Morphological Characteristics of Terminalia of the Wasp-Mimicking Fly, Stomorhina discolor (Fabricius)
Abstract
:1. Introduction
2. Materials and Methods
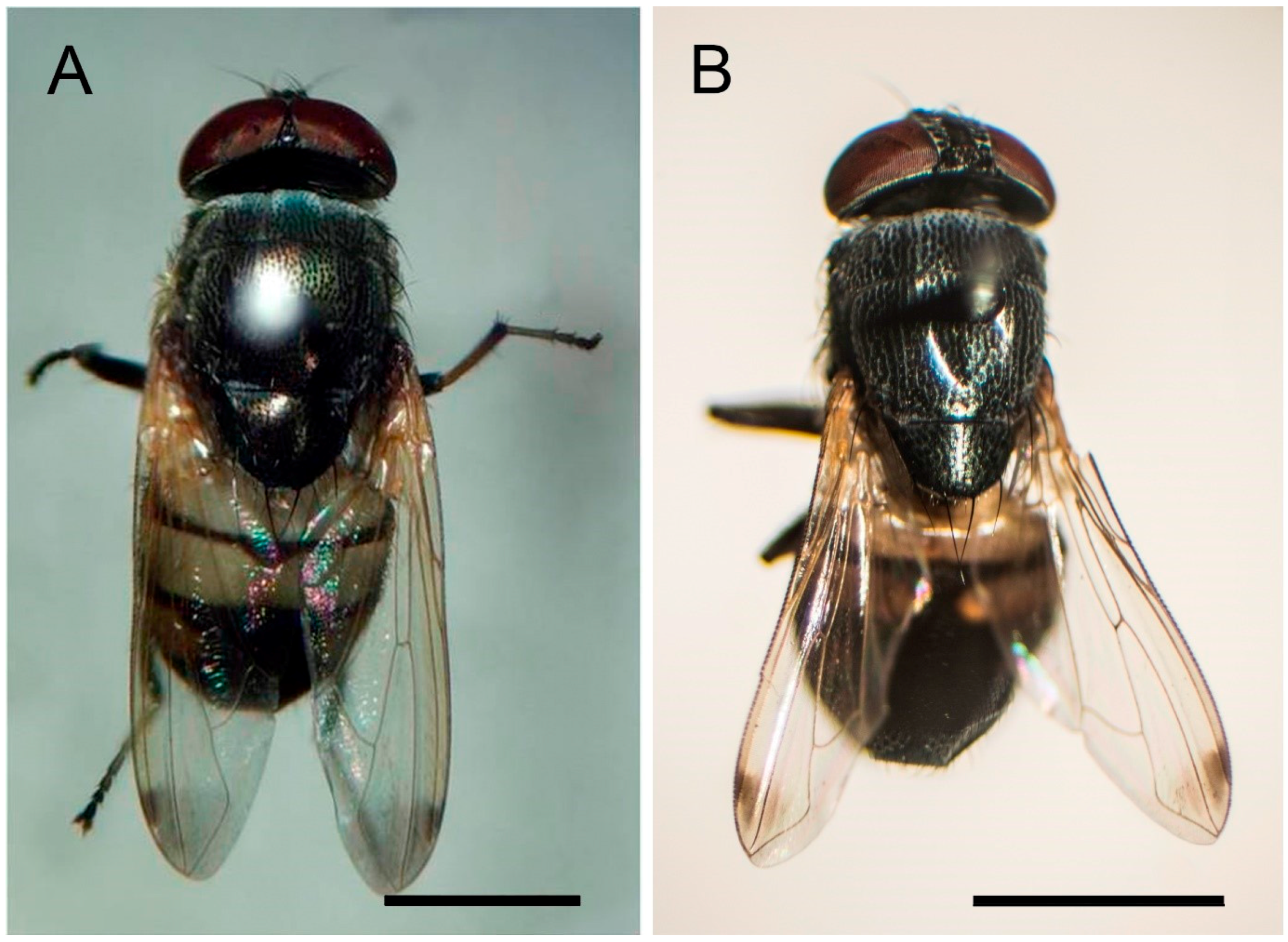
2.1. Wasp-Mimicking Fly, S. discolor
2.2. Light Microscopy
2.3. Scanning Electron Microscopy
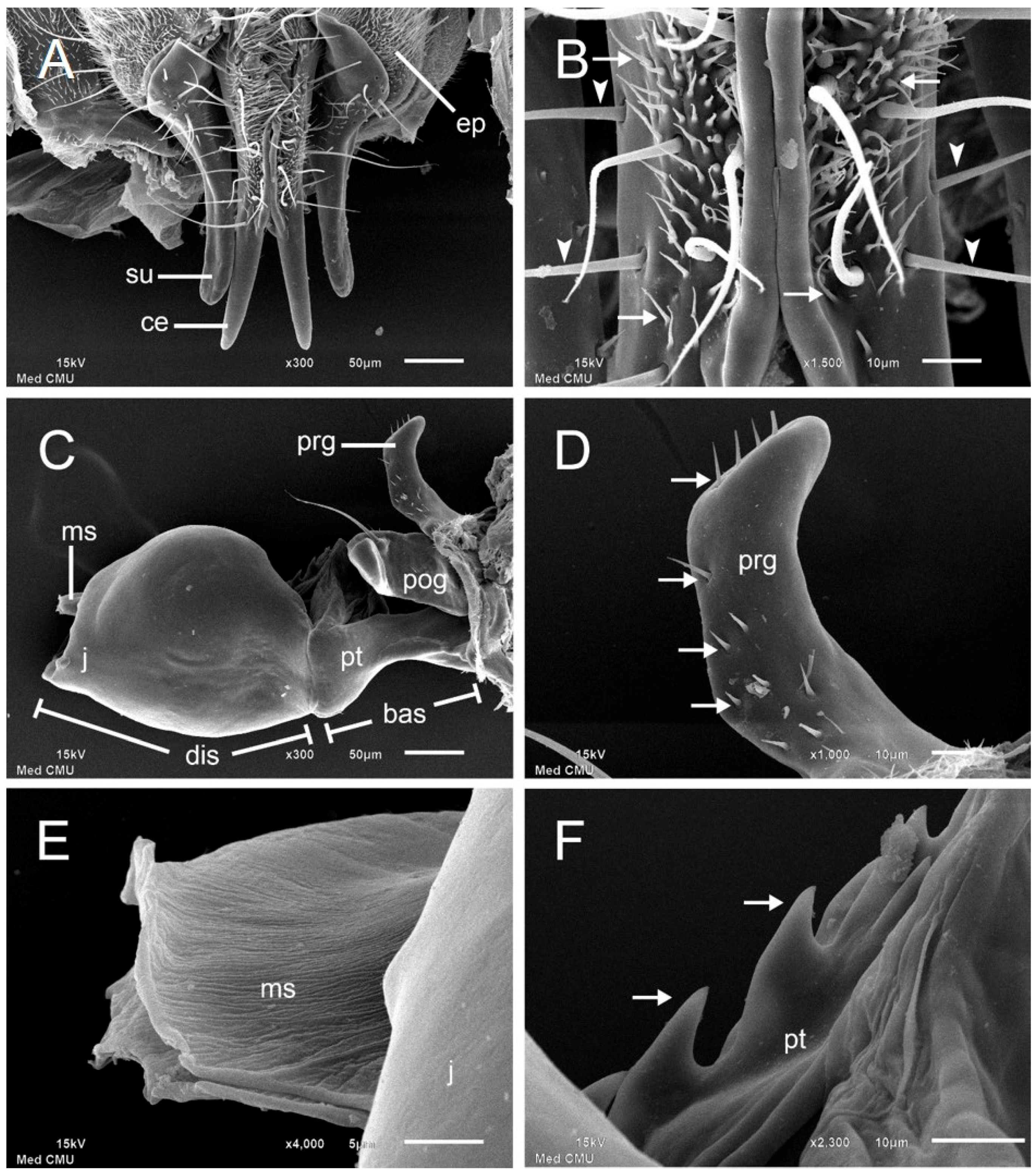
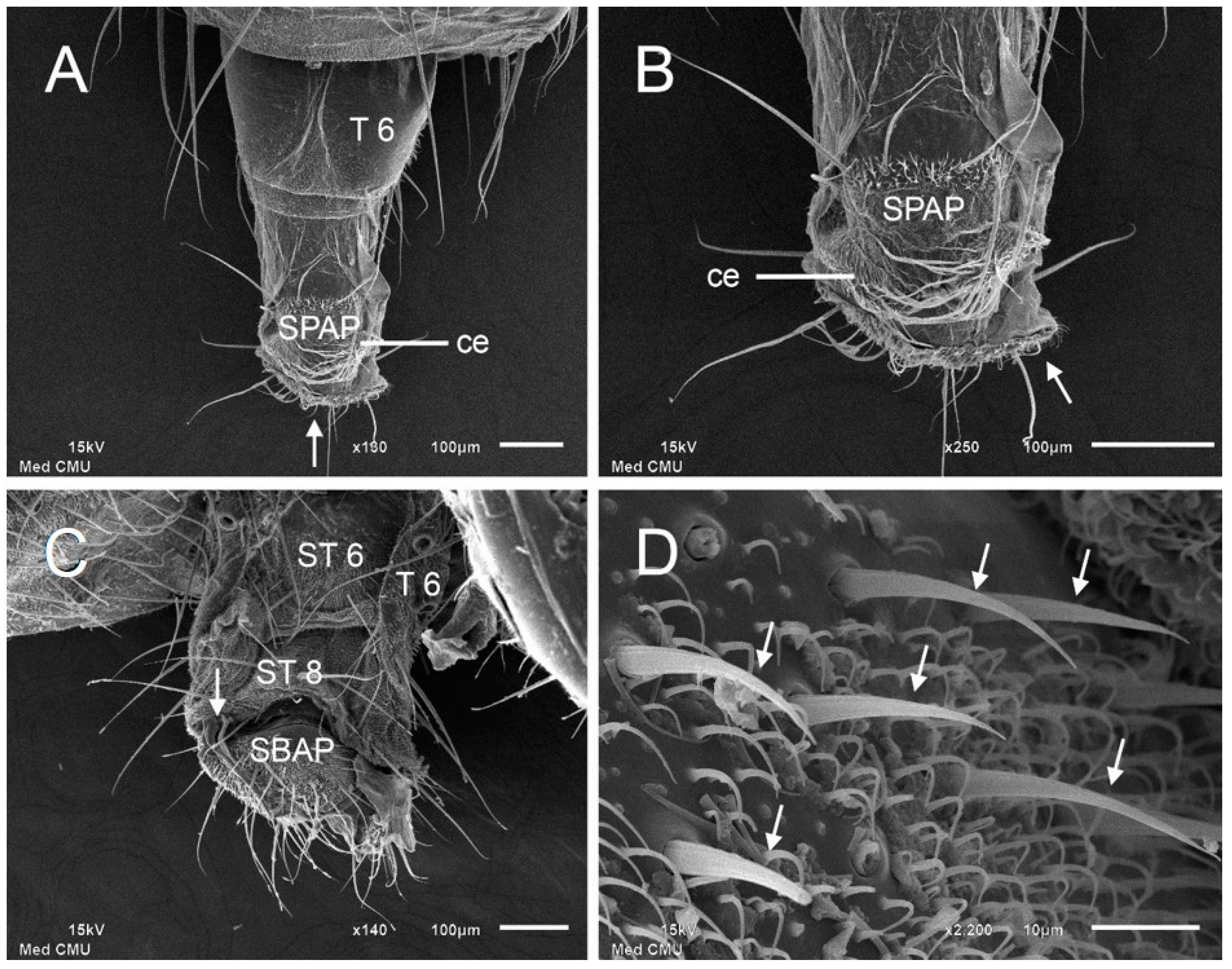
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dear, J.P. A revision of Australian Rhiniinae (Diptera: Calliphoridae). Aust. J. Zool. 1977, 25, 779–826. [Google Scholar] [CrossRef]
- Kurahashi, H.; Bunchu, N. The blow flies recorded from Thailand, with the description of a new species of Isomyia Walker (Diptera: Calliphoridae). Jpn. J. Syst. Entomol. 2011, 17, 237–278. [Google Scholar]
- Kurahashi, H.; Leh, M.U. The blow flies from Sarawak, East Malaysia (Diptera: Calliphoridae), with practical keys and a checklist. Sarawak Mus. J. 2009, 87, 299–300. [Google Scholar]
- Senior-White, R.; Aubertin, D.; Smart, J. Diptera: Family Calliphoridae. In The Fauna of British India, Including the Remainder of the Oriental Region; Sewell, R.B.S., Ed.; Taylor & Francis: London, UK, 1940. [Google Scholar]
- Verves, Y.G. A catalogue of Oriental Calliphoridae (Diptera). Int. J. Res. 2005, 16, 233–310. [Google Scholar]
