Meta-Analysis Comparing Wettability Parameters and the Effect of Wettability on Friction Coefficient in Lubrication
Abstract
:1. Introduction
2. Results and Discussion
2.1. Defining Parameters to Characterize Wettability
2.2. Comparing Characteristic Parameters for Wettability
2.3. Dimensionless Wetting Parameters
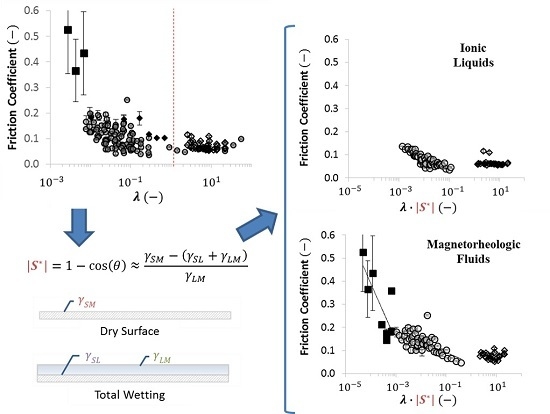
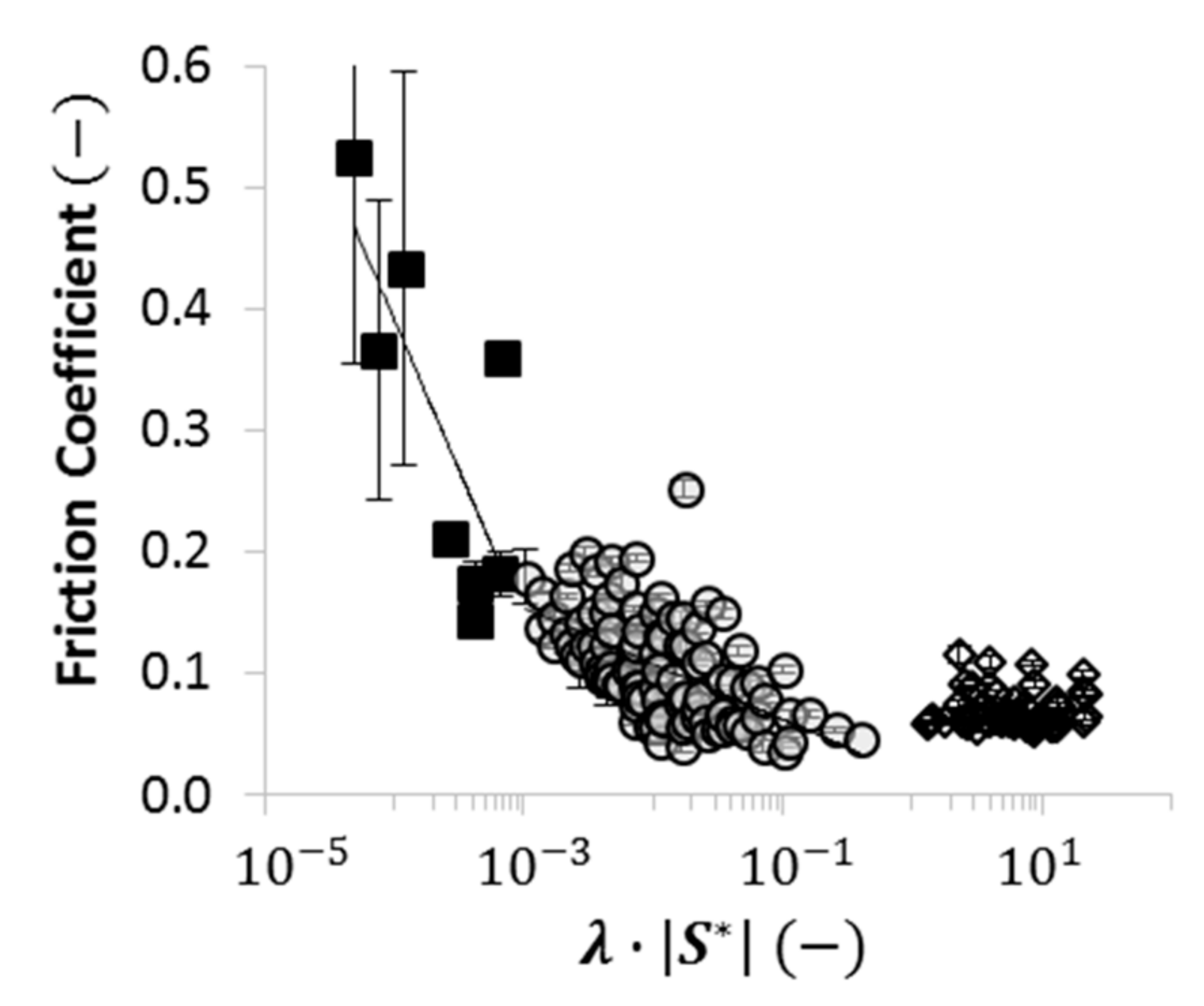
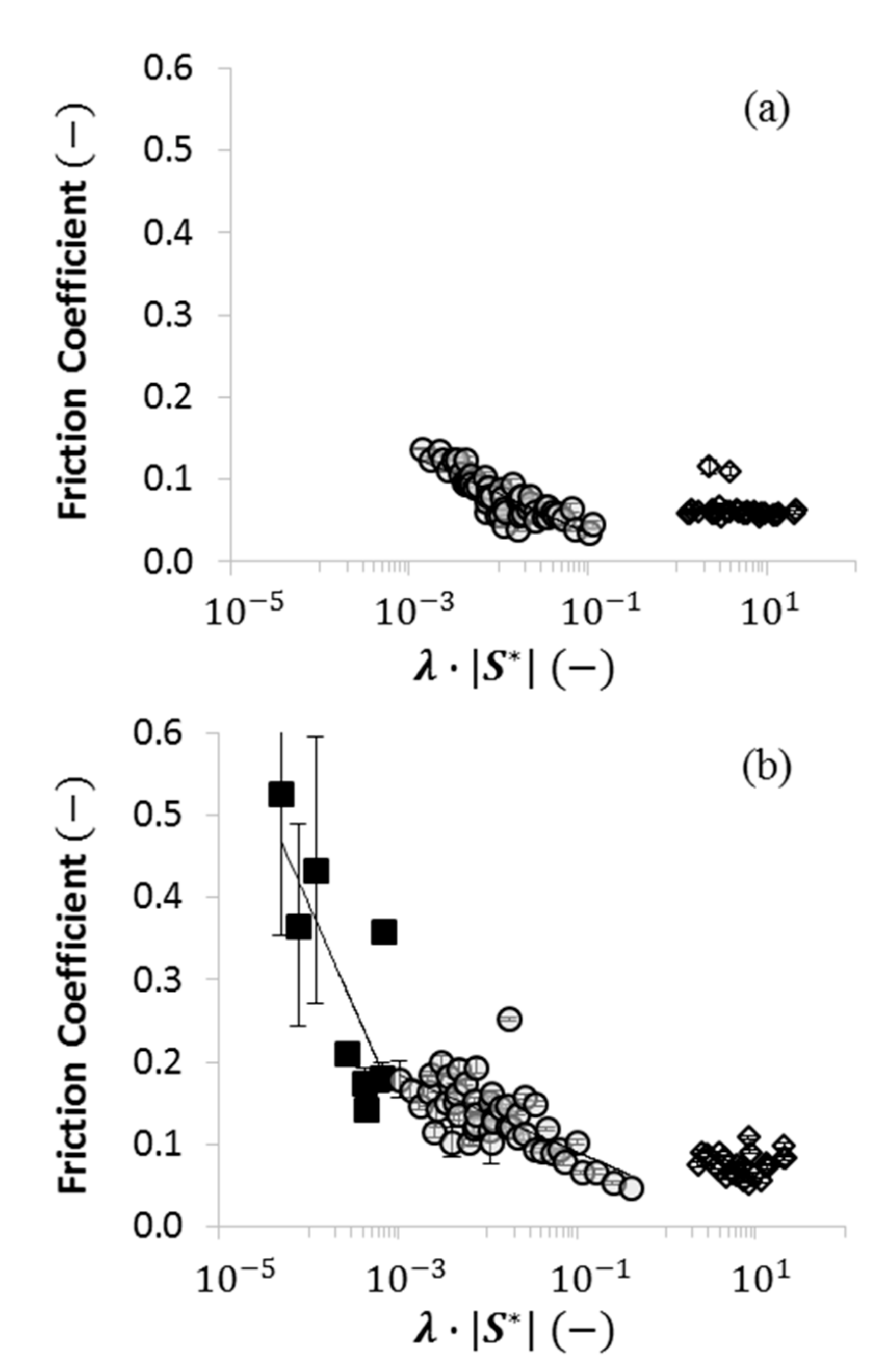
2.4. Using Wettability to Characterize Friction
- were low and consistent when was above approximately ;
- increased dramatically as decreased below approximately ; and
- increased moderately when .
