Insect Sex Determination Manipulated by Their Endosymbionts: Incidences, Mechanisms and Implications
Abstract
:1. Introduction
2. Diversity and Common Features of Insect Sex Determination

3. Sexual Aberrations of Insects
4. Involvement of Endosymbiotic Microorganisms
| Endosymbiont | Hosts | Phenotype | |||
|---|---|---|---|---|---|
| Kingdom | Phylum | Class | Species | ||
| Bacteria | Proteobacteria | α-Proteobacteria | Wolbachia pipientis | insects, crustaceans, arachnids, nematodes | CI, MK, PI, FM |
| Rickettsia sp. | insects | MK, PI | |||
| γ-Proteobacteria | Arsenophonus nasoniae | insects | MK | ||
| Firmicutes | Mollicutes | Spiroplasma poulsonii | insects | MK | |
| Spiroplasma ixodetis relative | insects | MK | |||
| Cytophaga-Flavobacterium-Bacteroides | Bacteroidetes | Cardinium hertigii | insects, arachnids, nematodes | CI, PI, FM | |
| Flavobacteria relative | insects | MK | |||
| Eukaryotes | Microsporidia | Dihaplophasea | Octosporea effeminans | crustaceans | FM |
| Thelohania herediteria | crustaceans | FM | |||
| Nosema granulosis | crustaceans | FM | |||
| Dictyocoela duebenum | crustaceans | FM | |||
| Amblyospora spp. | insects | MK | |||
| Parathelohania legeri | insects | MK | |||
| Parathelohania obesa | insects | MK | |||
| Nematoda | Adenophorea | Gasteromermis sp. | insects | FM | |
| Viruses | Unknown | Unknown | Unknown | crustaceans | MS |
| Unknown (RNA virus) | insects | MK | |||
| Endosymbiont | Class | Order | Species | Reference |
|---|---|---|---|---|
| (a) Feminizing bacteria | ||||
| Wolbachia pipientis | Insecta | Hemiptera | Zyginidia pullula | [28] |
| Lepidoptera | Eurema hecabe | [27] | ||
| E. madarina | [25,26,129] | |||
| Malacostraca | Isopoda | Armadillidium vulgare | [130,131,132] | |
| A. nasatum | [132,133,134] | |||
| Chaetophiloscia elongata | [135] | |||
| Porcellionides pruinosus | [135,136] | |||
| Sphaeroma rugicauda | [137] | |||
| Cardinium hertigii | Insecta | Hymenoptera | Encarsia hispida | [44] |
| Arachnida | Trombidiformes | Brevipalpus phoenicis | [40] | |
| B. californicus | [42] | |||
| (b) Feminizing microsporidia | ||||
| Octosporea effeminans | Malacostraca | Amphipoda | Gammarus duebeni | [46] |
| Thelohania herediteria | G. duebeni | [138] | ||
| Nosema granulosis | G. duebeni | [48] | ||
| Dictyocoela duebenum | G. duebeni | [52] | ||
| (c) Other feminizers | ||||
| Gasteromermis sp. | Insecta | Ephemeroptera | Baetis bicaudatus | [53] |
| f factor (unknown) | Malacostraca | Isopoda | Armadillidium vulgare | [33] |
| (d) Male-killing bacteria | ||||
| Wolbachia pipientis | Insecta | Coleoptera | Adalia bipunctata | [139] |
| Tribolium madens | [140] | |||
| Diptera | Drosophila bifasciata | [141] | ||
| D. borealis | [142] | |||
| D. innubila | [143] | |||
| Lepidoptera | Acraea encedon | [128] | ||
| A. encedana | [144] | |||
| Hypolimnas bolina | [69] | |||
| Ostrinia furnacalis | [64] | |||
| O. orientalis | [145] | |||
| O. scapulalis | [63,146] | |||
| O. zaguliaevi | [145] | |||
| Arachnida | Pseudoscorpionida | Cordylochernes scorpioides | [147] | |
| Spiroplasma ixodetis relatives | Insecta | Coleoptera | Adalia bipunctata | [148,149] |
| Anisosticta novemdecimpunctata | [150] | |||
| Harmonia axyridis | [151,152] | |||
| Menochilius sexmaculatus | [153] | |||
| Hemiptera | Acyrthosiphon pisum | [77,79] | ||
| Lepidoptera | Danaus chrysippus | [154] | ||
| Ostrinia zaguliaevi | [155] | |||
| Spiroplasma poulsonii | Diptera | Drosophila nebulosa | [156] | |
| D. neocardini | [157] | |||
| D. melanogaster | [158] | |||
| D. ornatifrons | [157] | |||
| D. paraguayensis | [157] | |||
| D. willistoni | [159] | |||
| Rickettsia spp. | Insecta | Coleoptera | Adalia bipunctata | [149,160,161] |
| A. decempunctata | [162] | |||
| Brachys tesselatus | [163] | |||
| Propylea japonica | [164] | |||
| Flavobacteria | Insecta | Coleoptera | Adonia variegate | [165] |
| Coccinula sinensis | [166,167] | |||
| Coleomegilla maculata | [168] | |||
| Arsenophonus nasoniae | Insecta | Hymenoptera | Nasonia vitripennis | [169,170] |