- Bunchu, N. Blow fly (Diptera: Calliphoridae) in Thailand: Distribution, morphological identification and medical importance appraisals. Int. J. Parasitol. Res. 2012, 4, 57–64. [Google Scholar] [CrossRef]
- Kutty, S.N.; Pape, T.; Wiegmann, B.M.; Meier, R. Molecular phylogeny of the Calyptrate (Diptera: Cyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine’s fly. Syst. Entomol. 2010, 35, 614–635. [Google Scholar] [CrossRef]
- Bernhard, M.; Haenni, J.P. Morphology and terminology of adult Diptera (other than terminalia). In Contributions to a Manual of Palaearctic Diptera. General and Applied Dipterology; László, P., Béla, D., Eds.; Science Herald: Budapest, Hungary, 2000; pp. 22–51. [Google Scholar]
- Zacharuk, R.Y. Antennal sensilla. In Comparative Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, NY, USA, 1985; pp. 1–69. [Google Scholar]
- Rognes, K. Blowflies (Diptera, Calliphoridae) of Fennoscandia and Denmark. In Fauna Entomologica Scandinavica; Scandinavian Science Press Ltd.: Leiden, The Netherlands, 1991. [Google Scholar]
- Ngern-Klun, R.; Sukontason, K.; Methanitikorn, R.; Vogtsberger, R.C.; Sukontason, K.L. Fine structure of Chrysomya nigripes (Diptera: Calliphoridae), a fly species of medical importance. Parasitol. Res. 2007, 100, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Acebes, A.; Cobb, M.; Feurler, J.F. Species-Specific