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Parameter/Abbreviation | Description | Unit |
| Subscript for adhesion work per unit area | ||
| a-C:H | Hydrogenated amorphous diamond-like carbon | |
| [BMIM][SCN] | 1-Butyl-3-methylimidazolium thiocyanate | |
| [BMIM][NTF2] | 1-Butyl-3-methylimidazolium bis(trifluoromethanesulfonyl imige/“Triflimide” | |
| [BMIM][BF4] | 1-Butyl-3-methylimidazolium tetrafluoroborate | |
| [BMIM][PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate | |
| [BMIM][CH3COO] | 1-Butyl-3-methylimidazolium | |
| BL | Boundary lubrication regime | |
| Subscript for cohesion work per unit area | ||
| D | Superscript for disperse component of | |
| [EMIM][NTf2] | 1-Ethyl-3-methylimdazolium bis(trifluoromethanesulfonyl) imide/Triflimide” | |
| [EMIM][CF3SO3] | 1-Ethyl-3-methylimdazolium trifluoromethanesulfonate/“Triflate” | |
| F-DLC | Hydrogenated amorphous diamond-like carbon doped with silicon and fluorine | |
| Lubricant film thickness | ||
| HL | Hydrodynamic lubrication regime | |
| IL | Ionic liquid | |
| IL104 | Trihexyltetradecylphosphonium bis(2,4,4-trimethylpentyl) phosphinate (Cytec) | |
| LM | Subscript for the interface between the liquid and the surrounding medium | |
| ML | Mixed lubrication regime | |
| MR | Magnetorhelogical | |
| N-DLC | N-doped diamond-like carbon | |
| OWRK | Owens–Wendt–Tabel–Kaelble | |
| Superscript for polar component of | ||
| PAO | Polyalphaolefin oil | |
| PAO4 | Low-viscosity polyalphaolefin oil (Chevron Philips) | |
| PAO9 | High-viscosity polyalphaolefin oil (Chevron Philips) | |
| PDMS | Polydimethylsiloxane | |
| POM | Polyoxymethylene | |
| Spreading parameter | ||
| Si-DLC | Hydrogenated amophorous diamond-like carbon | |
| SL | Subscript for the interface between the surface and the liquid | |
| SM | Subscript for the interface between the surface and the surrounding medium | |
| Spreading parameter derived by Kalin and Polajnar | ||
| ta-C | Non-hydrogenated tetrahedral amorphous diamond-like carbon | |
| [THTDP][NTf2] | Trihexyltetradecyl phosphonium bis(trifluoromethylsulfonyl) amide | |
| [THTDA][NTf2] | Trihexyltetradecyl ammonium bis(trifluoromethylsulfonyl) | |
| Work per unit area | ||
| [1TD3HI][NTf2] | 1-Tetradecyl-3-hexyl imidazolium bis(trifluoromethylsulfonyl) amide | |
| Surface tension (and surface energy) | or | |
| Surface roughness (average or root mean square) roughness | ||
| Contact angle | ||
| Specific film thickness | ||
| Superscript for a dimensionless parameter |
References
- Tzanakis, I.; Hadfield, M.; Thomas, B.; Noya, S.M.; Henshaw, I.; Austen, S. Future perspectives on sustainable tribology. Renew. Sustain. Energy Rev. 2012, 16, 4126–4140. [Google Scholar] [CrossRef]
- Stern, D.I. Energy and economic growth in the USA. Energy Econ. 1993, 15, 137–150. [Google Scholar] [CrossRef]
- Hutchings, I.M. Tribology, Friction and Wear of Engineering Materials; Edward Arnold: London, UK, 1992; ISBN 034056184X. [Google Scholar]
- Maru, M.M.; Tanaka, D.K. Consideration of stribeck diagram parameters in the investigation on wear and friction behavior in lubricated sliding. J. Braz. Soc. Mech. Sci. Eng. 2007, 29, 55–62. [Google Scholar] [CrossRef]
- Xie, Z.; Rao, Z.; Ta-Na; Liu, L.; Chen, R. Theoretical and experimental research on the friction coefficient of water lubricated bearing with consideration of wall slip effects. Mech. Ind. 2016, 17, 106. [Google Scholar] [CrossRef]
