Tribocorrosion of Fe-Based Amorphous Coating in Simulated Body Fluids
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Characterization
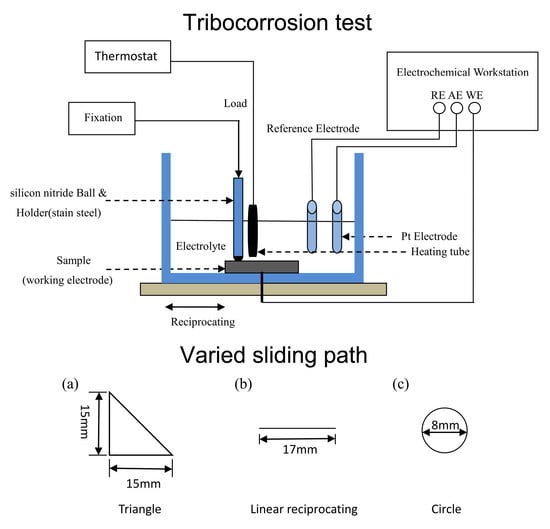
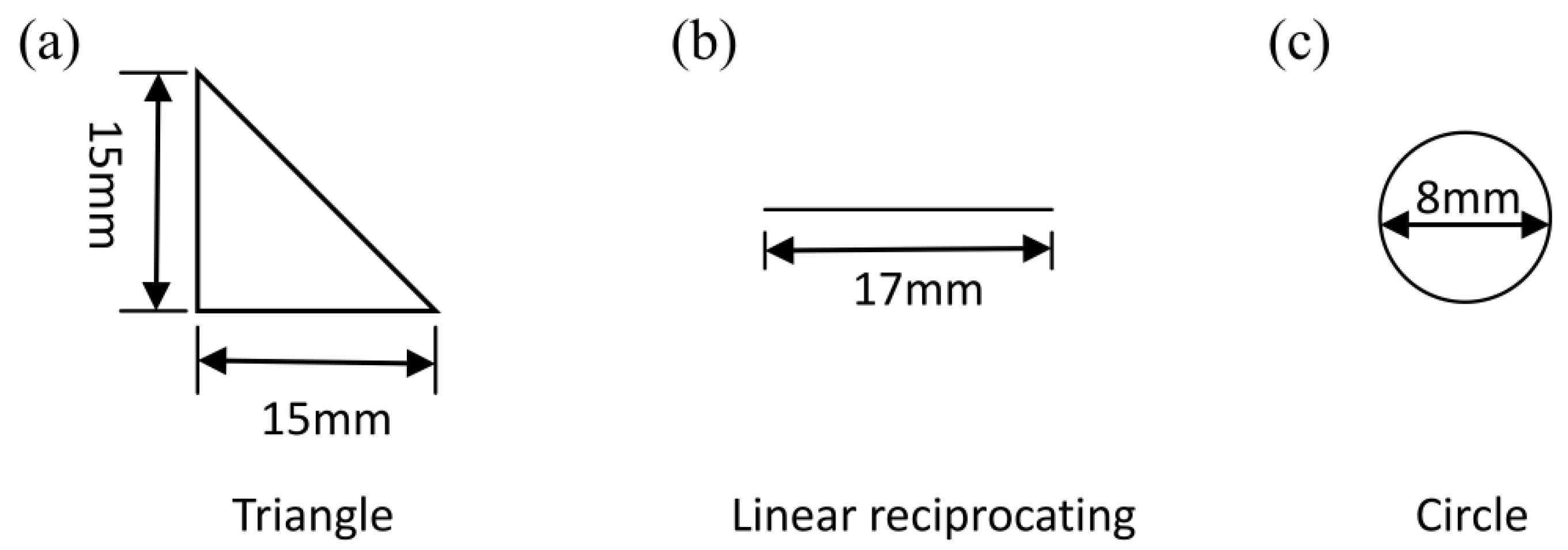
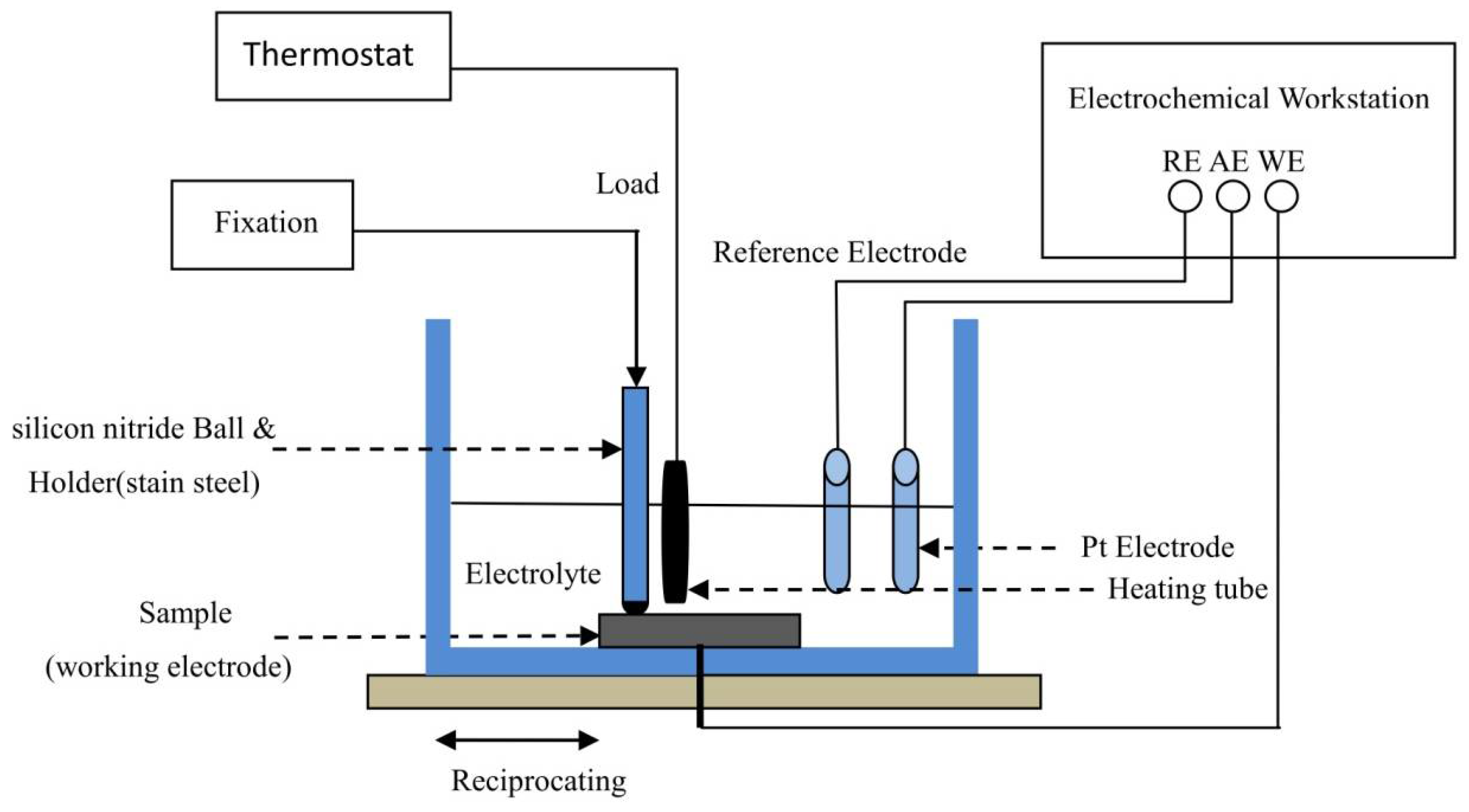
2.3. TribocorrosionTests
3. Results and Discussion
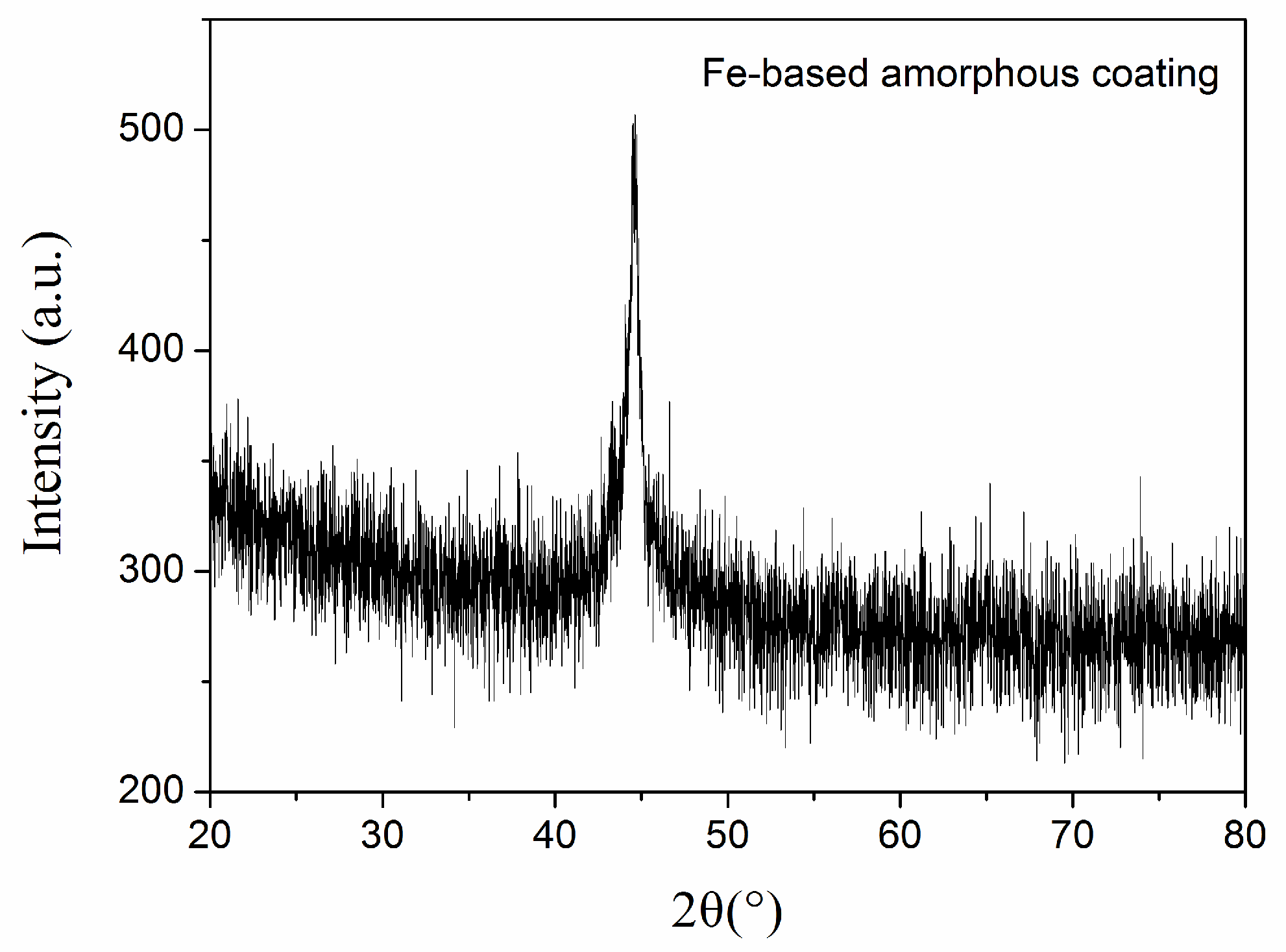
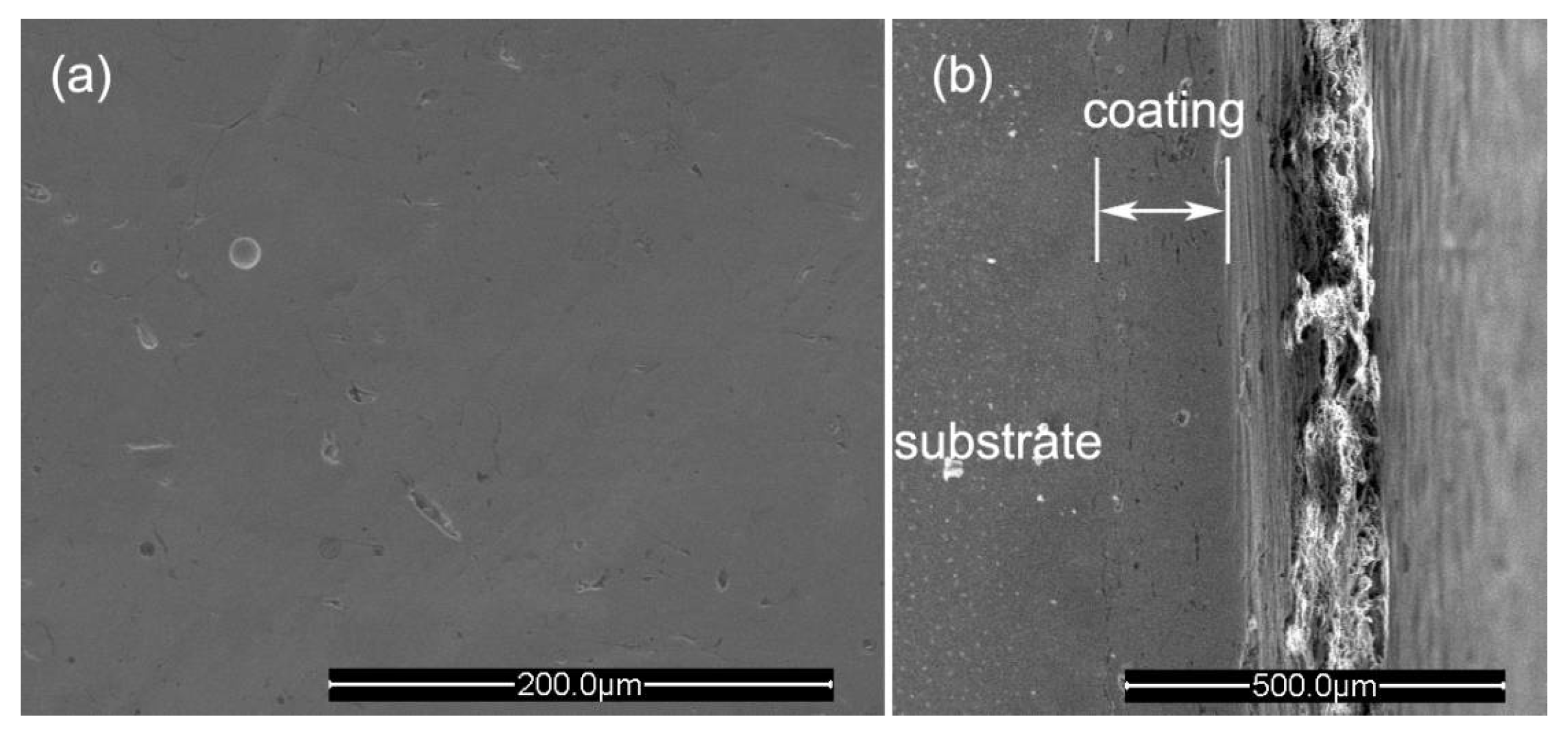
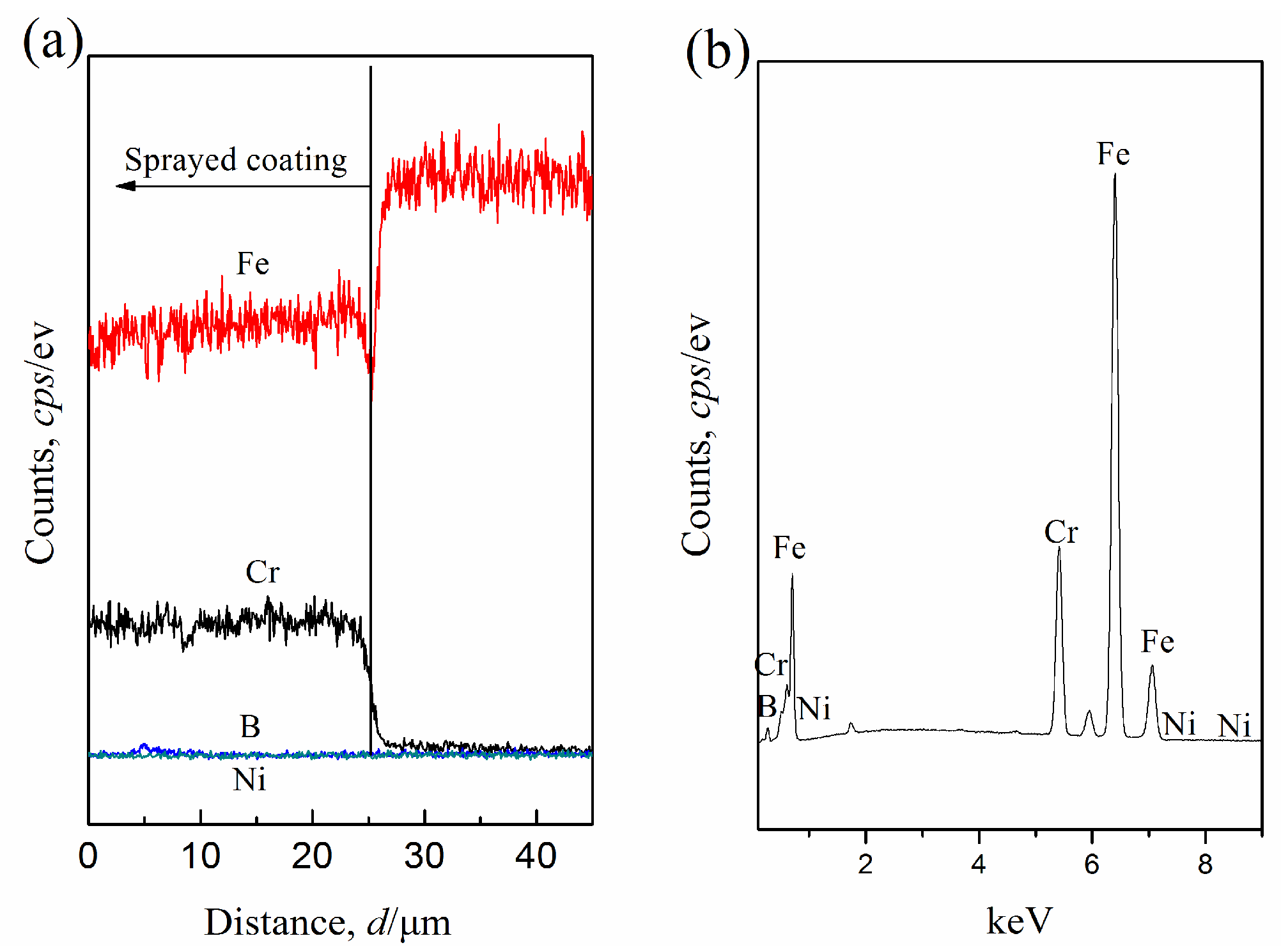
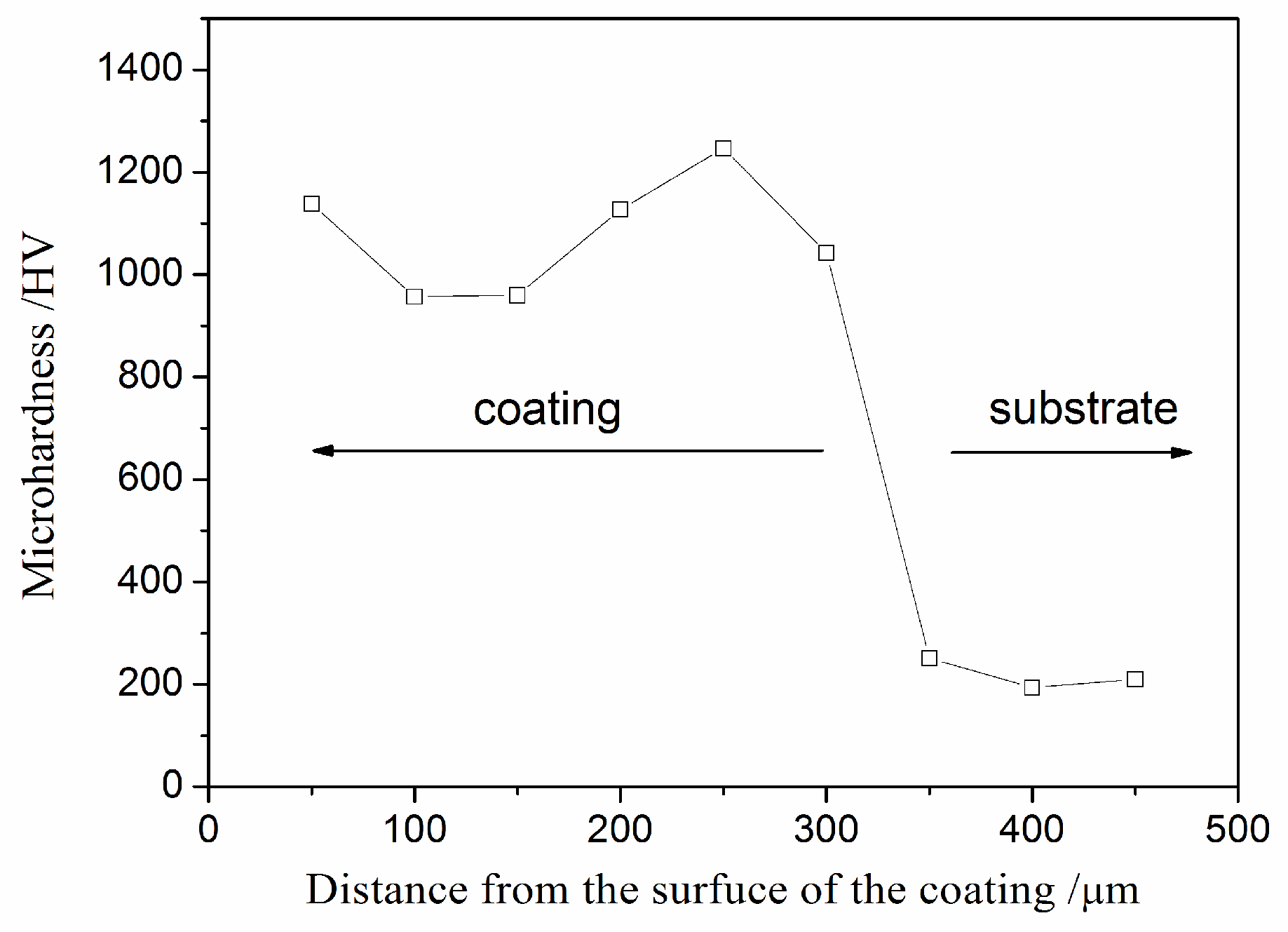
3.1. Microstructure and Hardness
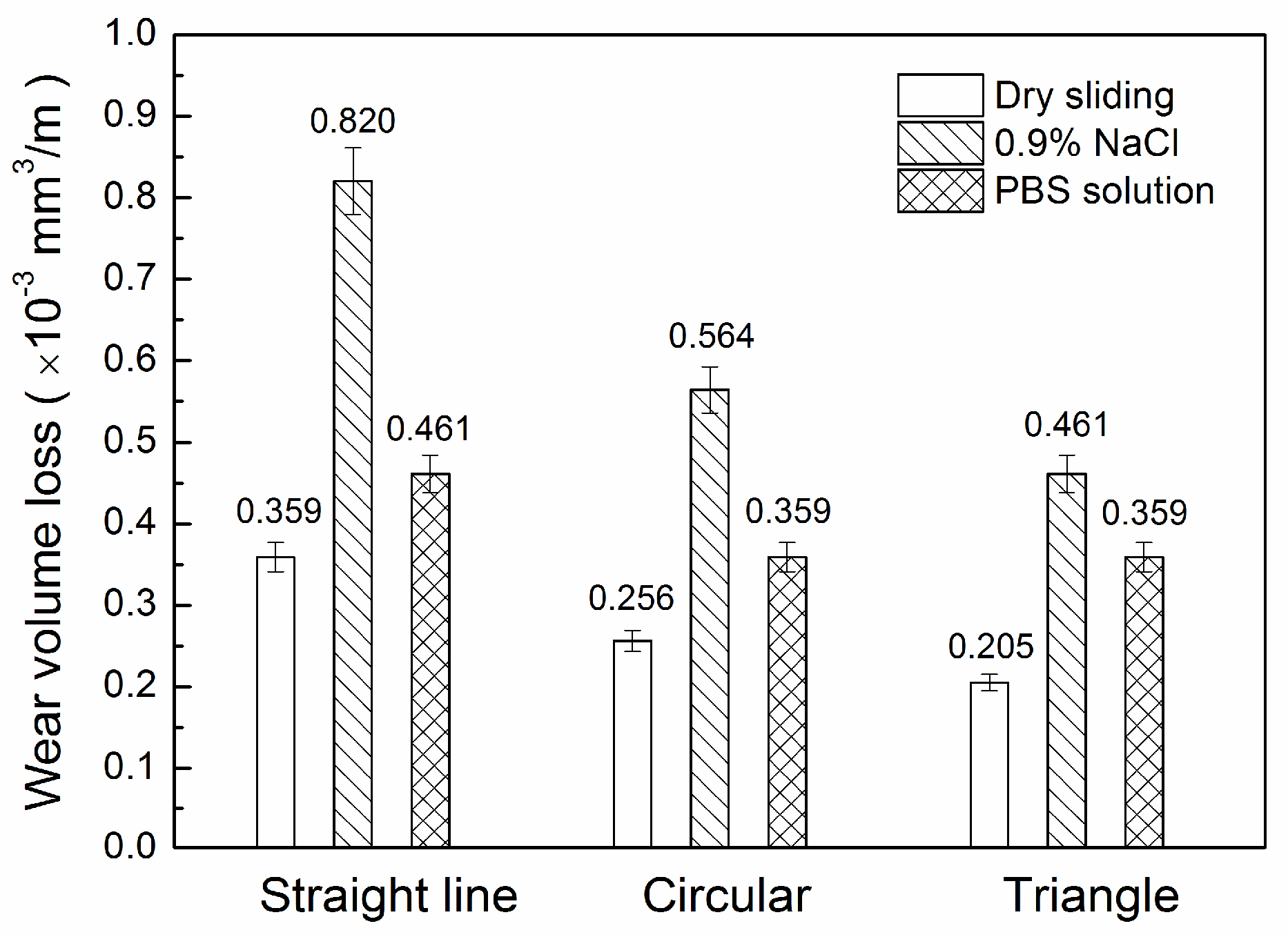
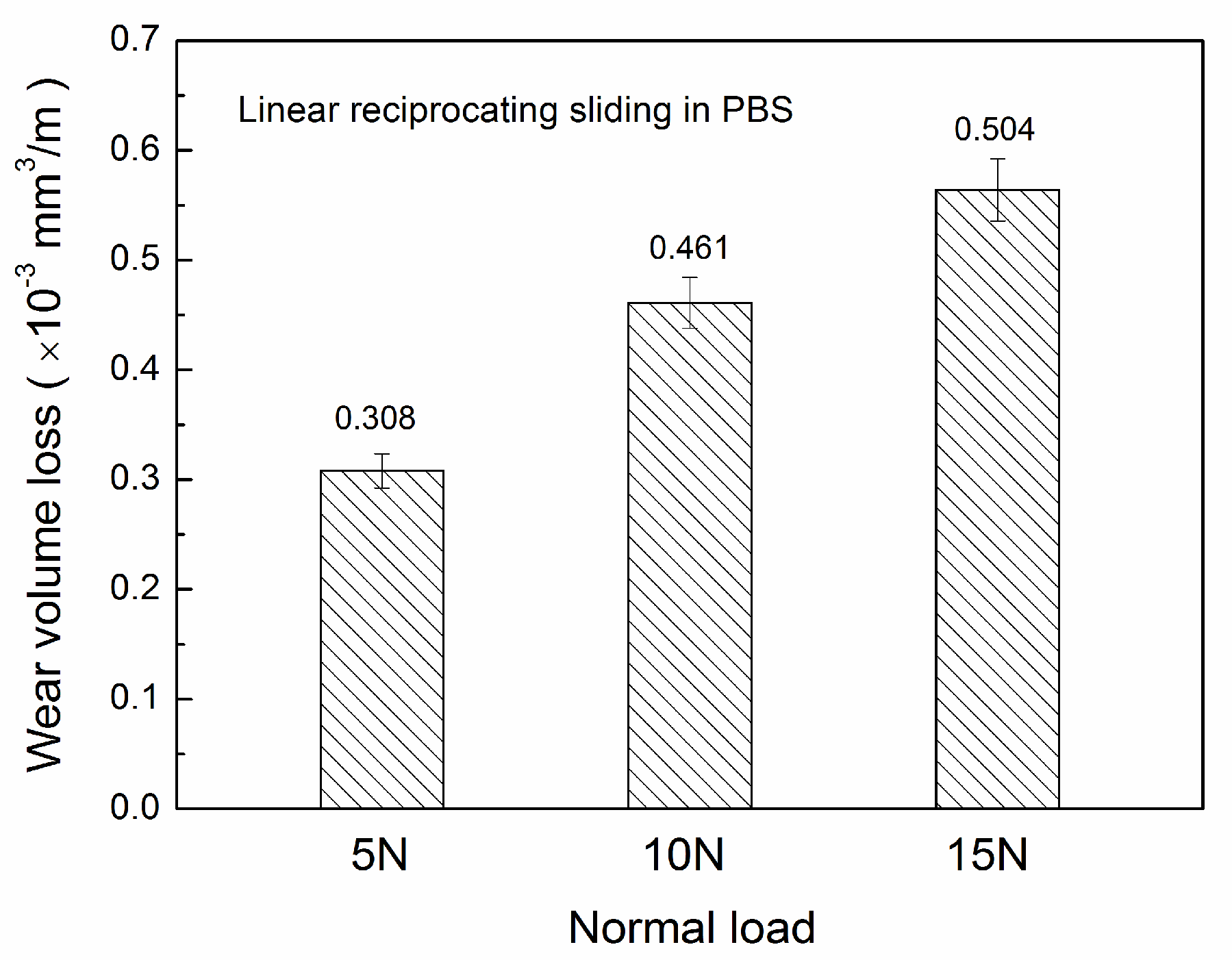
3.2. Wear Loss
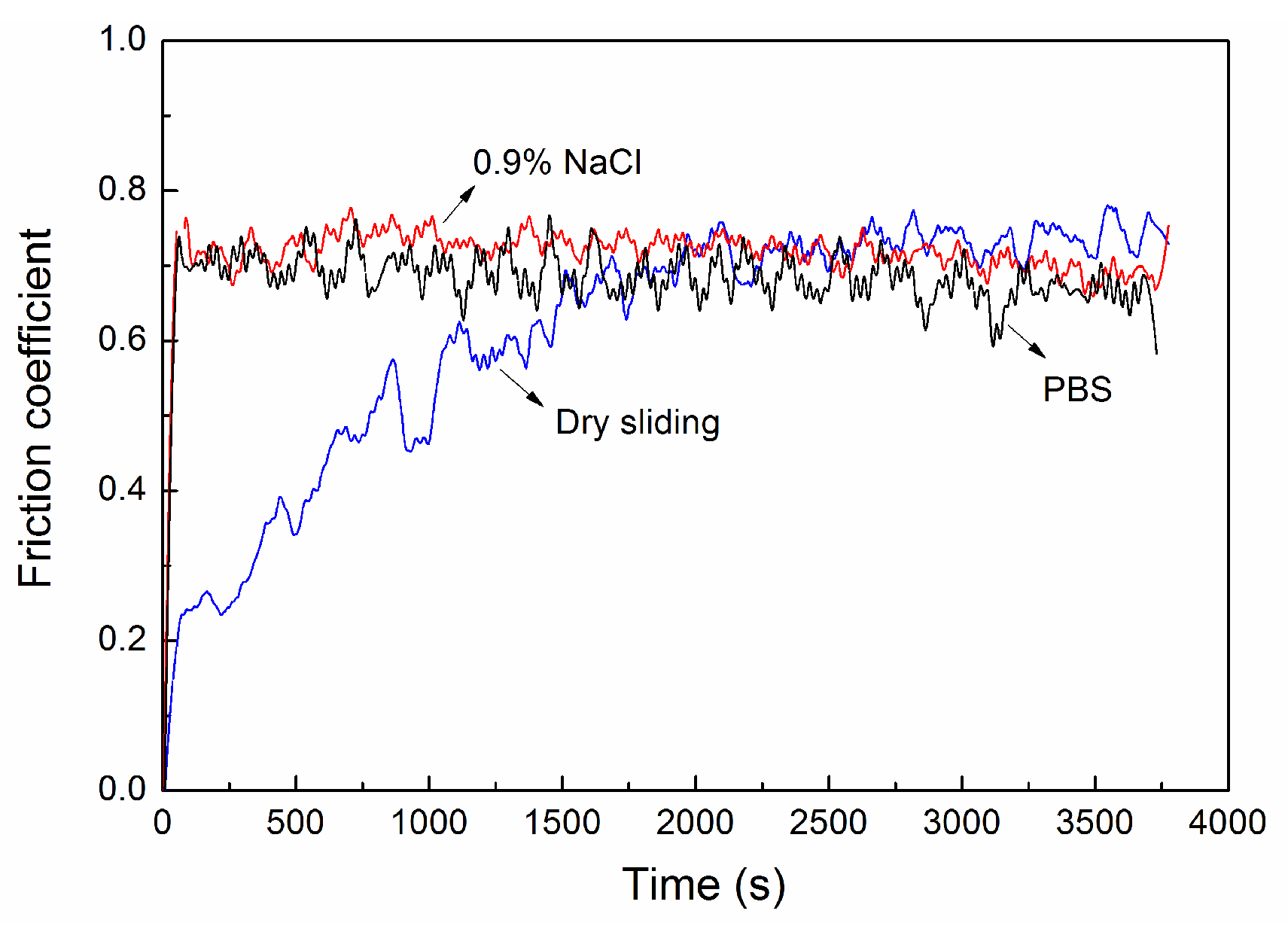
3.3. Friction Coefficient
