Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa destructor and Viruses
Abstract
:1. Introduction
2. Material and Methods
2.1. Bioassay to Test for Effects of Propolis on Virus Infections and Mite Loads
2.1.1. Experimental Site and Set-Up
2.1.2. Data Collection and Sampling
2.1.3. Bee Sampling and Determination of Mite Infestation Levels
2.1.4. Determination of Viral Loads
2.2. Bioassay to Test for Effect of Propolis on V. destructor Mite Survival
Collection of V. destructor Mites and Performance of Bioassays
2.3. Statistical Analyses
3. Results
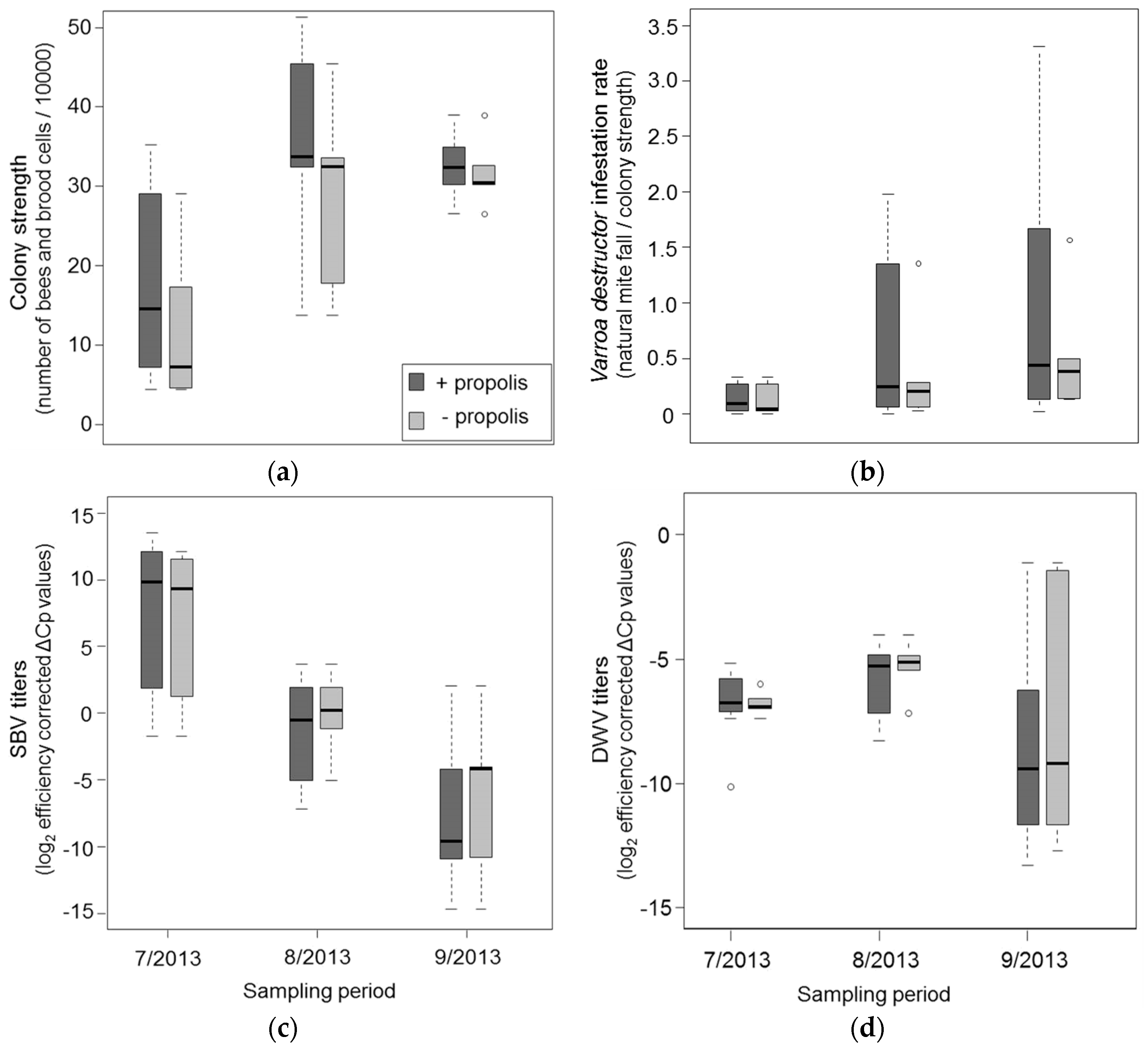
3.1. Resin Intake and Colony Strength
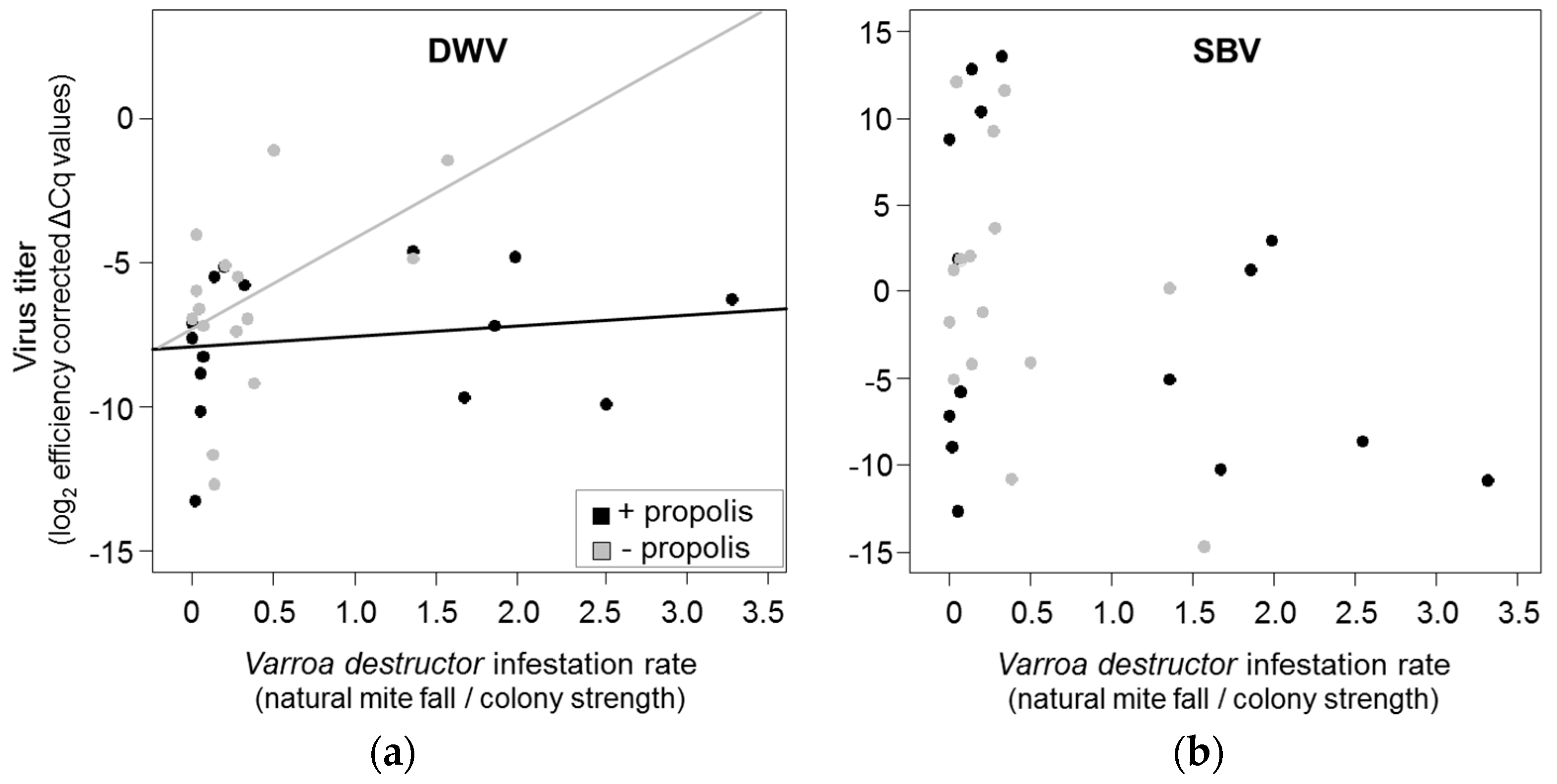
3.2. Effects of Propolis Manipulation on Virus Infections and Mite Loads
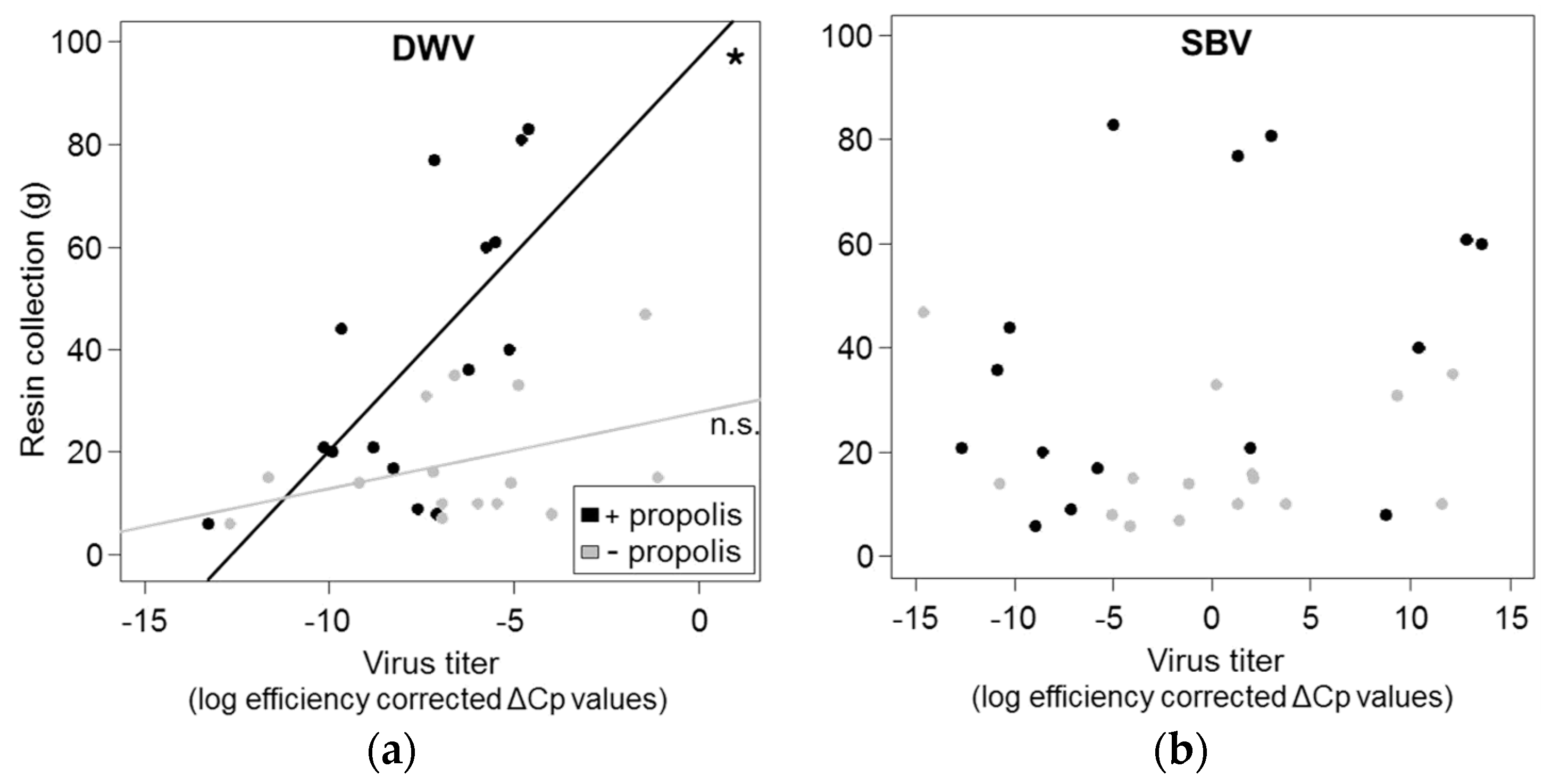
3.3. Correlation between Resin Intake and Pathogen Loads
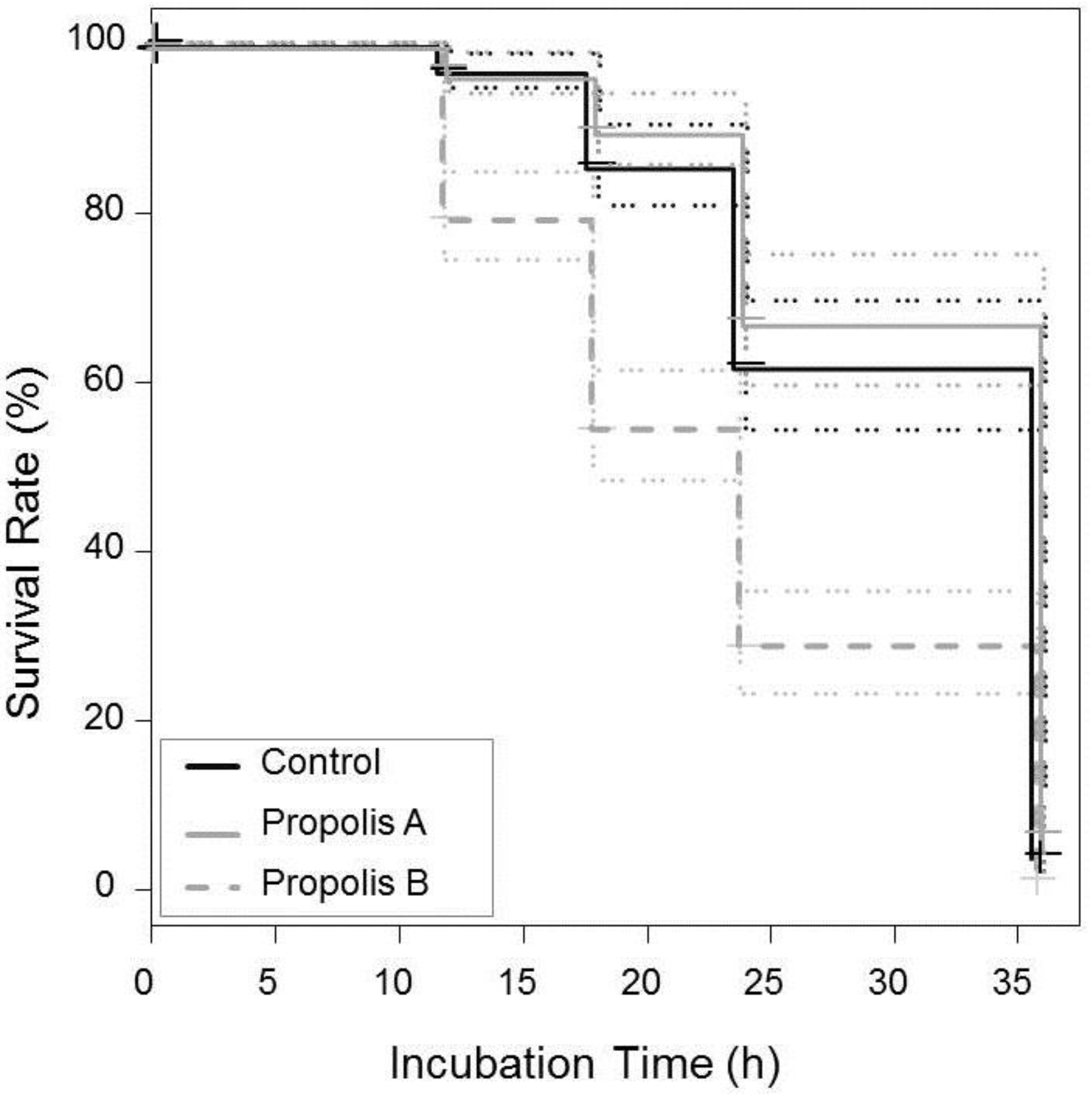
3.4. Effect of Propolis on V. destructor Mite Survival
4. Discussion
4.1. Effects of Propolis on Virus Infections and Mite Loads
4.2.Correlation between Resin Intake and Pathogen Loads
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ellis, J.D.; Evans, J.D.; Pettis, J. Colony losses, managed colony population decline, and Colony Collapse Disorder in the United States. J. Apic. Res. 2010, 49, 134–136. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Caron, D.; Pettis, J. A survey of managed honey bee colony losses in the USA, fall 2009 to winter 2010. J. Apic. Res. 2011, 50, 1–10. [Google Scholar] [CrossRef]