- Bombard, A.J.F.; Gonçalves, F.R.; Shahrivar, K.; Ortiz, A.L.; de Vicente, J. Tribological behavior of ionic liquid-based magnetorheological fluids in steel and polymeric point contacts. Tribol. Int. 2015, 81, 309–320. [Google Scholar] [CrossRef]
- De Vicente, J.; Stokes, J.R.; Spikes, H.A. The frictional properties of Newtonian fluids in rolling—Sliding soft-EHL contact. Tribol. Lett. 2005, 20, 273–286. [Google Scholar] [CrossRef]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2013; ISBN 9780123977762. [Google Scholar]
- Kalin, M.; Polajnar, M. The correlation between the surface energy, the contact angle and the spreading parameter, and their relevance for the wetting behaviour of DLC with lubricating oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The effect of wetting and surface energy on the friction and slip in oil-lubricated contacts. Tribol. Lett. 2013, 52, 185–194. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The wetting of steel, DLC coatings, ceramics and polymers with oils and water: The importance and correlations of surface energy, surface tension, contact angle and spreading. Appl. Surf. Sci. 2014, 293, 97–108. [Google Scholar] [CrossRef]
- Blanco, D.; Bartolomé, M.; Ramajo, B.; Viesca, J.L.; González, R.; Hernández Battez, A. Wetting Properties of Seven Phosphonium Cation-Based Ionic Liquids. Ind. Eng. Chem. Res. 2016, 55, 9594–9602. [Google Scholar] [CrossRef] [Green Version]
- Brochard, F. Motions of droplets on solid surfaces inducted by chemical or thermal gradients. Langmuir 1989, 5, 432–438. [Google Scholar] [CrossRef]
- Blanco, D.; Viesca, J.L.; Mallada, M.T.; Ramajo, B.; González, R.; Battez, A.H. Wettability and corrosion of [NTf 2] anion-based ionic liquids on steel and PVD (TiN, CrN, ZrN) coatings. Surf. Coat. Technol. 2016, 302, 24–32. [Google Scholar] [CrossRef]
- Matczak, L.; Johanning, C.; Gil, E.; Smith, T.W.; Schertzer, M.J.; Iglesias Victoria, P. Effect of cation nature on the lubricating and physicochemical properties of three ionic liquids. Tribol. Int. 2018, 124, 23–33. [Google Scholar] [CrossRef]
- Chen, W.; Foster, A.S.; Alava, M.J.; Laurson, L. Stick-Slip Control in Nanoscale Boundary Lubrication by Surface Wettability. Phys. Rev. Lett. 2015, 114, 95502. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, K.; Zhang, S.; Amann, T.; Zhang, C. Anti-spreading behavior of 1,3-diketone lubricating oil on steel surfaces. Tribol. Int. 2018, 121, 108–113. [Google Scholar] [CrossRef]
- Lawes, S.D.; Hainsworth, S.V.; Blake, P.; Ryder, K.S.; Abbott, A.P. Lubrication of steel/steel contacts by choline chloride ionic liquids. Tribol. Lett. 2010, 37, 103–110. [Google Scholar] [CrossRef]
- Qu, J.; Bansal, D.G.; Yu, B.; Howe, J.Y.; Luo, H.; Dai, S.; Li, H.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; et al. Antiwear performance and mechanism of an oil-miscible ionic liquid as a lubricant additive. ACS Appl. Mater. Interfaces 2012, 4, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Bonaccurso, E.; Kappl, M.; Butt, H.-J. Hydrodynamic force measurements: Boundary slip of water on hydrophilic surfaces and electrokinetic effects. Phys. Rev. Lett. 2002, 88, 76103–76104. [Google Scholar] [CrossRef] [PubMed]
- Pit, R.; Hervet, H.; Leger, L. Direct experimental evidence of slip in hexadecane: Solid interface. Phys. Rev. Lett. 2000, 85, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Udagawa, Y.; Udagawa, H. Drag reduction of Newtonian fluid in a circular pipe with a highly water-repellent wall. J. Fluid Mech. 1999, 381, 225–238. [Google Scholar] [CrossRef]
- Maali, A.; Bhushan, B. Nanorheology and boundary slip in confined liquids using atomic force microscopy. J. Phys. Condens. Matter 2008, 20, 315201. [Google Scholar] [CrossRef]