| (e) Male-killing microsporidia | ||||
| Parathelohania legeri | Diptera | Anopheles quadimaculatus | [171] | |
| Parathelohania obesa | A. quadimaculatus | [172] | ||
| Amblyospora spp. | Aedes spp. | [173,174] | ||
| Amblyospora spp. | Culex spp. | [174,175,176,177] | ||
| Amblyospora spp. | Culiseta spp. | [174] | ||
| (f) Other male killers | ||||
| Unknown virus | Insecta | Lepidoptera | Homona magnanima | [59,178] |
| Armadillidium vulgare | [56] | |||
| Porcellio dilatatus | [56] | |||
| P. laevis | [56] | |||
| (g) Parthenogenesis-inducing bacteria | ||||
| Wolbachia pipientis | Insecta | Hymenoptera | Aphytis diaspidis | [179,180] |
| A. lignaensis | [179,181] | |||
| Aponanagyrus diversicornis | [91] | |||
| Asobara japonica | [92] | |||
| Diplolepsis rosae | [182] | |||
| Encarsia formosa | [90] | |||
| Eretmocerus mundus | [183] | |||
| Gronotoma micromorpha | [184] | |||
| Muscidifurax uniraptor | [185,186] | |||
| Telenomus nawai | [187] | |||
| Trichogramma brevicapilliun | [188] | |||
| T. chilonis | [189] | |||
| T. cordubensis | [188,189] | |||
| T. deion | [93,188,189] | |||
| T. embryophagum | [188,189] | |||
| T. evanescens | [188,189] | |||
| T. kaykai | [190] | |||
| T. oleae | [132,189] | |||
| T. platneri | [188,189] | |||
| T. pretiosum | [93,188,189] | |||
| Insecta | Thysanoptera | Franklinothrips vespiformis | [88] | |
| Arachnida | Trombidiformes | Bryobia praetiosa | [40] | |
| Bryobia sp. | [40] | |||
| Cardinium hertigii | Insecta | Hymenoptera | Encarsia hispida | [44] |
| E. pergandiella | [191] | |||
| E. protransvena | [191,192] | |||
| Arachnida | Trombidiformes | Brevipalpus phoenicis | [43] | |
| Rickettsia spp. | Insecta | Hymenoptera | Neochrysocharis formosa | [96,193] |
| Pnigalio soemius | [194] | |||
5. Endosymbiont-Induced Feminization: Examples Are Scarce but May Potentially Be More Common
5.1. Feminization of Butterflies by Wolbachia
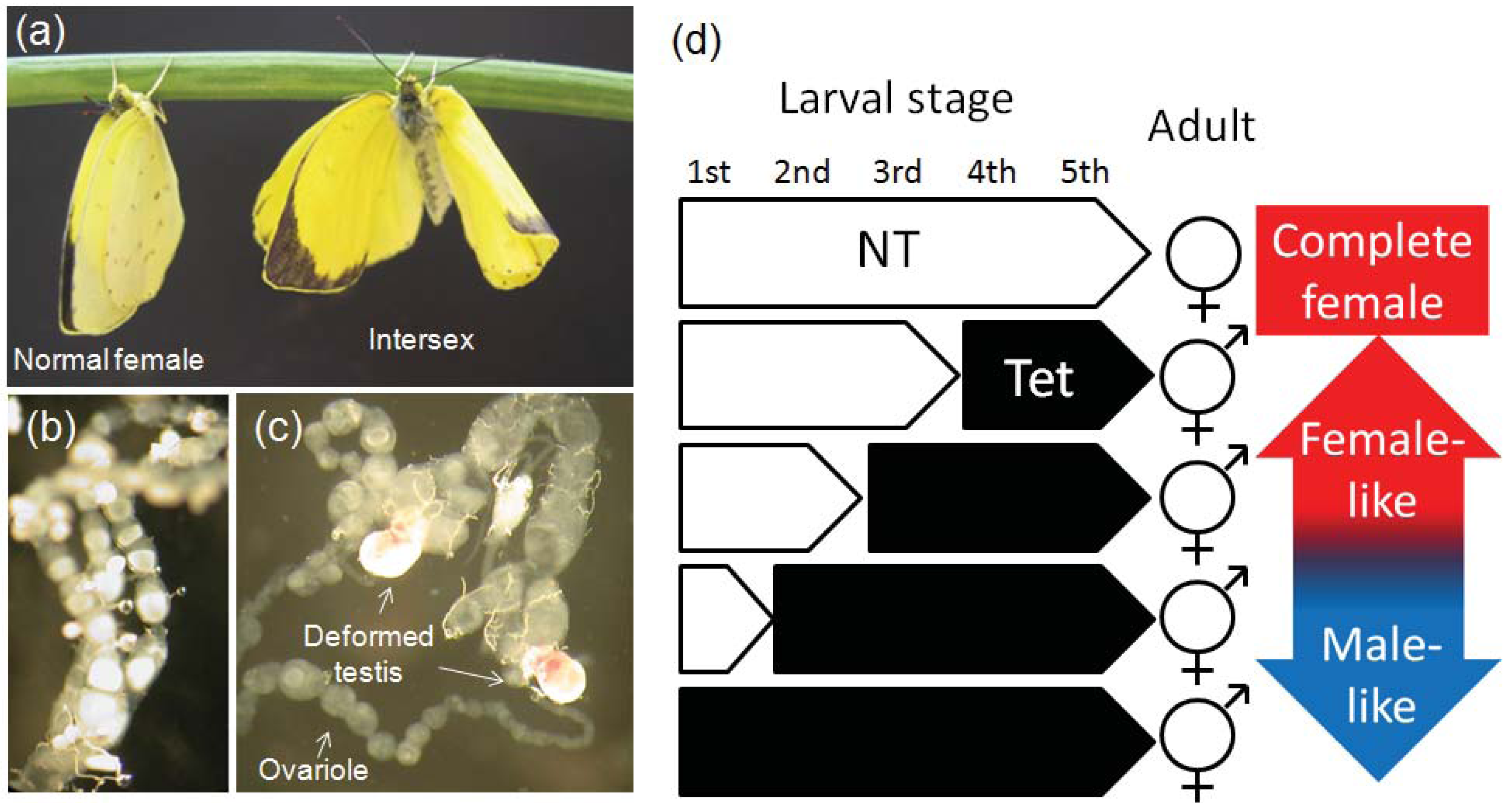
5.2. Feminization of Leafhoppers by Wolbachia
5.3. Feminization of Woodlice by Wolbachia
5.4. Feminization of Mites and Wasps by Cardinium Bacteria
5.5. Feminization of Shrimps by Microsporidian Parasites
5.6. Feminization Induced by Non-Microbes
5.7. Masculinization Induced by Viruses
6. Endosymbiont-Induced Male Killing: An Easily Evolved Trait?
6.1. Function of Dosage Compensation is Necessary for Male Killing
6.2. Male Killing by Lethal Effect of Feminization?

6.3. Hidden Male Killing
6.4. Is Timing of Male Killing Important?
7. Parthenogenesis Induction: Conversion of Genetic Males to Genetic Females or Feminization Following Diploidization?
7.1. Mechanisms of Microbe-Induced Parthenogenesis
8. Other Phenotypes
8.1. Cytoplasmic Incompatibility
8.2. Beneficial Effects of Wolbachia and Spiroplasma on Hosts
9. Transition of the Biological Systems of Arthropods by Sex-Associated Microbes

10. Population-Level Effects of Endosymbiotic Microbes on Their Hosts
11. Conclusions
Acknowledgments
References
- Bull, J.J. Evolution of Sex Determining Mechanisms; The Benjamin/Cummings Publishing Company, Inc.: Menlo Park, CA, USA, 1983; p. 316. [Google Scholar]
- Werren, J.H.; Beukeboom, L.W. Sex determination, sex ratios, and genetic conflict. Ann. Rev. Ecol. Evol. Syst. 1998, 29, 233–261. [Google Scholar] [CrossRef]
- Heimpel, G.E.; de Boer, J.G. Sex determination in the hymenoptera. Ann. Rev. Entomol. 2008, 53, 209–230. [Google Scholar] [CrossRef]
- Schütt, C.; Nöthiger, R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development 2000, 127, 667–677. [Google Scholar]
- Nöthiger, R.; Steinmann-Zwicky, M. A single principle for sex determination in insects. Cold Spring Harb. Symp. Quant. Biol. 1985, 50, 615–621. [Google Scholar] [CrossRef]
- Hoy, M.A. Insect Molecular Genetics, 2nd ed.; Academic Press/Elsevier: San Diego, CA, USA, 2003; p. 560. [Google Scholar]
- Verhulst, E.C.; van de Zande, L.; Beukeboom, L.W. Insect sex determination: It all evolves around transformer. Curr. Opin. Genet. Dev. 2010, 20, 376–383. [Google Scholar] [CrossRef]
- Laugé, G. Sex determination: Genetic and epigenetic factors. In Comprehensive Insect Physiology, Biochemistry and Pharmacology, Vol. 1. Embryogenesis and Reproduction; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 295–318. [Google Scholar]
- Narita, S.; Pereira, R.A.S.; Kjellberg, F.; Kageyama, D. Gynandromorphs and intersexes: Potential to understand the mechanism of sex determination in arthropods. Terrestrial Arthropod Rev. 2010, 3, 63–96. [Google Scholar] [CrossRef]
- Brust, R. Temperature-induced intersexes in Aedes mosquitoes: Comparative study of species from Manitoba. Can. Entomol. 1968, 100, 879–891. [Google Scholar] [CrossRef]
- Horsfall, W.R.; Anderson, J.F. Suppression of male characteristics of mosquitoes by thermal means. Science 1961, 133, 1830. [Google Scholar]
- Horsfall, W.R.; Anderson, J.F. Thermally induced genital appendages on mosquitoes. Science 1963, 141, 1183. [Google Scholar]
- Goldschmidt, R. Lymantria. Bibliogr. Genet. 1934, 11, 1–186. [Google Scholar]
- Mosbacher, G.C. Die Intersexualität bei Lymantria dispar L. (Lepidoptera). Z. Morphol. Tiere 1973, 76. [Google Scholar]
- Mosbacher, G.C. Sex specific cell differentiation in different types of intersexes of Lymantria dispar L. In Intersexuality in the Animal Kingdom; Reinboth, R., Ed.; Springer: Berlin, Germany, 1975; pp. 146–157. [Google Scholar]
- Seiler, J. Sexuality as developmental process. In Genetics Today. Proc. XI Int. Congress of Genetics, The Hague 1963; Geerts, S.J., Ed.; Pergamon: Oxford, UK, 1965; pp. 199–207. [Google Scholar]
- Werren, J.H. Biology of Wolbachia. Ann. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Microbial Manipulator of Arthropod Reproduction. Ann. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Ann. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef]
- Weiss, B.L.; Wang, J.; Aksoy, S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011, 9, e1000619. [Google Scholar] [CrossRef]
- Baldo, L.; Ayoub, N.A.; Hayashi, C.Y.; Russell, J.A.; Stahlhut, J.K.; Werren, J.H. Insight into the routes of Wolbachia invasion: High levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol. Ecol. 2008, 17, 557–569. [Google Scholar]
- Russell, J.A.; Goldman-Huertas, B.; Moreau, C.S.; Baldo, L.; Stahlhut, J.K.; Werren, J.H.; Pierce, N.E. Specialization and geographic isolation among Wolbachia symbionts from ants and lycaenid butterflies. Evolution 2009, 63, 624–640. [Google Scholar] [CrossRef]
- Stahlhut, J.K.; Desjardins, C.A.; Clark, M.E.; Baldo, L.; Russell, J.A.; Werren, J.H.; Jaenike, J. The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol. Ecol. 2010, 19, 1940–1952. [Google Scholar]
- Hiroki, M.; Tagami, Y.; Miura, K.; Kato, Y. Multiple infection with Wolbachia inducing different reproductive manipulations in the butterfly Eurema hecabe. Proc. R. Soc. Lond. B 2004, 271, 1751–1755. [Google Scholar] [CrossRef]
- Narita, S.; Kageyama, D.; Nomura, M.; Fukatsu, T. Unexpected mechanism of symbiont-induced reversal of insect sex: Feminizing Wolbachia continuously acts on the butterfly Eurema hecabe during larval development. Appl. Environ. Microbiol. 2007, 73, 4332–4341. [Google Scholar]
- Narita, S.; Kageyama, D.; Hiroki, M.; Sanpei, T.; Hashimoto, S.; Kamitoh, T.; Kato, Y. Wolbachia-induced feminisation newly found in Eurema hecabe, a sibling species of Eurema mandarina (Lepidoptera: Pieridae). Ecol. Entomol. 2011, 36, 309–317. [Google Scholar] [CrossRef]
- Negri, I.; Pellecchia, M.; Mazzoglio, P.J.; Patetta, A.; Alma, A. Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc. R. Soc. Lond. B 2006, 273, 2409–2416. [Google Scholar]
- Negri, I.; Franchini, A.; Gonella, E.; Daffonchio, D.; Mazzoglio, P.J.; Mandrioli, M.; Alma, A. Unravelling the Wolbachia evolutionary role: The reprogramming of the host genomic imprinting. Proc. R. Soc. Lond. B 2009, 276, 2485–2491. [Google Scholar]
- Legrand, J.J.; Legrand-Hamelin, E.; Juchault, P. Sex determination in Crustacea. Biol. Rev. 1987, 62, 439–470. [Google Scholar] [CrossRef]
- Rigaud, T. Inherited microorganisms and sex determination of arthropod hosts. In Influential Passengers; O’Neill, S.L., Hoffmann, A.A., Werren, J.H., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 81–101. [Google Scholar]
- Thierry, R.; Juchault, P.; Mocquard, J.P. The evolution of sex determination in isopod crustaceans. BioEssays 1997, 19, 409–416. [Google Scholar] [CrossRef]
- Juchault, P.; Mocquard, J.P. Transfer of a parasitic sex factor to the nuclear genome of the host: A hypothesis on the evolution of sex-determining mechanisms in the terrestrial Isopod Armadillidium vulgare Latr. J. Evol. Biol. 1993, 6, 511–528. [Google Scholar]
- Rigaud, T.; Juchault, P. Genetic control of the vertical transmission of a cytoplasmic sex factor in Armadillidium vulgare Latr. (Crustacea, Oniscidea). Heredity 1992, 68, 47–52. [Google Scholar] [CrossRef]
- Rigaud, T.; Juchault, P. Conflict between feminizing sex ratio distorters and an autosomal masculinizing gene in the terrestrial isopod Armadillidium vulgare Latr. Genetics 1993, 133, 247–252. [Google Scholar]
- Martin, G.; Sorokine, O.; Moniatte, M.; Bulet, P.; Hetru, C.; Van Dorsselaer, A. The structure of a glycosylated protein hormone responsible for sex determination in the isopod, Armadillidium vulgare. Eur. J. Biochem. 1999, 262, 727–736. [Google Scholar] [CrossRef]
