The Effect of Agglomeration Reduction on the Tribological Behavior of WS2 and MoS2 Nanoparticle Additives in the Boundary Lubrication Regime
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Dynamic Light Scattering
2.2.2. Friction and Wear Testing
3. Results
3.1. Dispersion of Nanoparticles in Oil
3.2. Friction Behavior
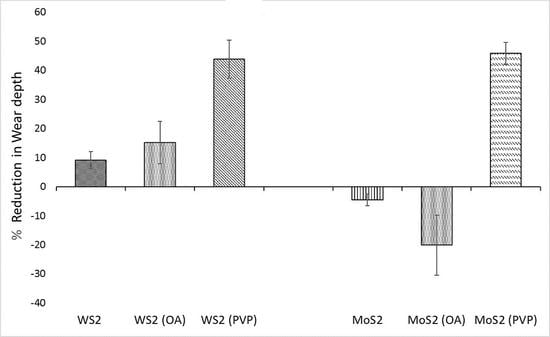
3.3. Wear Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asrul, M.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A. Tribological properties and lubricant mechanism of nanoparticle in engine oil. Procedia Eng. 2013, 68, 320–325. [Google Scholar] [CrossRef]
- Venkataraman, M. The Effect of Colloidal Stability on the Heat Transfer Characteristics of Nanosilica Dispersed Fluids. Master’s Thesis, University of Central Florida, Orlando, FL, USA, 2005; p. 93. [Google Scholar]
- Hwang, Y.; Lee, J.; Lee, J.; Jeong, Y.; Cheong, S.; Ahn, Y.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 1–4. [Google Scholar] [CrossRef]
- Rapoport, L.; Nepomnyashchy, O.; Lapsker, I.; Verdyan, A.; Soifer, Y.; Popovitz-Biro, R.; Tenne, R. Friction and wear of fullerene-like WS2 under severe contact conditions: Friction of ceramic materials. Tribo. Lett. 2005, 19, 143–149. [Google Scholar] [CrossRef]
- Hansson, P.; Lindman, B. Surfactant-polymer interactions. Curr. Opin. Colloid Interface Sci. 1996, 1, 604–613. [Google Scholar] [CrossRef]
- Feng, J.; Mao, J.; Wen, X.; Tu, M. Ultrasonic-assisted in situ synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J Alloys Compd. 2011, 509, 9093–9097. [Google Scholar] [CrossRef]
- Chen, X.; Boulos, R.A.; Eggers, P.K.; Raston, C.L. p-Phosphonic acid calix[8]arene assisted exfoliation and Stabilization of 2D materials in water. Chem. Commun. 2012, 48, 11407–11409. [Google Scholar] [CrossRef]
- Rao, Y. Particuology Nanofluids : Stability, phase diagram, rheology and applications. Particuology 2010, 8, 549–555. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, I.H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Effect of aggregation on the viscosity of copper oxide-gear oil nanofluids. Int. J. Therm. Sci. 2011, 50, 1741–1747. [Google Scholar] [CrossRef]
- Haddad, Z.; Abid, C.; Oztop, H.F.; Mataoui, A. A review on how the researchers prepare their nano fluids. Int. J. Therm. Sci. 2014, 76, 168–189. [Google Scholar] [CrossRef]
- Kalin, M.; Kogovšek, J.; Remškar, M. Mechanisms and improvements in the friction and wear behavior using MoS 2 nanotubes as potential oil additives. Wear 2012, 280–281, 36–45. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Liu, Y.; Gunsel, S.; Luo, J. Ultrathin MoS2 Nanosheets with Superior Extreme Pressure Property as Boundary Lubricants. Sci. Rep. 2015, 5, 12869. [Google Scholar] [CrossRef] [PubMed]
- Lahouij, I.; Vacher, B.; Martin, J.M.; Dassenoy, F. IF-MoS2 based lubricants: Influence of size, shape and crystal structure. Wear 2012, 296, 558–567. [Google Scholar] [CrossRef]
- Abate, F.; D’Agostino, V.; Di Giuda, R.; Senatore, A. Tribological behaviour of MoS2 and inorganic fullerene-like WS2 nanoparticles under boundary and mixed lubrication regimes. Tribol. Surf. Interfaces 2010, 4, 91–98. [Google Scholar] [CrossRef]
- Cizaire, L.; Vacher, B.; Le Mogne, T.; Martin, J.M.; Rapoport, L.; Margolin, A.; Tenne, R. Mechanisms of ultra-low friction by hollow inorganic fullerene-like MoS2 nanoparticles. Surf. Coat. Technol. 2002, 160, 282–287. [Google Scholar] [CrossRef]
- Yadgarov, L.; Petrone, V.; Rosentsveig, R.; Feldman, Y.; Tenne, R.; Senatore, A. Tribological studies of rhenium doped fullerene-like MoS2 nanoparticles in boundary, mixed and elasto-hydrodynamic lubrication conditions. Wear 2013, 297, 1103–1110. [Google Scholar] [CrossRef]
