Dispersion Stability and Lubrication Performance Correlation of Vegetable Oil-In-Water Emulsions with Nanoparticle-Shielded Oil Droplets
Abstract
:1. Introduction
2. Methodology
2.1. Materials and Preparation of Emulsions
2.2. Characterisation of NPs and VO/W Emulsions
2.3. Lubricity of VO/W Emulsions
3. Results and Discussion
3.1. Size Distribution of the Dispersed Droplets
3.2. UV–Vis Spectrophotometry Analysis
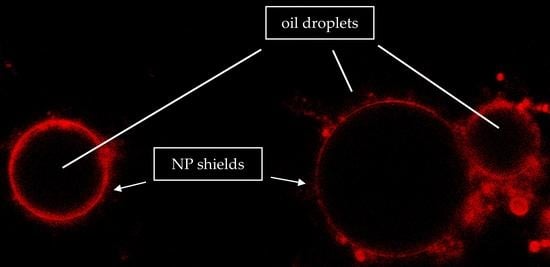
3.3. CLSM Analysis of Emulsions
3.4. Friction and Wear
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Taheri, R.; Kosasih, B.; Zhu, H.; Tieu, A. Surface film adsorption and lubricity of soybean oil in water emulsion and triblock copolymer solution: A comparative study. Lubricants 2017, 5, 1. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.; Perez, J. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Alves, S.; Barros, B.; Trajano, M.; Ribeiro, K.; Moura, E. Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribol. Int. 2013, 65, 28–36. [Google Scholar] [CrossRef]
- Doll, K.M.; Sharma, B.K. Emulsification of Chemically Modified Vegetable Oils for Lubricant Use. J. Surfactants Deterg. 2011, 14, 131–138. [Google Scholar] [CrossRef]
- Pashley, R.M. Effect of Degassing on the Formation and Stability of Surfactant-Free Emulsions and Fine Teflon Dispersions. J. Phys. Chem. B 2003, 107, 1714–1720. [Google Scholar] [CrossRef]
- Israelachvili, J.; Pashley, R. The hydrophobic interaction is long range, decaying exponentially with distance. Nature 1985, 300, 341–342. [Google Scholar] [CrossRef]
- Kamogawa, K.; Akatsuka, H.; Matsumoto, M.; Yokoyama, S.; Sakai, T.; Sakai, H.; Abe, M. Surfactant-free O/W emulsion formation of oleic acid and its esters with ultrasonic dispersion. Colloids Surf. A 2001, 180, 41–53. [Google Scholar] [CrossRef]
- Ridel, L.; Bolzinger, A.; Delepine, N.G.; Dugas, P.Y.; Chevalier, Y. Pickering emulsions stabilized by charged nanoparticles. Soft Matter 2016, 12, 7564–7576. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Stability of oil-in-water emulsions stabilised by silica particles. Phys. Chem. Chem. Phys. 1999, 1, 3007–3016. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Catastrophic Phase Inversion of Water-in-Oil Emulsions Stabilized by Hydrophobic Silica. Langmuir 2000, 16, 2539–2547. [Google Scholar] [CrossRef]
- Simovic, S.; Prestidge, C. Hydrophilic Silica Nanoparticles at PDMS Droplet-Water Interface. Langmuir 2003, 19, 3785–3792. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.-A.; Chevalier, Y. Effects of solid particle content on properties of o/w Pickering emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, G.; Oli, Y.; Zhang, Z.; Xue, Q. Study on tribological properties of oleic acid-modified TiO2 nanoparticle in water. Wear 2002, 252, 454–458. [Google Scholar] [CrossRef]
- Wu, Y.; Kao, M. Using TiO2 nanofluid additive for engine lubrication oil. Ind. Lubr. Tribol. 2011, 63, 440–445. [Google Scholar] [CrossRef]
- Riazian, M.; Montazeri, N.; Biazar, E. Nano Structural Properties of TiO2-SiO2. Orient. J. Chem. 2011, 27, 903–910. [Google Scholar]
- Wei, D.; Dave, R.; Pfeffer, R. Mixing and characterization of nanosized powders: An assessment of different techniques. J. Nanopart. Res. 2002, 4, 21–41. [Google Scholar] [CrossRef]
- Taheri, R.; Kosasih, B.; Zhu, H.; Tieu, A.K. Suspension stability and lubricity of eco-friendly vegetable oil-in-water emulsions stabilised by TiSiO4 nanoparticles. In Proceedings of the 6th World Tribology Congress, Beijing, China, 17–22 September 2017. [Google Scholar]