- Raj, R.; Enright, R.; Zhu, Y.; Adera, S.; Wang, E.N. Unified Model for Contact Angle Hysteresis on Heterogeneous and Superhydrophobic Surfaces. Langmuir 2012, 28, 15777–15788. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, A.; Trouba, C.; Liu, H.; Li, L. Characterization of the Intrinsic Water Wettability of Graphite Using Contact Angle Measurements: Effect of Defects on Static and Dynamic Contact Angles. Langmuir 2017, 33, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Extrand, C.; Kumagai, Y. An experimental study of contact angle hysteresis. J. Colloid Interface Sci. 1997, 191, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Wu, R.; Li, D.; Hair, M.; Neumann, A. Study of the advancing and receding contact angles: Liquid sorption as a cause of contact angle hysteresis. Adv. Colloid Interface Sci. 2002, 96, 169–191. [Google Scholar] [CrossRef]
- Bormashenko, E. Wetting of real solid surfaces: New glance on well-known problems. Colloid Polym. Sci. 2013, 291, 339–342. [Google Scholar] [CrossRef]
- Bermudez, M.; Jimenez, A. Surface interactions in lubrication of titanium, aluminium, and titanium-aluminium alloys with the ionic liquid [C(2)mim]Tf2N under increasing temperature. Proc. Inst. Mech. Eng. Part J. 2012, 226, 977–990. [Google Scholar] [CrossRef]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Mahrova, M.; Conte, M.; Roman, E.; Nevshupa, R. Critical Insight into Mechanochemical and Thermal Degradation of Imidazolium-Based Ionic Liquids with Alkyl and Monomethoxypoly(ethylene glycol) Side Chains. J. Phys. Chem. C 2014, 118, 22544–22552. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P. Lubrication of Inconel 600 with ionic liquids at high temperature. Tribol. Int. 2009, 42, 1744–1751. [Google Scholar] [CrossRef]
- Iglesias, P.; Bermudez, M.; Carrion, F.; Martinez-Nicolas, G. Friction and wear of aluminium–steel contacts lubricated with ordered fluids-neutral and ionic liquid crystals as oil additives. Wear 2004, 256, 386–392. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, J.; Kang, K.H.; Kang, I.S.; Lee, S.J. Electrowetting-induced droplet detachment from hydrophobic surfaces. Langmuir 2014, 30, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar]
- Burkhart, C.T.; Maki, K.L.; Schertzer, M.J. Effects of Interface Velocity, Diffusion Rate, and Radial Velocity on Colloidal Deposition Patterns Left by Evaporating Droplets. ASME J. Heat Transf. 2017, 139, 111505. [Google Scholar] [CrossRef]
- Kudtarkar, K.; Johnson, M.; Iglesias, P.; Smith, T.W.; Schertzer, M.J. Effects of chemicla composition on the electromechanical properties of microfluidically synthesized hydrogel beads. ASME J. Fluids Eng. 2018, 140, 101103. [Google Scholar] [CrossRef]





| Source | Surface | Lubricant | Temp. | θ (°) | S | SP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polar | Disperse | Total | Polar | Disperse | Total | (°C) | (mN/m) | (mN/m) | ||||
| [10] | Steel | PAO4 | 4.8 | 24.2 | 29.0 | 12.1 | 31.4 | 43.5 | 11.1 | −0.02 | 12.34 | |
| [10] | Steel | PAO9 | 6.5 | 23.7 | 30.2 | 12.1 | 31.4 | 43.5 | 11.3 | −0.02 | 11.86 | |
| [10] | ta-C | PAO4 | 4.8 | 24.2 | 29.0 | 16.5 | 31.9 | 48.4 | 3.5 | 0.00 | 15.38 | |
| [10] | ta-C | PAO9 | 6.5 | 23.7 | 30.2 | 16.5 | 31.9 | 48.4 | 8.5 | −0.01 | 15.31 | |
| [10] | Si-DLC | PAO4 | 4.8 | 24.2 | 29.0 | 8.6 | 31.8 | 40.4 | 4.3 | 0.00 | 10.32 | |
| [10] | Si-DLC | PAO9 | 6.5 | 23.7 | 30.2 | 8.6 | 31.8 | 40.4 | 10.9 | −0.02 | 9.44 | |
