facilitated, slightly reducing viscosity. However, at higher concentrations, due to agglomeration or increased particle size, the movement between oil layers is hindered, resulting in elevated viscosity. Sui et al. [
22] examined the viscosification effects of four types of SiO
2 on PAO100, noting that while nanoparticles modified with different functional groups did not significantly impact PAO at 100 °C, variations in nanoparticle size did affect PAO viscosity at this temperature.
In the realm of nanofluid viscosity, comprehensive research has elucidated the influence of nanomaterials on the viscosity attributes of fluids. The viscosity of nanofluids is intricately linked to the nanomaterials’ size, density, ultrasonic treatment time, and interfacial interactions. For example, Abdelhalim et al. [
23] observed an increase in the viscosity of nanofluids with the incorporation of larger Au nanoparticles, reinforcing the notion that nanoparticle size is a critical determinant of nanofluid viscosity. Dehghani et al. [
24] compared the viscosities of WO
3 and Al
2O
3 nanoparticles dispersed in deionized water and liquid paraffin, noting higher viscosities in the former, which may be attributed to WO
3’s greater density and reduced Brownian motion velocity, underscoring density’s role in influencing lubrication characteristics. Zhang et al. [
25] conducted an experimental investigation into the viscosity of hydrophilic TiO
2–water and hydrophobic TiO
2–water nanofluids, discovering that hydrophilic nanoparticles form water-attracting layers more swiftly than hydrophobic ones, leading to higher viscosities in nanofluids with hydrophilic nanoparticles.
These investigations enhance our comprehension of how nanoparticles affect fluid viscosity and offer empirical data elucidating the mechanisms by which nanoparticles modulate viscosity. Nonetheless, a consolidated consensus on the impact of nanoparticles on viscosity and the associated mechanisms remains elusive. Therefore, to gain a deeper understanding of nanomaterials’ effects on lubricant viscosity, further research is imperative. In this study, we examine the impact of dialkyl dithiophosphate (HDDP) and copper HDDP-modified (CuDDP) nanoparticles on the kinematic viscosity of various base oils. Diverging from prior research, we introduce the aniline point as a metric of lubricant polarity. By integrating the aniline point, we formulate a novel equation intended to characterize the viscosity of base oils infused with nanoparticles across different volume fractions. This innovative methodology offers a fresh lens through which to view the impact of nanoparticles on lubricant viscosity. Through rigorous analysis of the correlations between predicted dynamic viscosity and measured data, we aim to uncover regularity and determinants. This endeavor enhances our understanding of how nanoparticles influence lubricant viscosity. Finally, we undertake a thorough comparison of the experimental outcomes with the computational results to validate the accuracy of the newly developed formulae. This comparison is instrumental in ascertaining the practical applicability of our theoretical model and establishes a groundwork for future inquiries.
2. Materials and Methods
2.1. Material Characterization
Fourier-transform infrared (FTIR) spectroscopy (Tensor II, Bruker, Billerica, MS, USA) covering a wavelength range of 400 cm−1 to 4000 cm−1 was employed to ascertain the composition of the modifier. The thermal stability and modifier content of the sample were examined using a thermogravimetric analyzer (TGA/DSC3+, Mettler Toledo, Greifensee, Switzerland). The thermal analysis was conducted in a nitrogen atmosphere, with a heating rate of 10 °C per minute, spanning from 25 °C to 900 °C. To eliminate impurities, the sample was maintained at 100 °C for 5 min before the analysis. Additionally, the morphology and size of the CuDDP nanoparticles were characterized using transmission electron microscopy (TEM, JEM-F200, JEOL, Tokyo, Japan). This technique provided detailed insights into the nanostructure of CuDDP nanoparticles, which is crucial for understanding its interactions and performance in lubricant applications.
2.2. Sample Preparation
In our experiments, oil-soluble copper nanoparticles (CuNPs) and CuNPs surface-coated with oil-soluble dialkyl dithiophosphate (CuDDP) prepared by the Nanomaterials Engineering and Technology Research Center of Henan University (Kaifeng, China) were used as the nanoadditives. CuDDP nanoparticles were synthesized by means of a redox surface modification technique [
26]. They were dispersed in base oils 150N, alkylated naphthalene (AN5), and diisooctyl sebacate (DIOS), as well as polyalphaolefins (PAO4, PAO6, PAO10, PAO40, and PAO100) at mass fractions of 0.5%, 1.0%, 1.5%, 2.0% and 2.5%. HDPP was dispersed in base oils 150N, AN5, DIOS and PAO6 at mass fractions of 0.5%, 1.0%, 1.5%, 2.0% and 2.5%, respectively. The eight base oils employed in the experiment were obtained from Qingdao Lubemater Group (Shandong, China). The typical physical properties of these oils are delineated in
Table 1. The lubricant sample was mixed ultrasonically for 15 min to achieve uniform dispersion of the nanoadditives CuDDP and HDDP. CuDDP had good dispersion stability in the base oil used in the experiment, and no samples showed obvious precipitation after standing for 7 days.