- Okuno, A.; Hasegawa, Y.; Ohira, T.; Katakura, Y.; Nagasawa, H. Characterization and cDNA cloning of androgenic gland hormone of the terrestrial isopod Armadillidium vulgare. Biochem. Biophys. Res. Comm. 1999, 264, 419–423. [Google Scholar] [CrossRef]
- Helle, W.; Bolland, H.R.; Heitmans, W.R.B. Chromosomes and types of parthenogenesis in the false spider mites (Acari: Tenuipalpidae). Genetica 1980, 54, 45–50. [Google Scholar] [CrossRef]
- Weeks, A.R.; Marec, F.; Breeuwer, J.A.J. A mite species that consists entirely of haploid females. Science 2001, 292, 2479–2482. [Google Scholar]
- Weeks, A.R.; Breeuwer, J.A.J. Wolbachia-induced parthenogenesis in a genus of phytophagous mites. Proc. R. Soc. Lond. B 2001, 268, 2245–2251. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Perlman, S.J.; Kelly, S.E.; Katzir, N.; Hunter, M.S. Characterization of a “Bacteroidetes” symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): Proposal of “Candidatus Cardinium hertigii.”. Int. J. Syst. Evol. Microbiol. 2004, 54, 961. [Google Scholar] [CrossRef]
- Chigira, A.; Miura, K. Detection of “Candidatus Cardinium” bacteria from the haploid host Brevipalpus californicus (Acari: Tenuipalpidae) and effect on the host. Exp. Appl. Acarol. 2005, 37, 107–116. [Google Scholar] [CrossRef]
- Groot, T.V.M.; Breeuwer, J.A.J. Cardinium symbionts induce haploid thelytoky in most clones of three closely related Brevipalpus species. Exp. Appl. Acarol. 2006, 39, 257–271. [Google Scholar] [CrossRef]
- Giorgini, M.; Monti, M.M.; Caprio, E.; Stouthamer, R.; Hunter, M.S. Feminization and the collapse of haplodiploidy in an asexual parasitoid wasp harboring the bacterial symbiont Cardinium. Heredity 2009, 102, 365–371. [Google Scholar] [CrossRef]
- Traut, W. Zur Geschlechtsbestimmung bei Gammarus duebeni und verwandten Arten. Z. wiss. Zool. 1962, 167, 1–72. [Google Scholar]
- Bulnheim, H.P.; Vávra, J. Infection by the Microsporidian Octosporea effeminans sp. n., and its sex determining influence in the amphipod Gammarus duebeni. J. Parasitol. 1968, 54, 241–248. [Google Scholar] [CrossRef]
- Dunn, A.M.; Rigaud, T. Horizontal transfer of parasitic sex ratio distorters between crustacean hosts. Parasitology 1998, 117, 15–19. [Google Scholar] [CrossRef]
- Terry, R.S.; Smith, J.E.; Dunn, A.M. Impact of a Novel, Feminising Microsporidium on its Crustacean Host. J. Euk. Microbiol. 1998, 45, 497–501. [Google Scholar] [CrossRef]
- Dunn, A.M.; Hatcher, M.J.; Terry, R.S.; Tofts, C. Evolutionary ecology of vertically transmitted parasites: Transovarial transmission of a microsporidian sex ratio distorter in Gammarus duebeni. Parasitology 1995, 111, S91–S109. [Google Scholar] [CrossRef]
- Rodgers-Gray, T.P.; Smith, J.E.; Ashcroft, A.E.; Isaac, R.E.; Dunn, A.M. Mechanisms of parasite-induced sex reversal in Gammarus duebeni. Int. J. Parasitol. 2004, 34, 747–753. [Google Scholar] [CrossRef]
- Dunn, A.; Adams, J.; Smith, J. Transovarial transmission and sex-ratio distortion by a microsporidian parasite in a shrimp. J. Invertebr. Pathol. 1993, 61, 248–252. [Google Scholar] [CrossRef]
- Terry, R.S.; Smith, J.E.; Sharpe, R.G.; Rigaud, T.; Littlewood, D.T.J.; Ironside, J.E.; Rollinson, D.; Bouchon, D.; MacNeil, C.; Dick, J.T.; Dunn, A.M. Widespread vertical transmission and associated host sex-ratio distortion within the eukaryotic phylum Microspora. Proc. R. Soc. Lond. B 2004, 271, 1783–1789. [Google Scholar]
- Vance, S.A. Morphological and Behavioural sex reversal in mermithid-infected mayflies. Proc. R. Soc. Lond. B 1996, 263, 907–912. [Google Scholar] [CrossRef]
- Baudoin, M. Host castration as a parasitic strategy. Evolution 1975, 29, 335–352. [Google Scholar] [CrossRef]
- Kathirithamby, J. The effects of stylopisation on the sexual development of Javesella dubia (Kirschbaum) (Homoptera: Delphacidae). Biol. J. Linn. Soc. 1978, 10, 163–179. [Google Scholar] [CrossRef]
- Juchault, P.; Louis, C.; Martin, G.; Noulin, G. Masculinization of female isopods (Crustacea) correlated with non-Mendelian inheritance of cytoplasmic viruses. Proc. Natl. Acad. Sci. USA 1991, 88, 10460–10464. [Google Scholar]
- Hurst, G.D.; Jiggins, F.M. Male-killing bacteria in insects: Mechanisms, incidence, and implications. Emerg. Inf. Dis. 2000, 6, 329–336. [Google Scholar] [CrossRef]
- Hurst, L.D. The incidences and evolution of cytoplasmic male killers. Proc. R. Soc. Lond. B 1991, 244, 91. [Google Scholar] [CrossRef]
- Nakanishi, K.; Hoshino, M.; Nakai, M.; Kunimi, Y. Novel RNA sequences associated with late male killing in Homona magnanima. Proc. R. Soc. Lond. B 2008, 275, 1249–1254. [Google Scholar] [CrossRef]
- Gilfillan, G.D.; Dahlsveen, I.K.; Becker, P.B. Lifting a chromosome: Dosage compensation in Drosophila melanogaster. FEBS Lett. 2004, 567, 8–14. [Google Scholar] [CrossRef]
- Veneti, Z.; Bentley, J.K.; Koana, T.; Braig, H.R.; Hurst, G.D.D. A functional dosage compensation complex required for male killing in Drosophila. Science 2005, 307, 1461–1463. [Google Scholar]
- Kageyama, D.; Ohno, S.; Hoshizaki, S.; Ishikawa, Y. Sexual mosaics induced by tetracycline treatment in the Wolbachia-infected adzuki bean borer, Ostrinia scapulalis. Genome 2003, 46, 983–989. [Google Scholar] [CrossRef]
- Kageyama, D.; Traut, W. (2004) Opposite sex-specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proc. R. Soc. Lond. B 2004, 271, 251–258. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kageyama, D.; Hoshizaki, S.; Ishikawa, Y. Sex-specific death in the Asian corn borer moth (Ostrinia furnacalis) infected with Wolbachia occurs across larval development. Genome 2007, 50, 645–652. [Google Scholar] [CrossRef]
- Sugimoto, T.N.; Fujii, T.; Kayukawa, T.; Sakamoto, H.; Ishikawa, Y. Expression of a doublesex homologue is altered in sexual mosaics of Ostrinia scapulalis moths infected with Wolbachia. Insect Biochem. Mol. Biol. 2010, 40, 847–854. [Google Scholar] [CrossRef]
- Sugimoto, T.N.; Ishikawa, Y. A male-killing Wolbachia carries a feminizing factor and is associated with degradation of the sex-determining system of its host. Biol. Lett. 2012. [Google Scholar]
- Kageyama, D., Hoshizaki. Female-biased sex ratio in the Asian corn borer, Ostrinia furnacalis: Evidence for the occurrence of feminizing bacteria in an insect. Heredity 1998, 81, 311–316. [Google Scholar] [CrossRef]
- Kageyama, D.; Nishimura, G.; Hoshizaki, S.; Ishikawa, Y. Feminizing Wolbachia in an insect, Ostrinia furnacalis (Lepidoptera: Crambidae). Heredity 2002, 88, 444–449. [Google Scholar] [CrossRef]
- Dyson, E.A.; Kamath, M.K.; Hurst, G.D.D. Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): Evidence for horizontal transmission of a butterfly male killer. Heredity 2002, 88, 166–171. [Google Scholar] [CrossRef]
- Mitsuhashi, W.; Saiki, T.; Wei, W.; Kawakita, H.; Sato, M. Two novel strains of Wolbachia coexisting in both species of mulberry leafhoppers. Insect Mol. Biol. Biol. 2002, 11, 577–584. [Google Scholar] [CrossRef]
- Dyson, E.A.; Hurst, G.D.D. Persistence of an extreme sex-ratio bias in a natural population. Proc. Natl. Acad. Sci. USA 2004, 101, 6520–6523. [Google Scholar]
- Hornett, E.A.; Charlat, S.; Duplouy, A.M.R.; Davies, N.; Roderick, G.K.; Wedell, N.; Hurst, G.D. Evolution of male-killer suppression in a natural population. PLoS Biol. 2006, 4, e283. [Google Scholar] [CrossRef] [Green Version]
- Charlat, S.; Hornett, E.A.; Fullard, J.H.; Davies, N.; Roderick, G.K.; Wedell, N.; Hurst, G.D. Extraordinary flux in sex ratio. Science 2007, 317, 214. [Google Scholar]
- Mitsuhashi, W.; Ikeda, H.; Muraji, M. Fifty-year trend towards suppression of Wolbachia-induced male-killing by its butterfly host, Hypolimnas bolina. J. Insect Sci. 2011, 11, 1–15. [Google Scholar]
- Hornett, E.A.; Duplouy, A.M.R.; Davies, N.; Roderick, G.K.; Wedell, N.; Hurst, G.D.; Charlat, S. You can’t keep a good parasite down: Evolution of a male-killer suppressor uncovers cytoplasmic incompatibility. Evolution 2008, 62, 1258–1263. [Google Scholar] [CrossRef]
- Jaenike, J. Spontaneous emergence of a new Wolbachia phenotype. Evolution 2007, 61, 2244–2252. [Google Scholar] [CrossRef]
- Fukatsu, T.; Tsuchida, T.; Nikoh, N.; Koga, R. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 2001, 67, 1284–1291. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Shibao, H.; Matsumoto, T.; Fukatsu, T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002, 11, 2123–2135. [Google Scholar] [CrossRef]
- Simon, J.C.; Boutin, S.; Tsuchida, T.; Koga, R.; Le Gallic, J.F.; Frantz, A.; Outreman, Y.; Fukatsu, T. Facultative symbiont infections affect aphid reproduction. PLoS One 2011, 6, e21831. [Google Scholar]
- Elnagdy, S.; Majerus, M.E.N.; Handley, L.-J.L. The value of an egg: Resource reallocation in ladybirds (Coleoptera: Coccinellidae) infected with male-killing bacteria. J. Evol. Biol. 2011, 24, 2164–2172. [Google Scholar]
- Hurst, G.D.D.; Majerus, M.E.N. Why do maternally inherited microorganisms kill males? Heredity 1993, 71, 81–95. [Google Scholar] [CrossRef]
- Dunn, A.M.; Smith, J.E. Microsporidian life cycles and diversity: The relationship between virulence and transmission. Microb. Inf. 2001, 3, 381–388. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Hoffmann, A.A.; Werren, J.H. Influential Passengers; Oxford University Press: Oxford, UK, 1997; p. 214. [Google Scholar]
- Bourtzis, K.; Miller, T. Insect Symbiosis; CRC Press: Boca Raton, FL, USA, 2003; p. 368. [Google Scholar]