| [10] | N-DLC | PAO4 | 4.8 | 24.2 | 29.0 | 7.8 | 32.5 | 40.2 | 6.8 | −0.01 | 10.24 | |
| [10] | N-DLC | PAO9 | 6.5 | 23.7 | 30.2 | 7.8 | 32.5 | 40.2 | 13.8 | −0.03 | 9.25 | |
| [10] | a-C:H | PAO4 | 4.8 | 24.2 | 29.0 | 4.3 | 31.8 | 36.1 | 7.6 | −0.01 | 6.55 | |
| [10] | a-C:H | PAO9 | 6.5 | 23.7 | 30.2 | 4.3 | 31.8 | 36.1 | 13.6 | −0.03 | 5.06 | |
| [10] | F-DLC | PAO4 | 4.8 | 24.2 | 29.0 | 1.2 | 16.8 | 18.0 | 53.2 | −0.40 | −12.96 | |
| [10] | F-DLC | PAO9 | 6.5 | 23.7 | 30.2 | 1.2 | 16.8 | 18.0 | 56.5 | −0.45 | −14.99 | |
| [7] | Steel | PAO | 0.0 | 27.0 | 27.0 | 2.2 | 39.6 | 41.8 | 10.8 | −0.02 | 13.20 | |
| [7] | Steel | [EMIM][NTf2] | 11.4 | 25.6 | 37.0 | 2.2 | 39.6 | 41.8 | 34.5 | −0.18 | −0.46 | |
| [7] | Steel | [EMIM][CF3SO3] | 17.8 | 23.5 | 41.3 | 2.2 | 39.6 | 41.8 | 35.3 | −0.18 | −9.10 | |
| [7] | Steel | [BMIM][SCN] | 39.2 | 7.8 | 47.0 | 2.2 | 39.6 | 41.8 | 46.4 | −0.31 | −40.15 | |
| [7] | Steel | [BMIM][NTF2] | 0.9 | 32.7 | 33.6 | 2.2 | 39.6 | 41.8 | 38.9 | −0.22 | 7.44 | |
| [7] | Steel | [BMIM][BF4] | 20.7 | 24.1 | 44.8 | 2.2 | 39.6 | 41.8 | 48.8 | −0.34 | −14.12 | |
| [7] | Steel | [BMIM][PF6] | 5.2 | 38.9 | 44.1 | 2.2 | 39.6 | 41.8 | 52.6 | −0.39 | −3.02 | |
| [7] | Steel | [BMIM][CH3COO] | 9.9 | 26.5 | 36.4 | 2.2 | 39.6 | 41.8 | 33.6 | −0.17 | 1.36 | |
| [7] | Steel | IL I04 | 6.0 | 22.3 | 28.3 | 2.2 | 39.6 | 41.8 | 6.4 | −0.01 | 10.21 | |
| [7] | POM | PAO | 0.0 | 27.0 | 27.0 | 4.5 | 35.4 | 39.9 | 12.4 | −0.02 | 13.20 | |
| [7] | POM | [EMIM][NTf2] | 11.4 | 25.6 | 37.0 | 4.5 | 35.4 | 39.9 | 38.2 | −0.21 | 0.72 | |
| [7] | POM | [EMIM][CF3SO3] | 17.8 | 23.5 | 41.3 | 4.5 | 35.4 | 39.9 | 42.2 | −0.26 | −6.97 | |
| [7] | POM | [BMIM][SCN] | 39.2 | 7.8 | 47.0 | 4.5 | 35.4 | 39.9 | 48.8 | −0.34 | −34.07 | |
| [7] | POM | [BMIM][NTF2] | 0.9 | 32.7 | 33.6 | 4.5 | 35.4 | 39.9 | 33.4 | −0.17 | 4.88 | |
| [7] | POM | [BMIM][BF4] | 20.7 | 24.1 | 44.8 | 4.5 | 35.4 | 39.9 | 50.6 | −0.37 | −11.88 | |
| [7] | POM | [BMIM][PF6] | 5.2 | 38.9 | 44.1 | 4.5 | 35.4 | 39.9 | 53.5 | −0.41 | −4.41 | |
| [7] | POM | [BMIM][CH3COO] | 9.9 | 26.5 | 36.4 | 4.5 | 35.4 | 39.9 | 49.9 | −0.36 | 1.78 | |
| [7] | POM | IL I04 | 6.0 | 22.3 | 28.3 | 4.5 | 35.4 | 39.9 | 7.7 | −0.01 | 9.89 | |
| [7] | PDMS | PAO | 0.0 | 27.0 | 27.0 | 0.1 | 18.8 | 18.9 | 34.5 | −0.18 | −9.10 | |
| [7] | PDMS | [EMIM][NTf2] | 11.4 | 25.6 | 37.0 | 0.1 | 18.8 | 18.9 | 83.7 | −0.89 | −27.99 | |
| [7] | PDMS | [EMIM][CF3SO3] | 17.8 | 23.5 | 41.3 | 0.1 | 18.8 | 18.9 | 86.1 | −0.93 | −38.02 | |
| [7] | PDMS | [BMIM][SCN] | 39.2 | 7.8 | 47.0 | 0.1 | 18.8 | 18.9 | 102.9 | −1.22 | −65.76 | |
| [7] | PDMS | [BMIM][NTF2] | 0.9 | 32.7 | 33.6 | 0.1 | 18.8 | 18.9 | 73.8 | −0.72 | −16.89 | |
| [7] | PDMS | [BMIM][BF4] | 20.7 | 24.1 | 44.8 | 0.1 | 18.8 | 18.9 | 92.3 | −1.04 | −44.10 | |
| [7] | PDMS | [BMIM][PF6] | 5.2 | 38.9 | 44.1 | 0.1 | 18.8 | 18.9 | 87.0 | −0.95 | −32.47 | |
| [7] | PDMS | [BMIM][CH3COO] | 9.9 | 26.5 | 36.4 | 0.1 | 18.8 | 18.9 | 88.1 | −0.97 | −26.28 | |
| [7] | PDMS | IL I04 | 6.0 | 22.3 | 28.3 | 0.1 | 18.8 | 18.9 | 51.7 | −0.38 | −14.12 | |
| [16] | Steel | [THTDA][NTf2] | 11.0 | 15.6 | 26.6 | 2.2 | 39.6 | 41.8 | 25 | 26.6 | −0.11 | 6.34 |
| [16] | Steel | [THTDA][NTf2] | 10.6 | 18.1 | 28.7 | 2.2 | 39.6 | 41.8 | 40 | 28.7 | −0.12 | 5.87 |
| [16] | Steel | [THTDA][NTf2] | 10.9 | 7.2 | 18.0 | 2.2 | 39.6 | 41.8 | 100 | 18.0 | −0.05 | 7.41 |
| [16] | Steel | [THTDP][NTf2] | 9.8 | 4.0 | 13.8 | 2.2 | 39.6 | 41.8 | 25 | 13.8 | −0.03 | 6.81 |
| [16] | Steel | [THTDP][NTf2] | 11.1 | 6.5 | 17.7 | 2.2 | 39.6 | 41.8 | 40 | 17.7 | −0.05 | 6.72 |