- Kosior, A.; Celary, W.; Olejniczak, P.; Fijal, J.; Krol, W.; Solarz, W.; Plonka, P. The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx 2007, 41, 79–88. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. Long-Term Global Trends in Crop Yield and Production Reveal No Current Pollination Shortage but Increasing Pollinator Dependency. Curr. Biol. 2008, 18, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Frazier, M.; Mullin, C.; Frazier, J.; Ashcraft, S. What have pesticides got to do with it? Am. Bee J. 2008, 148, 521–523. [Google Scholar]
- Orantes-Bermejo, F.J.; Pajuelo, A.G.; Megias, M.M.; Fernandez-Pinar, C.T. Pesticide residues in beeswax and beebread samples collected from honey bee colonies (Apis mellifera L.) in Spain. Possible implications for bee losses. J. Apic. Res. 2010, 49, 243–250. [Google Scholar] [CrossRef]
- Martin, S.J. The role of Varroa and viral pathogens in the collapse of honeybee colonies: A modelling approach. J. Appl. Ecol. 2001, 38, 1082–1093. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; de Miranda, J.; Fries, I.; Le Conte, Y.; Neumann, P.; Paxton, R.J. Research strategies to improve honeybee health in Europe. Apidologie 2010, 41, 227–242. [Google Scholar] [CrossRef]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, pests, and economics: Drivers of honey bee colony declines and losses. Ecohealth 2013, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Pohorecka, K.; Bober, A.; Skubida, M.; Zdanska, D.; Toroj, K. A comparative study of environmental conditions, bee management and epidemiological situation in apiaries in the level of colony losses. J. Apic. Sci. 2014, 58, 107–132. [Google Scholar] [CrossRef]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Meixner, M.D.; Costa, C.; Kryger, P.; Hatjina, F.; Bouga, M.; Ivanova, E.; Buchler, R. Conserving diversity and vitality for honey bee breeding. J. Apic. Res. 2010, 49, 85–92. [Google Scholar] [CrossRef]
- De la Rua, P.; Jaffe, R.; Munoz, I.; Serrano, J.; Moritz, R.F.A.; Bernhard Kraus, F. Conserving genetic diversity in the honeybee: Comments on Harpur et al. (2012). Mol. Ecol. 2013, 22, 3208–3210. [Google Scholar] [CrossRef] [PubMed]
- Jones, R. European beekeeping in the 21st century: Strengths, weaknesses, opportunities, threats. Bee World 2004, 85, 77–80. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mutinelli, F. The spread of pathogens through trade in honey bees and their products (including queen bees and semen): Overview and recent developments. Rev. Sci. Tech. Off. Int. Epizoot. 2011, 30, 257–271. [Google Scholar] [CrossRef]