- Kageyama, D.; Anbutsu, H.; Shimada, M.; Fukatsu, T. Spiroplasma infection causes either early or late male killing in Drosophila, depending on maternal host age. Naturwissenschaften 2007, 94, 333–337. [Google Scholar] [CrossRef]
- Charlat, S.; Davies, N.; Roderick, G.K.; Hurst, G.D.D. Disrupting the timing of Wolbachia-induced male-killing. Biol. Lett. 2007, 3, 154–156. [Google Scholar] [CrossRef]
- Stouthamer, R. Wolbachia-induced parthenogenesis. In Influential Passengers; O’Neill, S.L., Hoffmann, A.A., Werren, J.H., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 102–124. [Google Scholar]
- Arakaki, N.; Miyoshi, T.; Noda, H. Wolbachia-mediated parthenogenesis in the predatory thrips Franklinothrips vespiformis (Thysanoptera: Insecta). Proc. R. Soc. Lond. B 2001, 268, 1011–1016. [Google Scholar] [CrossRef]
- Koivisto, R.K.K.; Braig, H.R. Microorganisms and parthenogenesis. Biol. J. Linn. Soc. 2003, 79, 43–58. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Roush, R.T.; Hunter, M.S. Male production induced by antibiotic treatment in Encarsia formosa (Hymenoptera: Aphelinidae), an asexual species. Experientia 1992, 48, 102–105. [Google Scholar] [CrossRef]
- Pijls, J.W.A.M.; Steenbergen, H.J.V.A.N.; Van, J.J.M. Asexuality cured: The relations and differences between sexual and asexual Apoanagyrus diversicornis. Heredity 1996, 76, 506–513. [Google Scholar] [CrossRef]
- Kremer, N.; Charif, D.; Henri, H.; Bataille, M.; Prévost, G.; Kraaijeveld, K.; Vavre, F. A new case of Wolbachia dependence in the genus Asobara: Evidence for parthenogenesis induction in Asobara japonica. Heredity 2009, 103, 248–256. [Google Scholar] [CrossRef]
- Stouthamer, R.; Kazmer, D.J. Cytogenetics of microbe-associated parthenogenesis and its consequences for gene flow in Trichogramma wasps. Heredity 1994, 73, 317–327. [Google Scholar] [CrossRef]
- Pannebakker, B.A.; Pijnacker, L.P.; Zwaan, B.J.; Beukeboom, L.W. Cytology of Wolbachia-induced parthenogenesis in Leptopilina clavipes (Hymenoptera: Figitidae). Genome 2004, 47, 299–303. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Zchori-Fein, E.; Werren, J.H.; Karr, T.L. Diploidy restoration in Wolbachia-infected Muscidifurax uniraptor (Hymenoptera: Pteromalidae). J. Invertebr. Pathol. 2002, 81, 166–174. [Google Scholar] [CrossRef]
- Adachi-Hagimori, T.; Miura, K.; Stouthamer, R. A new cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera. Proc. R. Soc. Lond. B 2008, 275, 2667–2673. [Google Scholar] [CrossRef]
- Verma, S.; Ruttner, F. Cytological analysis of thelytokous parthenogenesis in the Cape honeybee (Apis mellifera capensis Escholtz). Apidologie 1983, 17, 47–58. [Google Scholar]
- Pearcy, M.; Hardy, O.; Aron, S. Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity 2006, 96, 377–382. [Google Scholar] [CrossRef]
- Bourtzis, K.; Braig, H.; Karr, T. Cytoplasmic incompatibility. In Insect Symbiosis; Bourtzis, K., Miller, T., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 217–246. [Google Scholar]
- Poinsot, D.; Charlat, S.; Merçot, H. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: Confronting the models with the facts. BioEssays 2003, 25, 259–265. [Google Scholar] [CrossRef]
- Tram, U.; Fredrick, K.; Werren, J.; Sullivan, W. Paternal chromosome segregation during the first mitotic division determines Wolbachia-induced cytoplasmic incompatibility phenotype. J. Cell Sci. 2006, 119, 3655–3663. [Google Scholar] [CrossRef]
- Taylor, M.J.; Bandi, C.; Hoerauf, A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv. Parasitol. 2005, 60, 245–284. [Google Scholar] [CrossRef]
- Hoerauf, A.; Mand, S.; Fischer, K.; Kruppa, T.; Marfo-Debrekyei, Y.; Debrah, A.Y.; Pfarr, K.M.; Adjei, O.; Büttner, D.W. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med. Microbiol. Immunol. 2003, 192, 211–216. [Google Scholar]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X.-Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar]
- Dedeine, F.; Vavre, F.; Fleury, F.; Loppin, B.; Hochberg, M.E.; Bouletreau, M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA 2001, 98, 6247–6252. [Google Scholar]
- Starr, D.J.; Cline, T.W. A host-parasite interaction rescues Drosophila oogenesis defects. Nature 2002, 418, 76–79. [Google Scholar]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar]
- Osborne, S.E.; Leong, Y.S.; O’Neill, S.L.; Johnson, K.N. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009, 5, e1000656. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, e2. [Google Scholar]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 2010, 5, e11977. [Google Scholar] [CrossRef]
- Panteleev, D.I.; Goriacheva, I.I.; Andrianov, B.V.; Reznik, N.L.; Lazebnyĭ, O.E.; Kulikov, A.M. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Genetika 2007, 43, 1277–1280. [Google Scholar]
- Kambris, Z.; Cook, P.; Phuc, H.; Sinkins, S. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 2009, 326, 134–136. [Google Scholar]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; Hugo, L.E.; Johnson, K.N.; Kay, B.H.; McGraw, E.A.; van den Hurk, A.F.; Ryan, P.A.; O'Neill, S.L. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar]
- Kambris, Z.; Blagborough, A.M.; Pinto, S.B.; Blagrove, M.S.C.; Godfray, H.C.J.; Sinden, R.E.; Sinkins, S.P. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010, 6, e1001143. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; Cook, H.; Axford, J.; Callahan, A.G.; Kenny, N.; Omodei, C.; McGraw, E.A.; Ryan, P.A.; Ritchie, S.A.; Turelli, M.; O'Neill, S.L. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; Lloyd, A.L.; Ritchie, S.A.; O'Neill, S.L.; Hoffmann, A.A. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar]
- Iturbe-Ormaetxe, I.; Walker, T.; O’ Neill, S.L. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef]
- Jaenike, J.; Unckless, R.; Cockburn, S.N.; Boelio, L.M.; Perlman, S.J. Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science 2010, 329, 212–215. [Google Scholar]
- Rigaud, T.; Juchault, P.; Mocquard, J.-P. The evolution of sex determination in isopod crustaceans. BioEssays 1997, 19, 409–416. [Google Scholar] [CrossRef]
- Hashiyama, K.; Hayashi, Y.; Kobayashi, S. Drosophila Sex lethal gene initiates female development in germline progenitors. Science 2011, 333, 885–888. [Google Scholar]
- Clark, M.E.; Heath, B.D.; Anderson, C.L., Karr. Induced paternal effects mimic cytoplasmic incompatibility in Drosophila. Genetics 2006, 173, 727. [Google Scholar] [CrossRef]
- Xi, Z.; Gavotte, L.; Xie, Y.; Dobson, S. Genome-wide analysis of the interaction between the endosymbiotic bacterium Wolbachia and its Drosophila host. BMC Genomics 2008, 9, 1. [Google Scholar] [CrossRef]
- Nakamura, Y.; Gotoh, T.; Imanishi, S.; Mita, K.; Kurtti, T.J.; Noda, H. Differentially expressed genes in silkworm cell cultures in response to infection by Wolbachia and Cardinium endosymbionts. Insect Mol. Biol. 2011, 20, 279–289. [Google Scholar] [CrossRef]
- Hughes, G.L.; Ren, X.; Ramirez, J.L.; Sakamoto, J.M.; Bailey, J.A.; Jedlicka, A.E.; Rasgon, J.L. Wolbachia infections in Anopheles gambiae cells: Transcriptomic characterization of a novel host-symbiont interaction. PLoS Pathog. 2011, 7, e1001296. [Google Scholar] [CrossRef]
- Hatcher, M.J.; Taneyhill, D.E.; Dunn, A.M.; Tofts, C. Population dynamics under parasitic sex ratio distortion. Theor. Pop. Biol. 1999, 56, 11–28. [Google Scholar] [CrossRef]
- Saccheri, I.; Kuussaari, M.; Kankare, M.; Vikman, P.; Hanski, I. Inbreeding and extinction in a butterfly metapopulation. Nature 1998, 45, 1996–1999. [Google Scholar]
- Jiggins, F.M.; Hurst, G.D.; Majerus, M.E. Sex-ratio-distorting Wolbachia causes sex-role reversal in its butterfly host. Proc. R. Soc. Lond. B 2000, 267, 69–73. [Google Scholar] [CrossRef]
- Narita, S.; Nomura, M.; Kageyama, D. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: Transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol. Ecol. 2007, 61, 235–245. [Google Scholar] [CrossRef]
- Martin, G.; Juchault, P.; Legrand, J. Mise en évidence d’un microorganisme intracytoplasmique symbiote de l’oniscoïde Armadillidium vulgare Latr., dont la présence accompagne l’intersexualité ou la féminisation totale des mâles génétiques de la lignée thélygène. C. R. Acad. Sci. 1973, 276, 2313–2316. [Google Scholar]
- Rigaud, T.; Souty-Grosset, C.; Raimond, R.; Mocquard, J.P.; Juchault, P. Feminizing endocytobiosis in the terrestrial crustacean Armadillidium vulgare Latr. (Isopoda): Recent acquisitions. Endocytobiosis Cell Res. 1991, 7, 259–273. [Google Scholar]
- Rousset, F.; Bouchon, D.; Pintureau, B.; Juchault, P.; Solignac, M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. R. Soc. Lond. B 1992, 250, 91–98. [Google Scholar] [CrossRef]
- Juchault, P.; Legrand, J.J. Analyse génétique et physiologique de la détermination du sexe dans une population du crustacé Oniscoïde—Armadillidium nasatum Budde-Lund. Arch. Zool. Exp. Gén. 1979, 120, 25–43. [Google Scholar]
- Juchault, P.; Legrand, J.J. Sex determination and monogeny in terrestrial isopods Armadillidium vulgare (Latreille, 1804) and Armadillidium nasatum (Budde-Lund, 1885). Monogr. Monit. Zool. Italian. 1989, 4, 359–375. [Google Scholar]
- Juchault, P.; Frelon, M.; Bouchon, D.; Rigaud, T. New evidence for feminizing bacteria in terrestrial isopods: Evolutionary implications. C. R. Acad. Sci. 1994, 317, 225–230. [Google Scholar]