effects of single sensillum ablation on mating position in Drosophila. J. Exp. Biol. 2003, 206, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, M.; Wen, J.; Cai, J.; Jiang, H.; Liu, Y.; Aly, S.; Xiong, F. Molecular characterization and expression pattern of an odorant receptor from the myiasis-causing blowfly, Lucilia sericata (Diptera: Calliphoridae). Parasitol. Res. 2003, 110, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.A.; McIver, S.B. Fine structure and role in behavior of sensilla on the terminalia of Aedes aegypti (L.) (Diptera: Culicidae). J. Morphol. 1977, 151, 419–437. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Stomorhina discolor | Stomorhina lunata |
|---|---|---|
| Body length | 4.80–9.30 mm | 5.00–9.00 mm |
| Male genitalia | ||
| Cercus | slender with tapering tips | with cleft distal half |
| Surstylus | enlarged in proximal half tapered in distal half | narrow and pointed in lateral view |
| Pregonite | with short hair shafts at tip and posterior | with few setae posteriorly |
| Postgonite | with a strong preapical bristle | with a strong basal seta |
| Phallic tube | with dorsal serration | undetermined |
| Median stylus | with rough surface expanded at apex | undetermined |
| Female ovipositors | ||
| Tergite 7 | with broad sclerotize in between 2 half separates | with narrow sclerotize in between two half separates |
| Sternite 7 | rod-like with expanded at apex | square-like |
| Sternite 8 | narrow triangular | square-like with concave at apex |
| Subanal plate | broad triangular with expanded at base | broad triangular |
| Supra-anal plate | short and narrow triangular | short and broad triangular |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moophayak, K.; Sanit, S.; Chaiwong, T.; Sukontason, K.; Kurahashi, H.; Sukontason, K.L.; Vogtsberger, R.C.; Bunchu, N. Morphological Characteristics of Terminalia of the Wasp-Mimicking Fly, Stomorhina discolor (Fabricius). Insects 2017, 8, 11. https://doi.org/10.3390/insects8010011
Moophayak K, Sanit S, Chaiwong T, Sukontason K, Kurahashi H, Sukontason KL, Vogtsberger RC, Bunchu N. Morphological Characteristics of Terminalia of the Wasp-Mimicking Fly, Stomorhina discolor (Fabricius). Insects. 2017; 8(1):11. https://doi.org/10.3390/insects8010011
Chicago/Turabian StyleMoophayak, Kittikhun, Sangob Sanit, Tarinee Chaiwong, Kom Sukontason, Hiromu Kurahashi, Kabkaew L. Sukontason, Roy C. Vogtsberger, and Nophawan Bunchu. 2017. "Morphological Characteristics of Terminalia of the Wasp-Mimicking Fly, Stomorhina discolor (Fabricius)" Insects 8, no. 1: 11. https://doi.org/10.3390/insects8010011