2.3. Viscosity and Density Tests
A viscometer (SVM3001, Anton Paar, Styria, Austria) was employed to determine the kinematic viscosity of the lubricants. It uses the oscillating piston method to measure the density and dynamic viscosity of the sample and can adjust the temperature and calculate the kinematic viscosity automatically. At the end of the measurement, the measuring cell was fully washed with petroleum ether and anhydrous ethanol and dried by blowing. During the experimental procedure, the thermometer showed an expanded (k = 2) uncertainty of 0.03 °C. Relative expanded (k = 2) uncertainty of 0.35% was estimated for the kinematic viscosity.
The densities of CuDDP and PAO4 dispersions at various concentrations were quantified employing SVM3001. Subsequently, the densities of CuDDP were extrapolated utilizing the equations derived from curve fitting. The expanded (k = 2) uncertainty of density measurements performed with the SVM3001 was 0.0005 g·cm−3.
2.4. Aniline Point Test
The aniline point of the eight base oils used in the experiment was tested with a petroleum product aniline point tester (DZY-013A, Dalian Instruments and Meters Co., Ltd., Dalian, China), and details about the test method are described in GB/T262 “Determination of Aniline Point of Petroleum Products”.
4. Conclusions
Under the condition that the experimental temperature was 40 °C and the additive mass fraction ranged from 0.5% to 2.5%, we studied the effects of dialkyl dithiophosphate (HDDP) copper-modified (CuDDP) nanoparticles on the dynamic kinematic viscosity of mineral oils 150N, alkylated naphthalene (AN5), diisooctyl sebacate (DIOS), and polyalphaolefins (PAO4, PAO6, PAO10, PAO40, and PAO100). Based on classical formulae and experimental data, a novel equation was developed to quantify the interfacial interaction between the nanoparticles and base oil using aniline points, elucidating the impact of CuDDP nanoparticles on the viscosity of lubricating oil. The main findings are as follows.
CuDDP reduces the viscosity of higher-viscosity base oils, such as PAO40 and PAO100, and can increase the viscosity of lower-viscosity base oils, such as 150N, AN5, DIOS, PAO4, PAO6, and PAO10. The influence of CuDDP on the viscosity of base oils is governed by the interfacial slip effect and the nanoparticles’ shear resistance. When the interfacial slip effect predominates, it can lead to a more significant decrease in viscosity compared to the viscosity-increasing anti-shear effect of the nanoparticles. Conversely, when shear resistance is more pronounced, an increase in viscosity occurs.
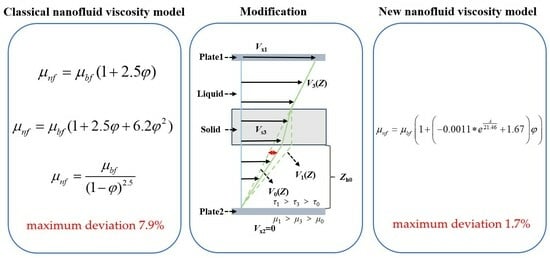
Experimental dynamic viscosity of eight base oils containing CuDDP was compared with values calculated using three classical nanofluid viscosity formulae. A significant deviation was observed between the predicted and experimental viscosity values, with the maximum deviation reaching 7.9%. This discrepancy indicates that traditional nanofluid viscosity equations cannot accurately characterize the effect of CuDDP on the viscosity of base oils.
A modified equation was developed by incorporating specific interfacial effects into Einstein’s viscosity formula and quantifying these effects using aniline points. This new equation elucidates the relationship between the base oil’s aniline points and the viscosity of CuDDP nanoparticles, yielding more accurate viscosity predictions for nanolubricants. The deviation in the predicted values from the experimental data is less than 1.7%, marking a significant improvement over traditional nanofluid viscosity models.