- Rigaud, T.; Antoine, D.; Marcadé, I.; Juchault, P. The effect of temperature on sex ratio in the isopod Porcellionides pruinosus: Environmental sex determination or a by-product of cytoplasmic sex determination? Evol. Ecol. 1997, 11, 205–215. [Google Scholar] [CrossRef]
- Martin, G.; Gruppe, S.G.; Laulier, M.; Bouchon, D.; Rigurd, D.; Juchault, P. (1994) Evidence for Wolbachia spp. in the estuarine isopod Sphaeroma rugicauda (Crustacea): A likely cytoplasmic sex ratio distorter. Endocytobiosis Cell Res. 1994, 10, 215–225. [Google Scholar]
- Bulnheim, H.-P. Interaction between genetic, external and parasitic factors in sex determination of the crustacean amphipod Gammarus duebeni. Helgoländer Wissenschaftliche Meeresuntersuchungen 1978, 31, 1–33. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Jiggins, F.M.; Schulenburg, J.H.; Bertrand, D.; West, S.A.; et al. Male-killing Wolbachia in two species of insect. Proc. R. Soc. Lond. B 1999, 266, 735–740. [Google Scholar]
- Fialho, R.F.; Stevens, L. Male-killing Wolbachia in a flour beetle. Proc. R. Soc. Lond. B 2000, 267, 1469–1473. [Google Scholar] [CrossRef]
- Hurst, G.D.; Johnson, A.P.; Schulenburg, J.H.; Fuyama, Y. Male-killing Wolbachia in Drosophila: A temperature-sensitive trait with a threshold bacterial density. Genetics 2000, 156, 699–709. [Google Scholar]
- Sheeley, S.L.; McAllister, B.F. Mobile male-killer: Similar Wolbachia strains kill males of divergent Drosophila hosts. Heredity 2009, 102, 286–292. [Google Scholar] [CrossRef]
- Jaenike, J.; Dyer, K.A.; Reed, L.K. Within-population structure of competition and the dynamics of male-killing Wolbachia. Evol. Ecol. Res. 2003, 5, 1023–1036. [Google Scholar]
- Jiggins, F.M.; Hurst, G.D.D.; Dolman, C.E.; Majerus, M.E.N. High-prevalence male-killing Wolbachia in the butterfly Acraea encedana. J. Evol. Biol. 2000, 13, 495–501. [Google Scholar]
- Kageyama, D.; Nishimura, G.; Ohno, S.; Takanashi, T.; Hoshizaki, S.; Ishikawa, Y. Wolbachia infection and an all-female trait in Ostrinia orientalis and Ostrinia zaguliaevi. Entomol. Exp. Appl. 2004, 111, 79–83. [Google Scholar] [CrossRef]
- Kageyama, D.; Nishimura, G.; Hoshizaki, S.; Ishikawa, Y. Two kinds of sex ratio distorters in a moth, Ostrinia scapulalis. Genome 2003, 46, 974–982. [Google Scholar] [CrossRef]
- Zeh, D.W.; Zeh, J.A.; Bonilla, M.M. Wolbachia, sex ratio bias and apparent male killing in the harlequin beetle riding pseudoscorpion. Heredity 2005, 95, 41–49. [Google Scholar] [CrossRef]
- Hurst, G.D.; Schulenburg, J.H.; Majerus, T.M.; Bertrand, D.; Zakharov, I.A.; Baungaard, J.; Völkl, W.; Stouthamer, R.; Majerus, M.E. Invasion of one insect species, Adalia bipunctata, by two different male-killing bacteria. Insect Mol. Biol. 1999, 8, 133–139. [Google Scholar]
- Majerus, M.E.; Schulenburg, J.H.; Zakharov, I.A. Multiple causes of male-killing in a single sample of the two-spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae) from Moscow. Heredity 2000, 84, 605–609. [Google Scholar] [CrossRef]
- Tinsley, M.C.; Majerus, M.E.N. A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitology 2006, 132, 757–765. [Google Scholar] [CrossRef]
- Matsuka, M.; Hashi, H.; Okada, I. Abnormal sex ratio found in the lady beetle Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Appl. Entomol. Zool. 1975, 10, 84–89. [Google Scholar]
- Majerus, T.M.; Schulenburg, J.H.; Majerus, M.E.; Hurst, G.D. Molecular identification of a male-killing agent in the ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Insect Mol. Biol. 1999, 8, 551–555. [Google Scholar] [CrossRef]
- Niijima, K.; Nakajima, K. Abnormal sex-ratio in Menochilus sexmaculatus (Fabricius). Bull. of the Faculty of Agriculture-Tamagawa Univ (Japan) 1981, 21, 59–67. [Google Scholar]
- Jiggins, F.M.; Hurst, G.D.; Jiggins, C.D.; Schulenburg, J.H.; Majerus, M.E. (2000) The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology 2000, 120, 439–446. [Google Scholar] [CrossRef]
- Tabata, J.; Hattori, Y.; Sakamoto, H.; Yukuhiro, F.; Fujii, T.; Kugimiya, S.; Mochizuki, A.; Ishikawa, Y.; Kageyama, D. Male killing and incomplete inheritance of a novel spiroplasma in the moth Ostrinia zaguliaevi. Microb. Ecol. 2011, 61, 254–263. [Google Scholar] [CrossRef]
- Bentley, J.K.; Veneti, Z.; Heraty, J.; Hurst, G.D.D. The pathology of embryo death caused by the male-killing Spiroplasma bacterium in Drosophila nebulosa. BMC Biol. 2007, 5, 9. [Google Scholar] [CrossRef]
- Montenegro, H.; Hatadani, L.M.; Medeiros, H.F.; Klaczko, L.B. Male killing in three species of the tripunctata radiation of Drosophila (Diptera: Drosophilidae). J. Zool. Syst. Evol. Res. 2006, 44, 130–135. [Google Scholar] [CrossRef]
- Pool, J.E.; Wong, A.; Aquadro, C.F. Finding of male-killing Spiroplasma infecting Drosophila melanogaster in Africa implies transatlantic migration of this endosymbiont. Heredity 2006, 97, 27–32. [Google Scholar] [CrossRef]
- Williamson, D.L.; Sakaguchi, B.; Hackett, K.J.; Whitcomb, R.F.; Tully, J.G.; Carle, P.; Bové, J.M.; Adams, J.R.; Konai, M.; Henegar, R.B. Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int. J. Syst. Bacteriol. 1999, 49, 611–618. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Majerus, M.E.N.; Walker, L.E. The importance of cytoplasmic male killing elements in natural populations of the two spot ladybird, Adalia bipunctata (Linnaeus) (Goleoptera: Goccinellidae). Biol. J. Linn. Soc. 1993, 49, 195–202. [Google Scholar]
- Werren, J.H.; Hurst, G.D.; Zhang, W.; Breeuwer, J.A.; Stouthamer, R.; Majerus, M.E. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J. Bacteriol. 1994, 176, 388–394. [Google Scholar]
- Schulenburg, J.H.; Habig, M.; Sloggett, J.J.; Webberley, K.M.; Bertrand, D.; Hurst, G.D.; Majerus, M.E. Incidence of male-killing Rickettsia spp. (alpha-proteobacteria) in the ten-spot ladybird beetle Adalia decempunctata L. (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 2001, 67, 270–277. [Google Scholar]
- Lawson, E.T.; Mousseau, T.A.; Klaper, R.; Hunter, M.D.; Werren, J.H. Rickettsia associated with male-killing in a buprestid beetle. Heredity 2001, 86, 497–505. [Google Scholar] [CrossRef]
- Majerus, T.M.; Majerus, M.E. Discovery and identification of a male-killing agent in the Japanese ladybird Propylea japonica (Coleoptera: Coccinellidae). BMC Evol. Biol. 2010, 10, 37. [Google Scholar] [CrossRef]
- Hurst, G.D.; Bandi, C.; Sacchi, L.; Cochrane, A.G.; Bertrand, D.; Karaca, I.; Majerus, M.E. Adonia variegata (Coleoptera: Coccinellidae) bears maternally inherited flavobacteria that kill males only. Parasitology 1999, 118, 125–134. [Google Scholar] [CrossRef]
- Majerus, T.M.O. The Evolutionary Genetics of Male killing in the Coccinellidae. PhD thesis, Department of Genetics, University of Cambridge, Cambridge, UK, 2001. [Google Scholar]
- Majerus, M.E.N.; Majerus, T.M.O. Female-biased sex ratio due to male-killing in the Japanese ladybird Coccinula sinensis. Ecol. Entomol. 2000, 25, 234–238. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Hammarton, T.C.; Bandi, C.; Majerus, T.M.O.; Bertrand, D.; Majerus, M.E.N. The diversity of inherited parasites of insects: The male-killing agent of the ladybird beetle Coleomegilla maculata is a member of the Flavobacteria. Genet. Res. 1997, 70, 1–6. [Google Scholar] [CrossRef]
- Werren, J.H.; Skinner, S.W.; Huger, A.M. Male-killing bacteria in a parasitic wasp. Science 1986, 231, 990–992. [Google Scholar]
- Ferree, P.M.; Avery, A.; Azpurua, J.; Wilkes, T.; Werren, J.H. A bacterium targets maternally inherited centrosomes to kill males in Nasonia. Curr. Biol. 2008, 18, 1409–1414. [Google Scholar]
- Hazard, E.I.; Weiser, J. Spores of Thelohania in adult female Anopheles: Development and transovarial transmission, and redescriptions of T. legeri Hesse and T. obesa Kudo. J. Euk. Microbiol. 1968, 15, 817–823. [Google Scholar] [CrossRef]
- Avery, S. Horizontal transmission of (Protozoa: Microsporida) to (Diptera: Culicidae). J. Invertebr. Pathol. 1989, 53, 424–426. [Google Scholar] [CrossRef]
- Andreadis, T.G. Life cycle, epizootiology, and horizontal transmission of (Microspora: Amblyosporidae) in a univoltine mosquito. J. Invertebr. Pathol. 1985, 46, 31–46. [Google Scholar] [CrossRef]
- Kellen, W.; Chapman, H.; Clark, T.; Lindegren, J. Host-parasite relationships of some from mosquitoes (Nosematidae: Microsporidia). J. Invertebr. Pathol. 1965, 7, 161–166. [Google Scholar] [CrossRef]
- Kellen, W.R.; Lipa, J.J. Thelohania californica n. sp. a microsporidian parasite of Culex tarsalis Coquillet. J. Insect. Pathol. 1960, 2, 1–12. [Google Scholar]
- Kellen, W.R.; Wills, W. The transovarian transmission of Thelohania californica Kellen and Lipa in Culex tarsalis Coquillet. J. Insect Pathol. 1962, 4, 321–326. [Google Scholar]
- Andreadis, T.G.; Hall, D.W. Development, ultrastructure, and mode of transmission of Amblyospora sp. (Microspora) in the mosquito. J. Protozool. 1979, 26, 444–452. [Google Scholar]
- Morimoto, S.; Nakai, M.; Ono, A.; Kunimi, Y. Late male-killing phenomenon found in a Japanese population of the oriental tea tortrix, Homona magnanima (Lepidoptera: Tortricidae). Heredity 2001, 87, 435–440. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Faktor, O.; Zeidan, M.; Gottlieb, Y.; Czosnek, H.; Rosen, D. Parthenogenesis-inducing microorganisms in Aphytis (Hymenoptera: Aphelinidae). Insect Mol. Biol. 1995, 4, 173–178. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Zchori-Fein, E.; Faktor, O.; Rosen, D. Phylogenetic analysis of parthenogenesis-inducing Wolbachia in the genus Aphytis (Hymenoptera: Aphelinidae). Insect Mol. Biol. 1998, 7, 393–396. [Google Scholar]