- An, V.; Irtegov, Y.; De Izarra, C. Study of tribological properties of nanolamellar WS2 and MoS2 as additives to lubricants. Int. Nanomater. 2014, 2014, 188. [Google Scholar]
- Verma, A.; Jiang, W.; Abu Safe, H.H.; Brown, W.D.; Malshe, A.P. Tribological behavior of deagglomerated active inorganic nanoparticles for advanced lubrication. Tribol. Trans. 2008, 51, 673–678. [Google Scholar] [CrossRef]
- Kedienhon, O. Characterization of Equilibrium Particle Size and Concentration in Oil-Based Nanolubricants. Master’s Thesis, Department of Mechanical Engineering, Howard University, Washingtong, DC, USA, 2012. [Google Scholar]
- Bari, R.; Parviz, D.; Khabaz, F.; Klaassen, C.D.; Metzler, S.D.; Hansen, M.J.; Khare, R.; Green, M.J. Liquid phase exfoliation and crumpling of inorganic nanosheets. Phys. Chem. Chem. Phys. 2015, 17, 9383–9393. [Google Scholar] [CrossRef]
- Guardia, L.; Paredes, J.I.; Rozada, R.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Production of aqueous dispersions of inorganic graphene analogues by exfoliation and stabilization with non-ionic surfactants. RSC Adv. 2014, 4, 14115–14127. [Google Scholar] [CrossRef]
- Moshkovith, A.; Perfiliev, V.; Lapsker, I.; Fleischer, N.; Tenne, R.; Rapoport, L. Friction of fullerene-like WS2 nanoparticles: Effect of agglomeration. Tribol. Lett. 2006, 24, 225–228. [Google Scholar] [CrossRef]
- Aldana, P.U.; Dassenoy, F.; Vacher, B.; Le Mogne, T.; Thiebaut, B. WS2 nanoparticles anti-wear and friction reducing properties on rough surfaces in the presence of ZDDP additive. Tribol. Int. 2016, 102, 213–221. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Dassenoy, F.; Belin, M.; Vacher, B.; Martin, J.M.; Fleischer, N. Ultralow-friction and wear properties of IF-WS2 under boundary lubrication. Tribol. Lett. 2005, 18, 477–485. [Google Scholar] [CrossRef]
- Moshkovith, A.; Perfiliev, V.; Verdyan, A.; Lapsker, I.; Popovitz-Biro, R.; Tenne, R.; Rapoporta, L. Sedimentation of IF-WS2 aggregates and a reproducibility of the tribological data. Tribol. Int. 2007, 40, 117–124. [Google Scholar] [CrossRef]
- Ratoi, M.; Niste, V.B.; Walker, J.; Zekonyte, J. Mechanism of action of WS2 lubricant nanoadditives in high-pressure contacts. Tribol. Lett. 2013, 52, 81–91. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Wang, J.; Ye, X.; Lei, W.; Xue, M.; Tang, H.; Li, C. Synthesis of Ultrathin WS2 Nanosheets and Their Tribological Properties as Lubricant Additives. Nanoscale Res. Lett. 2016, 11, 442. [Google Scholar] [CrossRef]
- Bogdan, V. WS2 Nanoparticles—Potential Replacement for ZDDP and Friction Modifier Additives. RSC Adv. 2014, 4, 21238–21245. [Google Scholar]
- Raichman, D.; Strawser, D.A.; Lellouche, J.P. Covalent functionalization/polycarboxylation of tungsten disulfide inorganic nanotubes (INTs-WS2). Nano Res. 2014, 8, 1454–1463. [Google Scholar] [CrossRef]
- Haba, D.; Griesser, T.; Müller, U.; Brunner, A.J. Comparative investigation of different silane surface functionalizations of fullerene-like WS2. J. Mater. Sci. 2015, 50, 5125–5135. [Google Scholar] [CrossRef]
- Liu, G.; Komatsu, N. Readily Available “Stock Solid” of MoS2 and WS2 Nanosheets through Solid-Phase Exfoliation for Highly Concentrated Dispersions in Water. ChemNanoMat 2016, 2, 500–503. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Mufti, R.A.; Zahid, R.; Yunus, R. AW/EP behavior of WS 2 nanoparticles added to vegetable oil—Based lubricant. In Proceedings of the Malaysian International Tribology Conference 2015, Penang, Malaysia, 16–17 November 2015; pp. 194–195. [Google Scholar]