- Vashisth, C.; Whitby, C.P.; Fornasiero, D.; Ralston, J. Interfacial displacement of nanoparticles by surfactant molecules in emulsions. J. Colloid Interface Sci. 2010, 349, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Taheri, R.; Kosasih, B.; Zhu, H.; Tieu, A.K. Phase behaviour and lubricity of aqueous PEO–PPO–PEO and PPO-PEO-PPO triblock copolymer solutions. Tribol. Trans. 2017, 60, 460–468. [Google Scholar] [CrossRef]
- Fox, N.; Stachowiak, G. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.; Clint, J. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Binks, B.; Whitby, C. Nanoparticle silica-stabilised oil-in-water emulsions: Improving emulsion stability. Colloids Surf. A 2005, 253, 105–115. [Google Scholar] [CrossRef]
- Simovic, S.; Prestidge, C.A. Adsorption of Hydrophobic Silica Nanoparticles at the PDMS Droplet-Water Interface. Langmuir 2003, 19, 8364–8370. [Google Scholar] [CrossRef]
- Sharma, P.; Baek, I.-H.; Cho, T.; Park, S.; Lee, K.B. Enhancement of thermal conductivity of ethylene glycol based silver nanofluids. Powder Technol. 2011, 208, 7–19. [Google Scholar] [CrossRef]
- Lacava, J.; Ouali, A.A.; Raillard, B.; Kraus, T. On the behaviour of nanoparticles in oil-in-water emulsions with different surfactants. Soft Matter 2014, 10, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Oh, S.G.; Suh, K.D.; Kim, D.G.; Sohn, D. Preparation of silver nanoparticles in hexagonal phase formed by nonionic Triton X-100 surfactant. Colloids Surf. A 2002, 210, 49–60. [Google Scholar] [CrossRef]
- Zareh-Desari, B.; Davoodi, B. Assessing the lubrication performance of vegetable oil-based nano-lubricants for environmentally conscious metal forming processes. J. Clean. Prod. 2016, 135, 1198–1209. [Google Scholar] [CrossRef]
- Katepalli, H.; Bose, A. Response of Surfactant Stabilized Oil-in-Water Emulsions to the Addition of Particles in an Aqueous Suspensio. Langmuir 2014, 30, 12736–12742. [Google Scholar] [CrossRef] [PubMed]
- Arditty, S.; Whitby, C.P.; Binks, B.P.; Schmitt, V.; Leal-Calderon, F. Some general features of limited coalescence in solid-stabilized emulsions. Eur. Phys. J. E 2003, 11, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, M.A. A review of two body abrasive wear. Wear 1974, 27, 1–17. [Google Scholar] [CrossRef]
- Peng, D.X.; Kang, Y.; Hwang, R.M.; Shyr, S.S.; Chang, Y.P. Tribological properties of diamond and SiO2 nanoparticles added in paraffin. Tribol. Int. 2009, 42, 911–917. [Google Scholar] [CrossRef]
- Trezona, R.I.; Allsopp, D.N.; Hutchings, I.M. Transitions between two-body and three-body abrasive wear: Influence of test conditions in the microscale abrasive wear test. Wear 1999, 225–229, 205–214. [Google Scholar] [CrossRef]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, R.; Kosasih, B.; Zhu, H.; Tieu, A.K. Dispersion Stability and Lubrication Performance Correlation of Vegetable Oil-In-Water Emulsions with Nanoparticle-Shielded Oil Droplets. Lubricants 2018, 6, 55. https://doi.org/10.3390/lubricants6020055
Taheri R, Kosasih B, Zhu H, Tieu AK. Dispersion Stability and Lubrication Performance Correlation of Vegetable Oil-In-Water Emulsions with Nanoparticle-Shielded Oil Droplets. Lubricants. 2018; 6(2):55. https://doi.org/10.3390/lubricants6020055
Chicago/Turabian StyleTaheri, Reza, Buyung Kosasih, Hongtao Zhu, and Anh Kiet Tieu. 2018. "Dispersion Stability and Lubrication Performance Correlation of Vegetable Oil-In-Water Emulsions with Nanoparticle-Shielded Oil Droplets" Lubricants 6, no. 2: 55. https://doi.org/10.3390/lubricants6020055