- Zchori-Fein, E.; Rosen, D.; Roush, R. Microorganisms associated with thelytoky in Aphytis lingnansensis compere (Hymenoptera: Aphelinidae). Int. J. Insect. Morphol. Embryol. 1994, 23, 169–172. [Google Scholar] [CrossRef]
- Schilthuizen, M.; Stouthamer, R. Distribution of Wolbachia among the guild associated with the parthenogenetic gall wasp Diplolepis rosae. Heredity 1998, 81, 270–274. [Google Scholar] [CrossRef]
- Barro, P.J.; Hart, P.J. Antibiotic curing of parthenogenesis in Eretmocerus mundus (Australian parthenogenic form). Entomol. Exp. Appl. 2001, 99, 225–230. [Google Scholar]
- Arakaki, N.; Oishi, T.; Noda, H. Parthenogenesis Induced by Wolbachia in Gronotoma micromorpha (Hymenoptera: Eucoilidae). Entomol. Sci. 2001, 4, 9–15. [Google Scholar]
- Stouthamer, R.; Breeuwert, J.A.; Luck, R.F.; Werren, J.H. Molecular identification of microorganisms associated with parthenogenesis. Nature 1993, 361, 66–68. [Google Scholar] [CrossRef]
- Stouthamer, R.; Lukoe, S.; Mak, F. Influence of parthenogenesis Wolbachia on host fitness. Nor. J. Agric. Sci. (Suppl.) 1994, 6, 117–122. [Google Scholar]
- Arakaki, N.; Noda, H.; Yamagishi, K. Wolbachia-induced parthenogenesis in the egg parasitoid Telenomus nawai. Entomol. Exp. Appl. 2000, 96, 177–184. [Google Scholar]
- Stouthamer, R.; Werren, J.H. Microbes associated with parthenogenesis in wasps of the genus Trichogramma. J. Invertebr. Pathol. 1993, 61, 6–9. [Google Scholar] [CrossRef]
- Stouthamer, R.; Luck, R.F.; Hamilton, W.D. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc. Natl. Acad. Sci. USA 1990, 87, 2424–2427. [Google Scholar]
- Schilthuizen, M.; Honda, J.; Stouthamer, R. Parthenogenesis-inducing Wolbachia in Trichogramma kaykai (Hymenoptera: Trichogrammatidae) originates from a single infection. Ann. Entomol. Soc. Am. 1998, 91, 410–414. [Google Scholar]
- Zchori-Fein, E.; Gottlieb, Y.; Kelly, S.E.; Brown, J.K.; Wilson, J.M.; Karr, T.L.; Hunter, M.S. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc. Natl. Acad. Sci. USA 2001, 98, 12555–12560. [Google Scholar]
- Giorgini, M. Induction of males in thelytokous populations of Encarsia meritoria and Encarsia protransvena: A systematic tool. BioControl 2001, 46, 427–438. [Google Scholar] [CrossRef]
- Hagimori, T.; Abe, Y.; Date, S.; Miura, K. The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Curr. Microbiol. 2006, 52, 97–101. [Google Scholar]
- Giorgini, M.; Bernardo, U.; Monti, M.M.; Nappo, A.G.; Gebiola, M. Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl. Environ. Microbiol. 2010, 76, 2589–2599. [Google Scholar] [CrossRef]
- Kondo, N.; Ijichi, N.; Shimada, M.; Fukatsu, T. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol. Ecol. 2002, 11, 167–180. [Google Scholar] [CrossRef]
- Keller, G.P.; Windsor, D.M.; Saucedo, J.M.; Werren, J.H. Reproductive effects and geographical distributions of two Wolbachia strains infecting the Neotropical beetle, Chelymorpha alternans Boh. (Chrysomelidae, Cassidinae). Mol. Ecol. 2004, 13, 2405–2420. [Google Scholar] [CrossRef]
- Giordano, R.; Jackson, J.J.; Robertson, H.M. The role of Wolbachia bacteria in reproductive incompatibilities and hybrid zones of Diabrotica beetles and Gryllus crickets. Proc. Natl. Acad. Sci. USA 1997, 94, 11439–11444. [Google Scholar] [CrossRef]
- Blickenstaff, C.C. Partial Inter sterility of Eastern and Western U.S. Strains of the Alfalfa Weevil. Ann. Entomol. Soc. Am. 1965, 58, 523–526. [Google Scholar]
- Hsiao, C.; Hsiao, T. Rickettsia as the cause of cytoplasmic incompatibility in the alfalfa weevil, Hypera postica. J. Insect Pathol. 1985, 45, 244–246. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Giordano, R.; Colbert, A.M.; Karr, T.L.; Robertson, H.M. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 1992, 89, 2699–2702. [Google Scholar]
- Fialho, R.; Stevens, L. Wolbachia Infections in the Flour Beetle Tribolium confusum: Evidence for a Common Incompatibility Type across Strains. J. Insect Pathol. 1996, 67, 195–197. [Google Scholar] [CrossRef]
- Fialho, R.F.; Stevens, L. Molecular evidence for single Wolbachia infections among geographic strains of the flour beetle Tribolium confusum. Proc. R. Soc. Lond. B 1997, 264, 1065–1068. [Google Scholar]
- Macdonald, W. Mosquito genetics in relation to filarial infections. Symposia of the British Society of Parasitology 1976, 14, 1–24. [Google Scholar]
- Wright, J.D.; Barr, A.R. The ultrastructure and symbiotic relationships of Wolbachia of mosquitoes of the Aedes scutellaris group. J. Ultra. Res. 1980, 72, 52–64. [Google Scholar] [CrossRef]
- Wright, J.; Wang, B. Observations on Wolbachiae in mosquitoes. J. Insect Pathol. 1980, 35, 200–208. [Google Scholar] [CrossRef]
- Trpis, M.; Perrone, J.; Reissig, M.; Parker, K. Control of cytoplasmic incompatibility in the Aedes scutellaris complex. J. Heredity 1981, 72, 313–317. [Google Scholar]
- Tesfa-Yohannes, T.M.; Rozeboom, L.E. Experimental crossing of Aedes (S.) polynesiensis Marks and A. scutellaris malayensis Colless (Diptera: Culicidae). J. Med. Entomol. 1974, 11, 323–331. [Google Scholar]
- Beckett, E.B.; Boothroyd, B.; Macdonald, W.W. A light and electron microscope study of rickettsia-like organisms in the ovaries of mosquitoes of the Aedes scutellaris group. Ann. Trop. Med. Parasitol. 1978, 72, 277–283. [Google Scholar]
- Meek, S.R. Occurrence of rickettsia-like symbionts among species of the Aedes scutellaris group (Diptera: Culicidae). Ann. Trop. Med. Parasitol. 1984, 78, 377–381. [Google Scholar]
- Meek, S.; Macdonald, W. Crossing relationships among seven members of the group of Aedes scutellaris (Walker)(Diptera: Culicidae). Bull. Entomol. Res. 1984, 74, 65–78. [Google Scholar] [CrossRef]
- Smith-White, S.; Woodhill, A. The nature and significance of non-reciprocal fertility in Aedes scutellaris and other mosquitoes. Proc. Linn. Soc. N. S. W. 1954, 79, 163–176. [Google Scholar]
- Jamnongluk, W.; Kittayapong, P.; Baisley, K.J.; O’Neill, S.L. Wolbachia infection and expression of cytoplasmic incompatibility in Armigeres subalbatus (Diptera: Culicidae). J. Med. Entomol. 2000, 37, 53–57. [Google Scholar] [CrossRef]
- Laven, H. Speciation and evolution in Culex pipiens. In Genetics of insect vectors of disease; Wright, J., Pal, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; pp. 251–275. [Google Scholar]
- Barr, A.R. Cytoplasmic incompatibility in natural populations of a mosquito, Culex pipiens L. Nature 1980, 283, 71–72. [Google Scholar] [CrossRef]
- Magnin, M.; Pasteur, N.; Raymond, M. Multiple incompatibilities within populations of Culex pipiens L. in southern France. Genetica 1987, 74, 125–130. [Google Scholar] [CrossRef]
- Irving-Bell, R.J. Cytoplasmic incompatibility within and between Culex molestus and Cx. quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 1983, 20, 44–48. [Google Scholar]
- O’Neill, S.L.; Paterson, H.E. Crossing type variability associated with cytoplasmic incompatibility in Australian populations of the mosquito Culex quinquefasciatus Say. Med. Vet. Entomol. 1992, 6, 209–216. [Google Scholar] [CrossRef]
- Bourtzis, K.; Nirgianaki, A.; Markakis, G.; Savakis, C. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 1996, 144, 1063–1073. [Google Scholar]
- Hoffmann, A.A.; Turelli, M. Unidirectional incompatibility in Drosophila simulans: Inheritance, geographic variation and fitness effects. Genetics 1988, 119, 435–444. [Google Scholar]
- Holden, P.R.; Brookfield, J.F.; Jones, P. Cloning and characterization of an ftsZ homologue from a bacterial symbiont of Drosophila melanogaster. Mol. Genet. Genomics 1993, 240, 213–220. [Google Scholar] [CrossRef]
- Werren, J.H.; Jaenike, J. Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity 1995, 75, 320–326. [Google Scholar] [CrossRef]
- Giordano, R.; O’Neill, S.L.; Robertson, H.M. Wolbachia infections and the expression of cytoplasmic incompatibility in Drosophila sechellia and D. mauritiana. Genetics 1995, 140, 1307–1317. [Google Scholar]
- Hoffmann, A.A.; Turelli, M.; Harshman, L.G. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 1990, 126, 933–948. [Google Scholar]
- Tagami, Y.; Doi, M.; Sugiyama, K.; Tatara, A.; Saito, T. Wolbachia-induced cytoplasmic incompatibility in Liriomyza trifolii and its possible use as a tool in insect pest control. Biol. Cont. 2006, 38, 205–209. [Google Scholar] [CrossRef]
- Kassem, H.A.; Hassan, A.N.; Abdel-Hamid, I.; Osman, G.; El Khalab, E.M.; Madkour, M.A. Wolbachia infection and the expression of cytoplasmic incompatibility in sandflies (Diptera: Psychodidae) from Egypt. Ann. Trop. Med. Parasitol. 2003, 97, 639–644. [Google Scholar] [CrossRef]
- Boller, E.F.; Russ, K.; Vallo, V.; Bush, G.L. Incompatible races of European cherry fruit fly, Rhagoletis cerasi (Diptera: Tephritidae), their origin and potential use in biological control. Entomol. Exp. Appl. 1976, 20, 237–247. [Google Scholar] [CrossRef]
- Riegler, M.; Stauffer, C. Wolbachia infections and superinfections in cytoplasmically incompatible populations of the European cherry fruit fly Rhagoletis cerasi (Diptera, Tephritidae). Mol. Ecol. 2002, 11, 2425–2434. [Google Scholar] [CrossRef]