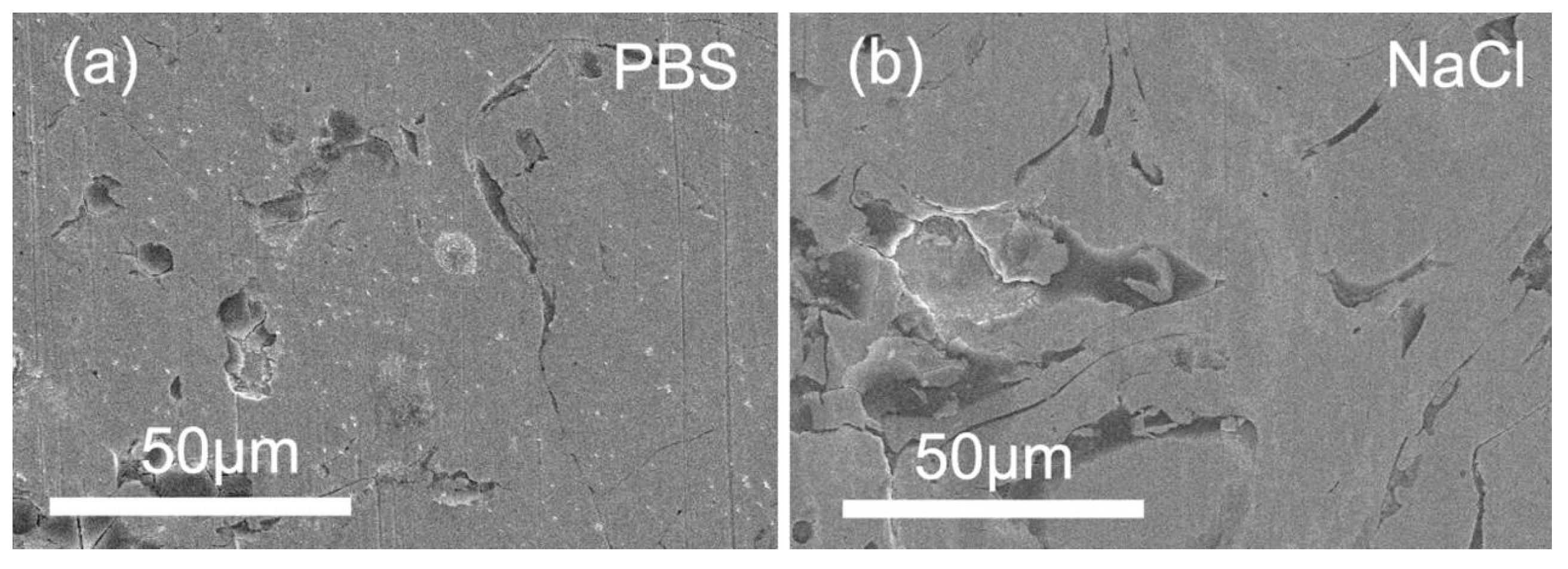
3.4. Wear Topography
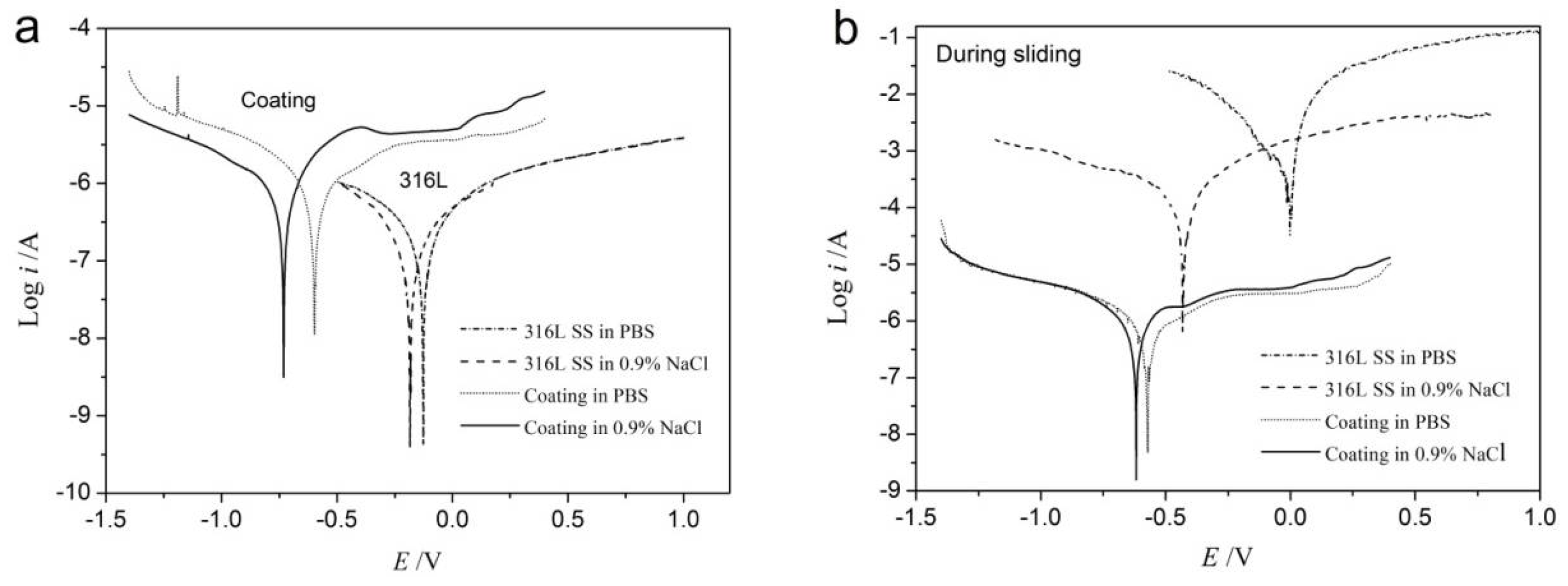
3.5. Tribocorrosion Behaviour
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zivic, F.; Babic, M.; Mitrovic, S.; Vencl, A. Continuous control as alternative route for wear monitoring by measuring penetration depth during linear reciprocating sliding of Ti6Al4V alloy. J. Alloys Compd. 2011, 509, 5748–5754. [Google Scholar] [CrossRef]
- Alves, S.A.; Bayón, R.; Viteri, V.S.D.; Garcia, M.P.; Igartua, A.; Fernandes, M.H.; Rocha, L.A. Tribocorrosion Behavior of Calcium and Phosphorous-Enriched Titanium Oxide Films and Study of Osteoblast Interactions for Dental Implants. J. Bio Tribo Corros. 