| [16] | Steel | [THTDP][NTf2] | 11.1 | 6.5 | 17.7 | 2.2 | 39.6 | 41.8 | 100 | 17.7 | −0.05 | 6.72 |
| [16] | Steel | [1TD3HI][NTf2] | 4.4 | 0.5 | 4.9 | 2.2 | 39.6 | 41.8 | 25 | 4.9 | 0.00 | 5.23 |
| [16] | Steel | [1TD3HI][NTf2] | 11.2 | 6.1 | 17.3 | 2.2 | 39.6 | 41.8 | 40 | 17.3 | −0.05 | 6.43 |
| [16] | Steel | [1TD3HI][NTf2] | 12.1 | 15.3 | 27.4 | 2.2 | 39.6 | 41.8 | 100 | 27.4 | −0.11 | 4.79 |
| Source | Surface | Lubricant | Type | Friction Coefficient at | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 (m/s) | 0.10 (m/s) | 0.20 (m/s) | 0.05 (m/s) | 0.10 (m/s) | 0.20 (m/s) | |||||
| [7] | Steel | PAO | MR | 0.018 | 0.52 | 0.36 | 0.43 | 0.003 | 0.004 | 0.007 |
| [7] | Steel | [EMIM][NTf2] | MR | 0.176 | 0.17 | 0.17 | 0.15 | 0.008 | 0.012 | 0.019 |
| [7] | Steel | [EMIM][CF3SO3] | MR | 0.183 | 0.15 | 0.15 | 0.15 | 0.009 | 0.015 | 0.023 |
| [7] | Steel | [BMIM][SCN] | MR | 0.310 | 0.20 | 0.19 | 0.19 | 0.010 | 0.015 | 0.024 |
| [7] | Steel | [BMIM][NTF2] | MR | 0.222 | 0.19 | 0.18 | 0.18 | 0.010 | 0.016 | 0.026 |
| [7] | Steel | [BMIM][BF4] | MR | 0.341 | 0.14 | 0.14 | 0.13 | 0.014 | 0.022 | 0.034 |
| [7] | Steel | [BMIM][PF6] | MR | 0.393 | 0.15 | 0.15 | 0.16 | 0.027 | 0.042 | 0.066 |
| [7] | Steel | [BMIM][CH3COO] | MR | 0.167 | 0.16 | 0.16 | 0.16 | 0.027 | 0.043 | 0.067 |
| [7] | Steel | IL I04 | MR | 0.006 | 0.18 | 0.18 | 0.18 | 0.068 | 0.106 | 0.166 |
| [7] | POM | PAO | MR | 0.023 | 0.21 | 0.14 | 0.36 | 0.012 | 0.018 | 0.029 |
| [7] | POM | [EMIM][NTf2] | MR | 0.214 | 0.12 | 0.11 | 0.26 | 0.033 | 0.051 | 0.081 |
| [7] | POM | [EMIM][CF3SO3] | MR | 0.259 | 0.12 | 0.12 | 0.12 | 0.040 | 0.062 | 0.098 |
| [7] | POM | [BMIM][SCN] | MR | 0.341 | 0.14 | 0.14 | 0.15 | 0.041 | 0.064 | 0.101 |
| [7] | POM | [BMIM][NTF2] | MR | 0.165 | 0.12 | 0.13 | 0.13 | 0.044 | 0.069 | 0.108 |
| [7] | POM | [BMIM][BF4] | MR | 0.365 | 0.11 | 0.10 | 0.09 | 0.059 | 0.092 | 0.145 |
| [7] | POM | [BMIM][PF6] | MR | 0.406 | 0.12 | 0.08 | 0.07 | 0.113 | 0.177 | 0.277 |
| [7] | POM | [BMIM][CH3COO] | MR | 0.356 | 0.09 | 0.10 | 0.10 | 0.115 | 0.180 | 0.283 |
| [7] | POM | IL I04 | MR | 0.009 | 0.12 | 0.10 | 0.10 | 0.284 | 0.446 | 0.699 |
| [7] | PDMS | PAO | MR | 0.176 | 0.07 | 0.06 | 0.05 | 0.911 | 1.430 | 2.243 |
| [7] | PDMS | [EMIM][NTf2] | MR | 0.890 | 0.08 | 0.08 | 0.07 | 2.576 | 4.043 | 6.344 |
| [7] | PDMS | [EMIM][CF3SO3] | MR | 0.931 | 0.09 | 0.08 | 0.08 | 3.125 | 4.903 | 7.694 |
| [7] | PDMS | [BMIM][SCN] | MR | 1.223 | 0.07 | 0.06 | 0.06 | 3.213 | 5.041 | 7.911 |
| [7] | PDMS | [BMIM][NTF2] | MR | 0.721 | 0.09 | 0.09 | 0.08 | 3.448 | 5.410 | 8.489 |
| [7] | PDMS | [BMIM][BF4] | MR | 1.040 | 0.06 | 0.06 | 0.06 | 4.614 | 7.240 | 11.361 |
| [7] | PDMS | [BMIM][PF6] | MR | 0.947 | 0.11 | 0.08 | 0.09 | 8.841 | 13.873 | 21.768 |
| [7] | PDMS | [BMIM][CH3COO] | MR | 0.966 | 0.09 | 0.07 | 0.09 | 9.027 | 14.165 | 22.228 |
| [7] | PDMS | IL I04 | MR | 0.380 | 0.06 | 0.07 | 0.10 | 22.308 | 35.005 | 54.929 |
| Source | Surface | Lubricant | Type | Speed (−) | λ (−) | |S*| (−) | Friction Coefficient (−) |
|---|---|---|---|---|---|---|---|
| [7] | PDMS | [EMIM][NTf2] | IL | 7.900 | 0.176 | 0.060 | |
| [7] | POM | [EMIM][NTf2] | IL | 0.101 | 0.176 | 0.079 | |
| [7] | Steel | [EMIM][NTf2] | IL | 0.024 | 0.176 | 0.124 | |
| [7] | PDMS | [EMIM][CF3SO3] | IL | 7.900 | 0.183 | 0.062 | |
| [7] | POM | [EMIM][CF3SO3] | IL | 0.101 | 0.183 | 0.060 | |
| [7] | Steel | [EMIM][CF3SO3] | IL | 0.024 | 0.183 | 0.095 | |
| [7] | PDMS | [BMIM][SCN] | IL | 7.900 | 0.310 | 0.060 | |
| [7] | POM | [BMIM][SCN] | IL | 0.101 | 0.310 | 0.054 | |