- Vasquez, C.J.; Stouthamer, R.; Jeong, G.; Morse, J.G. Discovery of a CI-inducing Wolbachia and its associated fitness costs in the biological control agent Aphytis melinus DeBach (Hymenoptera: Aphelinidae). Biol. Cont. 2011, 58, 192–198. [Google Scholar] [CrossRef]
- Dedeine, F.; Vavre, F.; Shoemaker, D.D.; Boulétreau, M. Intra-individual coexistence of a Wolbachia strain required for host oogenesis with two strains inducing cytoplasmic incompatibility in the wasp Asobara tabida. Evolution 2004, 58, 2167–2174. [Google Scholar]
- Mochiah, M.B.; Ngi-Song, A.J.; Overholt, W.A.; Stouthamer, R. Wolbachia infection in Cotesia sesamiae (Hymenoptera: Braconidae) causes cytoplasmic incompatibility: Implications for biological control. Biol. Cont. 2002, 25, 74–80. [Google Scholar] [CrossRef]
- White, J.A.; Kelly, S.E.; Perlman, S.J.; Hunter, M.S. Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: Disentangling the roles of Cardinium and Wolbachia symbionts. Heredity 2009, 102, 483–489. [Google Scholar] [CrossRef]
- Noda, H. Cytoplasmic incompatibility in a rice planthopper. J. Heredity 1984, 75, 345–348. [Google Scholar]
- Rousset, F.; Bouchon, D.; Pintureau, B.; Juchault, P.; Solignac, M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. R. Soc. Lond. B 1992, 250, 91–98. [Google Scholar] [CrossRef]
- Hoshizaki, S.; Shimada, T. PCR-based detection of Wolbachia, cytoplasmic incompatibility microorganisms, infected in natural populations of Laodelphax striatellus (Homoptera: Delphacidae) in central Japan: Has the distribution of Wolbachia spread recently? Insect Mo. Biol. 1995, 4, 237–243. [Google Scholar]
- Vavre, F.; Fleury, F.; Varaldi, J.; Fouillet, P.; Boulétreau, M. Evidence for female mortality in Wolbachia-mediated cytoplasmic incompatibility in haplodiploid insects: Epidemiologic and evolutionary consequences. Evolution 2000, 54, 191–200. [Google Scholar]
- Vavre, F.; Dedeine, F.; Quillon, M.; Fouillet, P.; Fleury, F.; Boulétreau, M. Within-species diversity of Wolbachia-induced cytoplasmic incompatibility in haplodiploid insects. Evolution 2001, 55, 1710–1714. [Google Scholar]
- Mouton, L.; Henri, H.; Boulétreau, M.; Vavre, F. Multiple infections and diversity of cytoplasmic incompatibility in a haplodiploid species. Heredity 2005, 94, 187–192. [Google Scholar] [CrossRef]
- Machtelinckx, T.; van Leeuwen, T.; Vanholme, B.; Gehesquière, B.; Dermauw, W.; Vandekerkhove, B.; Gheysen, G.; De Clercq, P. Wolbachia induces strong cytoplasmic incompatibility in the predatory bug Macrolophus pygmaeus. Insect Mol. Biol. 2009, 18, 373–381. [Google Scholar] [CrossRef]
- Breeuwer, J.A.; Werren, J.H. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 1990, 346, 558–560. [Google Scholar] [CrossRef]
- Breeuwer, J.A.; Stouthamer, R.; Barns, S.M.; Pelletier, D.A.; Weisburg, W.G.; Werren, J.H. Phylogeny of cytoplasmic incompatibility micro-organisms in the parasitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol. Biol. 1992, 1, 25–36. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; O’Hara, F.P.; Werren, J.H. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 2001, 409, 707–710. [Google Scholar] [CrossRef]
- Saul, G.B. An analysis of non-reciprocal cross incompatibility in Mormoniella vitripennis (Walker). Zeitschrift für Vererbungslehre 1961, 92, 28–33. [Google Scholar] [CrossRef]
- Watanabe, M.; Miura, K.; Hunter, M.S.; Wajnberg, E. Superinfection of cytoplasmic incompatibility-inducing Wolbachia is not additive in Orius strigicollis (Hemiptera: Anthocoridae). Heredity 2011, 106, 642–648. [Google Scholar] [CrossRef]
- Narita, S.; Shimajiri, Y.; Nomura, M. Strong cytoplasmic incompatibility and high vertical transmission rate can explain the high frequencies of Wolbachia infection in Japanese populations of Colias erate poliographus (Lepidoptera: Pieridae). Bull. Entomol. Res. 2009, 99, 385–391. [Google Scholar] [CrossRef]
- Sasaki, T.; Ishikawa, H. Wolbachia Infections and Cytoplasmic Incompatibility in the Almond Moth and the Mediterranean Flour Moth. Zool. Sci. 1999, 16, 739–744. [Google Scholar] [CrossRef]
- Narita, S.; Nomura, M.; Kato, Y.; Yata, O.; Kageyama, D. Molecular phylogeography of two sibling species of Eurema butterflies. Genetica 2007, 131, 241–253. [Google Scholar] [CrossRef]
- Hiroki, M.; Ishii, Y.; Kato, Y. Variation in the prevalence of cytoplasmic incompatibility-inducing Wolbachia in the butterfly Eurema hecabe across the Japanese archipelago. Evol. Ecol. Res. 2005, 7, 931–942. [Google Scholar]
- Narita, S.; Nomura, M.; Kato, Y.; Fukatsu, T. Genetic structure of sibling butterfly species affected by Wolbachia infection sweep: Evolutionary and biogeographical implications. Mol. Ecol. 2006, 15, 1095–1108. [Google Scholar] [CrossRef]
- Kamoda, S.; Masui, S.; Ishikawa, H.; Sasaki, T. Wolbachia infection and cytoplasmic incompatibility in the cricket Teleogryllus taiwanemma. J. Exp. Biol. 2000, 203, 2503–2509. [Google Scholar]
- Moret, Y.; Juchault, P.; Rigaud, T. Wolbachia endosymbiont responsible for cytoplasmic incompatibility in a terrestrial crustacean: Effects in natural and foreign hosts. Heredity 2001, 86, 325–332. [Google Scholar] [CrossRef]
- Legrand, J.; Martin, G.; Artault, J. Corrélation entre la présence d’un symbiote bactérien dans les ovocytes de Porcellio dilatatus petiti, et la stérilité du croisement P. d. petiti mâle × P. d. dilatatus femelle. Inst. Pasteur. Tunis. 1978, 55, 507–514. [Google Scholar]
- Gotoh, T.; Noda, H.; Hong, X-Y. Wolbachia distribution and cytoplasmic incompatibility based on a survey of 42 spider mite species (Acari: Tetranychidae) in Japan. Heredity 2003, 91, 208–216. [Google Scholar] [CrossRef]
- Gotoh, T.; Noda, H.; Fujita, T.; Iwadate, K.; Higo, Y.; Saito, S.; Ohtsuka, S. Wolbachia and nuclear-nuclear interactions contribute to reproductive incompatibility in the spider mite Panonychus mori (Acari: Tetranychidae). Heredity 2005, 94, 237–246. [Google Scholar] [CrossRef]
- Tsagkarakou, A.; Guillemaud, T.; Rousset, F.; Navajas, M. Molecular identification of a Wolbachia endosymbiont in a Tetranychus urticae strain (Acari: Tetranychidae). Insect Mol. Biol. 1996, 5, 217–221. [Google Scholar] [CrossRef]
- van Opijnen, T.; Breeuwer, J.A. High temperatures eliminate Wolbachia, a cytoplasmic incompatibility inducing endosymbiont, from the two-spotted spider mite. Exp. Appl. Acarol. 1999, 23, 871–881. [Google Scholar] [CrossRef]
- Gotoh, T.; Sugasawa, J.; Noda, H.; Kitashima, Y. Wolbachia-induced cytoplasmic incompatibility in Japanese populations of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2007, 42, 1–16. [Google Scholar] [CrossRef]
- Hunter, M.S.; Perlman, S.J.; Kelly, S.E. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. R. Soc. Lond. B 2003, 270, 2185–2190. [Google Scholar] [CrossRef]
- de Luna, C.J.; Moro, C.V.; Guy, J.H.; Zenner, L.; Sparagano, O.A.E. Endosymbiotic bacteria living inside the poultry red mite (Dermanyssus gallinae). Exp. Appl. Acarol. 2009, 48, 105–113. [Google Scholar] [CrossRef]
- Ros, V.I.D.; Breeuwer, J.A.J. The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni. Heredity 2009, 102, 413–422. [Google Scholar] [CrossRef]
- Xie, R.; Zhou, L.; Zhao, Z.; Hong, X.; Xiao-Yue, H. Male age influences the strength of Cardinium-induced cytoplasmic incompatibility expression in the carmine spider mite Tetranychus cinnabarinus. Appl. Entomol. Zool. 2010, 45, 417–423. [Google Scholar] [CrossRef]
- Kambris, Z.; Blagborough, A.M.; Pinto, S.B.; Blagrove, M.S.C.; Godfray, H.C.J.; Sinden, R.E.; Sinkins, S.P. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010, 6, e1001143. [Google Scholar]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Kambris, Z.; Cook, P.E.; Phuc, H.K.; Sinkins, S.P. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 2009, 326, 134–136. [Google Scholar]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PloS One 2010, 5, e11977. [Google Scholar] [CrossRef]
- Fytrou, A.; Schofield, P.G.; Kraaijeveld, A.R.; Hubbard, S.F. Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc. R. Soc. Lond. B 2006, 273, 791–796. [Google Scholar] [CrossRef]
- Osborne, S.E.; Leong, Y.S.; O’Neill, S.L.; Johnson, K.N. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009, 5, e1000656. [Google Scholar] [CrossRef]
- Panteleev, D.I.; Goriacheva, I.I.; Andrianov, B.V.; Reznik, N.L.; Lazebny, O.E.; Kulikov, A.M. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Genetika 2007, 43, 1277–1280. [Google Scholar]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, e2. [Google Scholar]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar]
- Jaenike, J.; Unckless, R.; Cockburn, S.N.; Boelio, L.M.; Perlman, S.J. Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science 2010, 329, 212–215. [Google Scholar]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X-Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar]
- Supali, T.; Djuardi, Y.; Pfarr, K.M.; Wibowo, H.; Taylor, M.J.; Hoerauf, A.; Houwing-Duistermaat, J.J.; Yazdanbakhsh, M.; Sartono, E. Doxycycline treatment of Brugia malayi-infected persons reduces microfilaremia and adverse reactions after diethylcarbamazine and albendazole treatment. Clin. Infect. Dis. 2008, 46, 1385–1393. [Google Scholar]
- Rao, R.; Well, G.J. In vitro effects of antibiotics on Brugia malayi worm survival and reproduction. J. Parasitol. 2002, 88, 605–611. [Google Scholar]