2015, 1, 1–23. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, H.S.; Jang, J.W.; Yi, S. Corrosion behavior in a 3.5 wt% NaCl solution of amorphous coatings prepared through plasma-spray and cold-spray coating processes. Met. Mater. Int. 2014, 20, 1053–1057. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk amorphous metal—An emerging engineering material. JOM 2002, 54, 40–43. [Google Scholar]
- Inoue, A.; Shinohara, Y.; Gook, J.S. Thermal and Magnetic Properties of Bulk Fe-based Glassy Alloys Prepared by Copper Mold Casting. Mater. Trans. JIM 1995, 36, 1427–1433. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, H.F.; Cheng, Y.; Wei, S.C.; Zheng, Y.F. Corrosion performances of a Nickel-free Fe-based bulk metallic glass in simulated body fluids. Electrochem. Commun. 2009, 11, 2187–2190. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Z.; Lin, P.; Cheng, J.; Zhang, J.; Zhang, X. Microstructure and Corrosion Resistance of Fe-Based Coatings Prepared by Twin Wires Arc Spraying Process. J. Therm. Spray Technol. 2013, 23, 333–339. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yano, S.; Kimura, H.; Inoue, A. Mechanical property of Fe-base metallic glass coating formed by gas tunnel type plasma spraying. Surf. Coat. Technol. 2008, 202, 2513–2518. [Google Scholar] [CrossRef]