| [7] | Steel | [BMIM][SCN] | IL | 0.024 | 0.310 | 0.090 | |
| [7] | PDMS | [BMIM][NTF2] | IL | 7.900 | 0.222 | 0.060 | |
| [7] | POM | [BMIM][NTF2] | IL | 0.101 | 0.222 | 0.080 | |
| [7] | Steel | [BMIM][NTF2] | IL | 0.024 | 0.222 | 0.092 | |
| [7] | PDMS | [BMIM][BF4] | IL | 7.900 | 0.341 | 0.059 | |
| [7] | POM | [BMIM][BF4] | IL | 0.101 | 0.341 | 0.067 | |
| [7] | Steel | [BMIM][BF4] | IL | 0.024 | 0.341 | 0.080 | |
| [7] | PDMS | [BMIM][PF6] | IL | 7.900 | 0.393 | 0.055 | |
| [7] | POM | [BMIM][PF6] | IL | 0.101 | 0.393 | 0.060 | |
| [7] | Steel | [BMIM][PF6] | IL | 0.024 | 0.393 | 0.057 | |
| [7] | PDMS | [BMIM][CH3COO] | IL | 7.900 | 0.167 | 0.059 | |
| [7] | POM | [BMIM][CH3COO] | IL | 0.101 | 0.167 | 0.056 | |
| [7] | Steel | [BMIM][CH3COO] | IL | 0.024 | 0.167 | 0.097 |
| Source | Surface | Lubricant | Type | Friction Coefficient at | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 (m/s) | 0.10 (m/s) | 0.50 (m/s) | 0.05 (m/s) | 0.10 (m/s) | 0.20 (m/s) | |||||
| [7] | Steel | [EMIM][NTf2] | IL | 0.176 | 0.14 | 0.13 | 0.12 | 0.008 | 0.012 | 0.019 |
| [7] | Steel | [EMIM][CF3SO3] | IL | 0.183 | 0.12 | 0.11 | 0.10 | 0.009 | 0.015 | 0.023 |
| [7] | Steel | [BMIM][SCN] | IL | 0.310 | 0.12 | 0.10 | 0.09 | 0.010 | 0.015 | 0.024 |
| [7] | Steel | [BMIM][NTF2] | IL | 0.222 | 0.12 | 0.10 | 0.09 | 0.010 | 0.016 | 0.026 |
| [7] | Steel | [BMIM][BF4] | IL | 0.341 | 0.09 | 0.08 | 0.06 | 0.014 | 0.022 | 0.034 |
| [7] | Steel | [BMIM][PF6] | IL | 0.393 | 0.06 | 0.04 | 0.05 | 0.027 | 0.042 | 0.066 |
| [7] | Steel | [BMIM][CH3COO] | IL | 0.167 | 0.10 | 0.06 | 0.04 | 0.027 | 0.043 | 0.067 |
| [7] | POM | [EMIM][NTf2] | IL | 0.214 | 0.10 | 0.08 | 0.08 | 0.033 | 0.051 | 0.081 |
| [7] | POM | [EMIM][CF3SO3] | IL | 0.259 | 0.09 | 0.08 | 0.06 | 0.040 | 0.062 | 0.098 |
| [7] | POM | [BMIM][SCN] | IL | 0.341 | 0.10 | 0.07 | 0.05 | 0.041 | 0.064 | 0.101 |
| [7] | POM | [BMIM][NTF2] | IL | 0.165 | 0.08 | 0.07 | 0.08 | 0.044 | 0.069 | 0.108 |
| [7] | POM | [BMIM][BF4] | IL | 0.365 | 0.06 | 0.07 | 0.05 | 0.059 | 0.092 | 0.145 |
| [7] | POM | [BMIM][PF6] | IL | 0.406 | 0.06 | 0.04 | 0.05 | 0.113 | 0.177 | 0.277 |
| [7] | POM | [BMIM][CH3COO] | IL | 0.356 | 0.06 | 0.07 | 0.03 | 0.115 | 0.180 | 0.283 |
| [7] | PDMS | [EMIM][NTf2] | IL | 0.890 | 0.12 | 0.06 | 0.06 | 2.576 | 4.043 | 6.344 |
| [7] | PDMS | [EMIM][CF3SO3] | IL | 0.931 | 0.07 | 0.06 | 0.06 | 3.125 | 4.903 | 7.694 |
| [7] | PDMS | [BMIM][SCN] | IL | 1.223 | 0.11 | 0.06 | 0.06 | 3.213 | 5.041 | 7.911 |
| [7] | PDMS | [BMIM][NTF2] | IL | 0.721 | 0.06 | 0.06 | 0.06 | 3.448 | 5.410 | 8.489 |
| [7] | PDMS | [BMIM][BF4] | IL | 1.040 | 0.06 | 0.06 | 0.06 | 4.614 | 7.240 | 11.361 |
| [7] | PDMS | [BMIM][PF6] | IL | 0.947 | 0.05 | 0.06 | 0.06 | 8.841 | 13.873 | 21.768 |
| [7] | PDMS | [BMIM][CH3COO] | IL | 0.966 | 0.06 | 0.06 | 0.06 | 9.027 | 14.165 | 22.228 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schertzer, M.J.; Iglesias, P. Meta-Analysis Comparing Wettability Parameters and the Effect of Wettability on Friction Coefficient in Lubrication. Lubricants 2018, 6, 70. https://doi.org/10.3390/lubricants6030070
Schertzer MJ, Iglesias P. Meta-Analysis Comparing Wettability Parameters and the Effect of Wettability on Friction Coefficient in Lubrication. Lubricants. 2018; 6(3):70. https://doi.org/10.3390/lubricants6030070
Chicago/Turabian StyleSchertzer, Michael J., and Patricia Iglesias. 2018. "Meta-Analysis Comparing Wettability Parameters and the Effect of Wettability on Friction Coefficient in Lubrication" Lubricants 6, no. 3: 70. https://doi.org/10.3390/lubricants6030070