- Bandi, C.; Trees, A.J.; Brattig, N.W. Wolbachia in filarial nematodes: Evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Vet. Parasitol. 2001, 98, 215–238. [Google Scholar] [CrossRef]
- Bandi, C.; McCall, J.W.; Genchi, C.; Corona, S.; Venco, L.; Sacchi, L. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 1999, 29, 357–364. [Google Scholar] [CrossRef]
- Genchi, C.; Sacchi, L.; Bandi, C.; Venco, L. Preliminary results on the effect of tetracycline on the embryogenesis and symbiotic bacteria (Wolbachia) of Dirofilaria immitis. An update and discussion. Parassitologia 1998, 40, 247–249. [Google Scholar]
- Hoerauf, A.; Nissen-Pähle, K.; Schmetz, C.; Henkle-Dührsen, K.; Blaxter, M.L.; Büttner, D.W.; Gallin, M.Y.; Al-Qaoud, K.M.; Lucius, R.; Fleischer, B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Invest. 1999, 103, 11–18. [Google Scholar] [CrossRef]
- Hoerauf, A.; Volkmann, L.; Nissen-Paehle, K.; Schmetz, C.; Autenrieth, I.; Büttner, D.W.; Fleischer, B. Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: Comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop. Med. Int. Health 2000, 5, 275–279. [Google Scholar] [CrossRef]
- Townson, S.; Hutton, D.; Siemienska, J.; Hollick, L.; Scanlon, T.; Tagboto, S.K.; Taylor, M.J. Antibiotics and Wolbachia in filarial nematodes: Antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Ann. Trop. Med. Parasitol. 2000, 94, 801–816. [Google Scholar] [CrossRef]
- Langworthy, N.G.; Renz, A.; Mackenstedt, U.; Henkle-Dührsen, K.; de Bronsvoort, M.B.; Tanya, V.N.; Donnelly, M.J.; Trees, A.J. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: Elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc. R. Soc. Lond. B 2000, 267, 1063–1069. [Google Scholar] [CrossRef]
- Hoerauf, A.; Mand, S.; Adjei, O.; Fleischer, B.; Büttner, D.W. Depletion of Wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet 2001, 357, 1415–1416. [Google Scholar]
- Hoerauf, A.; Mand, S.; Fischer, K.; Kruppa, T.; Marfo-Debrekyei, Y.; Debrah, A.Y.; Pfarr, K.M.; Adjei, O.; Büttner, D.W. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med. Microbiol. Immunol. 2003, 192, 211–216. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Borad, C.; Harari, A.R. Oogenesis in the date stone beetle, Coccotrypes dactyliperda, depends on symbiotic bacteria. Physiol. Entomol. 2006, 31, 164–169. [Google Scholar] [CrossRef]
- Son, Y.; Luckhart, S.; Zhang, X.; Lieber, M.J.; Lewis, E.E. Effects and implications of antibiotic treatment on Wolbachia-infected vine weevil (Coleoptera: Curculionidae). Agr. Forest. Entomol. 2008, 10, 147–155. [Google Scholar] [CrossRef]
- Timmermans, M.J.T.N.; Ellers, J. Wolbachia endosymbiont is essential for egg hatching in a parthenogenetic arthropod. Evol. Ecol. 2008, 23, 931–942. [Google Scholar] [CrossRef]
- Starr, D.J.; Cline, T.W. A host parasite interaction rescues Drosophila oogenesis defects. Nature 2002, 418, 76–79. [Google Scholar]
Supplementary Materilas
| Phenotype | Endosymbiont | Class | Order | Species | Reference |
|---|---|---|---|---|---|
| CI | Wolbachia | Insecta | Coleoptera | Callosobruchus chinensis | [195] |
| CI | Wolbachia | Insecta | Coleoptera | Chelymorpha alternans | [196] |
| CI | Wolbachia | Insecta | Coleoptera | D. virgifera | [197] |
| CI | Wolbachia | Insecta | Coleoptera | D. virgifera virgifera | [197] |
| CI | Wolbachia | Insecta | Coleoptera | D. virgifera zeae | [197] |
| CI | Wolbachia | Insecta | Coleoptera | Hypera postica | [198,199,200] |
| CI | Wolbachia | Insecta | Coleoptera | Tribolium confusum | [201,202] |
| CI | Wolbachia | Insecta | Diptera | Aedes albopictus | [200] |
| CI | Wolbachia | Insecta | Diptera | A. cooki | [209,204] |
| CI | Wolbachia | Insecta | Diptera | A. kesseli | [205,206] |
| CI | Wolbachia | Insecta | Diptera | A. malayensis | [207,208] |
| CI | Wolbachia | Insecta | Diptera | A. polynesiensis | [207,208] |
| CI | Wolbachia | Insecta | Diptera | A. pseudoscutellaris | [209,210] |
| CI | Wolbachia | Insecta | Diptera | A. s. scutellaris | [209,211] |
| CI | Wolbachia | Insecta | Diptera | Armigeres subalbatus | [212] |
| CI | Wolbachia | Insecta | Diptera | Culex pipiens | [213,214,215] |
| CI | Wolbachia | Insecta | Diptera | C. pipiens quinquefasciatus | [216,217] |
| CI | Wolbachia | Insecta | Diptera | Drosophila auraria | [218] |
| CI | Wolbachia | Insecta | Diptera | D. melanogaster | [219,220] |
| CI | Wolbachia | Insecta | Diptera | D. recens | [221] |
| CI | Wolbachia | Insecta | Diptera | D. sechellia | [222] |
| CI | Wolbachia | Insecta | Diptera | D. simulans | [219,224] |
| CI | Wolbachia | Insecta | Diptera | Liriomyza trifolii | [224] |
| CI | Wolbachia | Insecta | Diptera | Phlebotomus papatasi | [225] |
| CI | Wolbachia | Insecta | Diptera | Rhagoletis cerasi | [226,227] |
| CI | Wolbachia | Insecta | Hymenoptera | Aphytis melinus DeBach | [228] |
| CI | Wolbachia | Insecta | Hymenoptera | Asobara tabida | [229] |
| CI | Wolbachia | Insecta | Hymenoptera | Cotesia sesamiae | [230] |
| CI | Wolbachia | Insecta | Hymenoptera | Encarsia inaron | [231] |
| CI | Wolbachia | Insecta | Hemiptera | Laodelphax striatellus | [232,233,234] |
| CI | Wolbachia | Insecta | Hemiptera | Leptopilina heterotoma | [235,236,237] |
| CI | Wolbachia | Insecta | Hemiptera | Macrolophus pygmaeus | [238] |
| CI | Wolbachia | Insecta | Hemiptera | Nasonia giraulti | [239,240] |
| CI | Wolbachia | Insecta | Hemiptera | N. longicornis | [240,241] |
| CI | Wolbachia | Insecta | Hemiptera | N. vitripennis | [240,242] |
| CI | Wolbachia | Insecta | Hemiptera | Orius strigicollis | [243] |
| CI | Wolbachia | Insecta | Lepidoptera | Colias erate poliographus | [244] |
| CI | Wolbachia | Insecta | Lepidoptera | Ephestia cautella | [245] |
| CI | Wolbachia | Insecta | Lepidoptera | E. kuehniella | [246] |
| CI | Wolbachia | Insecta | Lepidoptera | Eurema hecabe | [247] |
| CI | Wolbachia | Insecta | Lepidoptera | E. madarina | [248,249] |
| CI | Wolbachia | Insecta | Orthoptera | Gryllus assimilis | [197] |
| CI | Wolbachia | Insecta | Orthoptera | G. integer | [197] |
| CI | Wolbachia | Insecta | Orthoptera | G. ovisopis | [197] |
| CI | Wolbachia | Insecta | Orthoptera | G. pennsylvanicus | [197] |
| CI | Wolbachia | Insecta | Orthoptera | G. rubens | [197] |
| CI | Wolbachia | Insecta | Orthoptera | Teleogryllus taiwanemma | [249] |
| CI | Wolbachia | Malacostraca | Isopoda | Cylisticus convexus | [250] |
| CI | Wolbachia | Malacostraca | Isopoda | Porcellio dilatatus | [233,251] |
| CI | Wolbachia | Arachnida | Trombidiformes | Oligonychus gotohi | [252] |
| CI | Wolbachia | Arachnida | Trombidiformes | Panonychus mori | [252,253] |
| CI | Wolbachia | Arachnida | Trombidiformes | Tetranychus urticae | [253,254] |
| CI | Cardinium | Insecta | Hymenoptera | Encarsia pergandiella | [257] |
| CI | Cardinium | Arachnida | Mesostigmata | Dermanyssus gallinae | [258] |
| CI | Cardinium | Arachnida | Trombidiformes | Bryobia sarothamni | [289] |
| CI | Cardinium | Arachnida | Trombidiformes | Eotetranychus suginamensis | [256] |
| CI | Cardinium | Arachnida | Trombidiformes | Tetranychus cinnabarinus | [260] |
| RP a | Wolbachia | Insecta | Diptera | Anopheles gambiae | [261] |
| RP b | Wolbachia | Insecta | Diptera | Aedes aegypti | [262,263,264] |
| RP c | Wolbachia | Insecta | Diptera | Culex quinquefasciatus | [265] |
| RP d | Wolbachia | Insecta | Diptera | Drosophila simulans | [266,267] |
| RP e | Wolbachia | Insecta | Diptera | D. melanogaster | [265,268,269,270] |
| RP f | Spiroplasma | Insecta | Diptera | D. hydei | [271] |
| RP g | Spiroplasma | Insecta | Diptera | D. neotestacea | [272] |
| M | Wolbachia | Insecta | Hemiptera | Cimex lectularius | [273] |
| M | Wolbachia | Secernentea | Spirurida | Brugia malayi | [274-271] |
| M | Wolbachia | Secernentea | Spirurida | B. pahangi | [277] |
| M | Wolbachia | Secernentea | Spirurida | Dirofilaria immitis | [277,278] |
| M | Wolbachia | Secernentea | Spirurida | Litomosoides sigmodontis | [279,280] |
| M | Wolbachia | Secernentea | Spirurida | Onchocerca gutturosa | [281] |
| M | Wolbachia | Secernentea | Spirurida | O. lienalis | [281] |
| M | Wolbachia | Secernentea | Spirurida | O. ochengi | [282] |
| M | Wolbachia | Secernentea | Spirurida | O. volvulus | [283] |
| M | Wolbachia | Secernentea | Spirurida | Wuchereria bancroft | [284] |
| O | Wolbachia | Insecta | Hymenoptera | Asobara tabida | [236] |
| O | Wolbachia | Insecta | Coleoptera | Coccotrypes dactyliperda | [285] |
| O | Wolbachia | Insecta | Coleoptera | Otiorhynchus sulcatus | [286] |
| O | Wolbachia | Insecta | Collembola | Folsomia candida | [287] |
| O | Wolbachia | Insecta | Diptera | Drosophila melanogaster (Sxl) | [288] |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kageyama, D.; Narita, S.; Watanabe, M. Insect Sex Determination Manipulated by Their Endosymbionts: Incidences, Mechanisms and Implications. Insects 2012, 3, 161-199. https://doi.org/10.3390/insects3010161
Kageyama D, Narita S, Watanabe M. Insect Sex Determination Manipulated by Their Endosymbionts: Incidences, Mechanisms and Implications. Insects. 2012; 3(1):161-199. https://doi.org/10.3390/insects3010161
Chicago/Turabian StyleKageyama, Daisuke, Satoko Narita, and Masaya Watanabe. 2012. "Insect Sex Determination Manipulated by Their Endosymbionts: Incidences, Mechanisms and Implications" Insects 3, no. 1: 161-199. https://doi.org/10.3390/insects3010161