- Lee, H.B.; Wu, M.Y. A Study on the Corrosion and Wear Behavior of Electrodeposited Ni–W–P Coating. Metall. Mater. Trans. A 2017, 48, 4667–4680. [Google Scholar] [CrossRef]
- Cheng, J.B.; Wang, Z.H.; Xu, B.S. Wear and Corrosion Behaviors of FeCrBSiNbW Amorphous/Nanocrystalline Coating Prepared by Arc Spraying Process. J. Therm. Spray Technol. 2012, 21, 1025–1031. [Google Scholar] [CrossRef]
- Ji, X.; Wang, H.; Bao, Y.; Zheng, D. Wear resistance of CuZr-based amorphous-forming alloys against bearing steel in 3.5% NaCl solution. Philos. Mag. 2017, 97, 3042–3054. [Google Scholar] [CrossRef]
- Tao, P.; Yang, Y.; Xie, Z.; He, Y. Research on friction and wear behavior of a bulk metallic glass under different sliding velocity. Mater. Lett. 2015, 156, 177–179. [Google Scholar] [CrossRef]
- Bennett, D.; Humphreys, L.; O’Brien, S.; Kelly, C.; Orr, J.; Beverland, D.E. The influence of wear paths produced by hip replacement patients during normal walking on wear rates. J. Orthop. Res. 2008, 26, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.I.; Mischler, S. Effect of the environment on wear ranking and corrosion of biomedical CoCrMo alloys. J. Mater. Sci. Mater. Med. 2011, 22, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, D.; Liang, X.; Chen, Y. Wear Behaviors of Arc-Sprayed FeBSiNb Amorphous Coatings. Tribol. Lett. 2015, 60, 22. [Google Scholar] [CrossRef]
| Element | C | Si | Mn | P | S | V | Nb |
|---|---|---|---|---|---|---|---|