- Kamatchi, R.; Venkatachalapathy, S. Parametric study of pool boiling heat transfer with nanofluids for the enhancement of critical heat flux: A review. Int. J. Therm. Sci. 2015, 87, 228–240. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Yella, A.; Tahir, M.N.; Meuer, S.; Zentel, R.; Berger, R.; Panthöfer, M.; Tremel, W. Synthesis, characterization, and hierarchical organization of tungsten oxide nanorods: Spreading driven by Marangoni flow. J. Am. Chem. Soc. 2009, 131, 17566–17575. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Role of interfacial layer and clustering on the effective thermal conductivity of CuO-gear oil nanofluids. Exp. Therm. Fluid Sci. 2011, 35, 1490–1495. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, L.; Li, X.; Huang, W. Layer-controllable WS2-reduced graphene oxide hybrid nanosheets with high electrocatalytic activity for hydrogen evolution. Nanoscale 2015, 7, 10391–10397. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Tsui, W.C.; Liu, T.C. Experimental analysis of tribological properties of lubricating oils with nanoparticle additives. Wear 2007, 262, 819–825. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, G.; Yang, G.B.; Chen, S.; Huang, B.Y.; Zhang, C.F. Surface adsorption phenomenon during the preparation process of nano WC and ultrafine cemented carbide. Int. J. Refract. Met. Hard Mater. 2007, 25, 166–170. [Google Scholar] [CrossRef]
- Xu, P.; Li, Z.; Zhang, X.; Yang, Z. Increased response to oxidative stress challenge of nano-copper-induced apoptosis in mesangial cells. J. Nanoparticle Res. 2014, 16. [Google Scholar] [CrossRef]
- Rodriguez-Devecchis, V.M.; Carbognani Ortega, L.; Scott, C.E.; Pereira-Almao, P. Use of Nanoparticle Tracking Analysis for Particle Size Determination of Dispersed Catalyst in Bitumen and Heavy Oil Fractions. Ind. Eng. Chem. Res. 2015, 54, 9877–9886. [Google Scholar] [CrossRef]
- Sonali, J.; Sandhyarani, N.; Sajith, V. Tribological properties and stabilization study of surfactant modified MoS2 nanoparticle in 15W40 engine oil. Int. J. Fluid Mech. Mach. 2014, 1, 1–5. [Google Scholar]
- Roy, S.; Sundararajan, S. The effect of heat treatment routes on the retained austenite and Tribomechanical properties of carburized AISI 8620 steel. Surf. Coat. Technol. 2016, 308, 236–243. [Google Scholar] [CrossRef]
- Rabaso, P.; Ville, F.; Dassenoy, F.; Diaby, M.; Afanasiev, P.; Cavoret, J.; Vacher, B.; Le Mongne, T. Boundary lubrication: Influence of the size and structure of inorganic fullerene-like MoS2nanoparticles on friction and wear reduction. Wear 2014, 320, 161–178. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Yang, G.B.; Chai, S.T.; Xiong, X.J.; Zhang, S.M.; Yu, L.G.; Zhang, P.Y. Preparation and tribological properties of surface modified Cu nanoparticles. Trans. Nonferrous Met. Soc. China 2012, 22, 366–372. [Google Scholar] [CrossRef]
- Chiñas-Castillo, F.; Spikes, H.A. Mechanism of Action of Colloidal Solid Dispersions. J. Tribol. 2003, 125, 552–557. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jazaa, Y.; Lan, T.; Padalkar, S.; Sundararajan, S. The Effect of Agglomeration Reduction on the Tribological Behavior of WS2 and MoS2 Nanoparticle Additives in the Boundary Lubrication Regime. Lubricants 2018, 6, 106. https://doi.org/10.3390/lubricants6040106
Jazaa Y, Lan T, Padalkar S, Sundararajan S. The Effect of Agglomeration Reduction on the Tribological Behavior of WS2 and MoS2 Nanoparticle Additives in the Boundary Lubrication Regime. Lubricants. 2018; 6(4):106. https://doi.org/10.3390/lubricants6040106
Chicago/Turabian StyleJazaa, Yosef, Tian Lan, Sonal Padalkar, and Sriram Sundararajan. 2018. "The Effect of Agglomeration Reduction on the Tribological Behavior of WS2 and MoS2 Nanoparticle Additives in the Boundary Lubrication Regime" Lubricants 6, no. 4: 106. https://doi.org/10.3390/lubricants6040106