| Content (wt %) | ≤0.2 | ≤0.55 | 1.0–1.6 | ≤0.045 | ≤0.045 | 0.02–0.15 | 0.015–0.06 |
| Element | C | Si | Mn | P | S | Cr | Ni | Mo |
|---|---|---|---|---|---|---|---|---|
| Content (wt %) | ≤0.08 | ≤1.0 | ≤2.0 | ≤0.035 | ≤0.03 | 16.0–18.5 | 10.0–14.0 | 2.0–3.0 |
| Element | B | Cr | Ni | Fe |
|---|---|---|---|---|
| Content (wt %) | 2.40 | 16.29 | 0.14 | 81.18 |
| Materials and Environments | Static | Dynamic | ||
|---|---|---|---|---|
| Icorr (μA/cm2) | Ecorr (V) | Icorr (μA/cm2) | Ecorr (V) | |
| 316L in PBS | 0.174 | −0.126 | 644.6 | −0.002 |
| 316L in 0.9% NaCl | 0.103 | −0.184 | 95.99 | −0.433 |
| Amorphous coating in PBS | 0.786 | −0.597 | 0.944 | −0.572 |
| Amorphous coating in 0.9% NaCl | 0.664 | −0.731 | 0.907 | −0.619 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Ji, X.; Ji, C.; Zhang, Y.; Wang, H. Tribocorrosion of Fe-Based Amorphous Coating in Simulated Body Fluids. Lubricants 2018, 6, 37. https://doi.org/10.3390/lubricants6020037
Luo C, Ji X, Ji C, Zhang Y, Wang H. Tribocorrosion of Fe-Based Amorphous Coating in Simulated Body Fluids. Lubricants. 2018; 6(2):37. https://doi.org/10.3390/lubricants6020037
Chicago/Turabian StyleLuo, Chanyuan, Xiulin Ji, Cuicui Ji, Yingtao Zhang, and Hui Wang. 2018. "Tribocorrosion of Fe-Based Amorphous Coating in Simulated Body Fluids" Lubricants 6, no. 2: 37. https://doi.org/10.3390/lubricants6020037