- Neumann, P.; Pettis, J.S.; Schäfer, M.O. Quo vadis Aethina tumida? Biology and control of small hive beetles. Apidologie 2016, 47, 427–466. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Siede, R. Honey Bee Viruses; Elsevier Academic Press Inc.: San Diego, CA, USA, 2007; Volume 70, pp. 33–80. [Google Scholar]
- Ellis, J.D.; Munn, P.A. The worldwide health status of honey bees. Bee World 2005, 86, 88–101. [Google Scholar] [CrossRef]
- Chantawannakul, P.; de Guzman, L.; Williams, G. Parasites and pests of honey bees in Asia. Apidologie 2016, 47, 301–324. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103 (Suppl. 1), 96–119. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cox-Foster, D. Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitology 2007, 134, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.V.; Allen, M.F. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with The parasitic mite Varroa Jacobsoni. Ann. Appl. Biol. 1988, 113, 237–244. [Google Scholar] [CrossRef]
- Fries, I.; Imdorf, A.; Rosenkranz, P. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie 2006, 37, 564–570. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Dead or alive: Deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 2011, 78, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, P.; Yañez, O.; Fries, I.; de Miranda, J.R. Varroa invasion and virus adaptation. Trends Parasitol. 2012, 28, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F.; Evans, J.D.; Chen, Y.P. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef] [PubMed]
- Ongus, J.R.; Peters, D.; Bonmatin, J.M.; Bengsch, E.; Vlak, J.M.; van Oers, M.M. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J. Gen. Virol. 2004, 85, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Buchler, R.; Berg, S.; Ritter, W.; Muhlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Di Prisco, G.; Zhang, X.; Pennacchio, F.; Caprio, E.; Li, J.L.; Evans, J.D.; DeGrandi-Hoffman, G.; Hamilton, M.; Chen, Y.P. Dynamics of persistent and acute Deformed wing virus infections in honey bees, Apis mellifera. Viruses 2011, 3, 2425–2441. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Walker, P.L.; Martin, S.J.; Gunn, A. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 1999, 73, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Q.; Yang, X.L.; Cox-Foster, D.; Cui, L.W. The role of varroa mites in infections of Kashmir bee virus (KBV) and Deformed wing virus (DWV) in honey bees. Virology 2005, 342, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Cox-Foster, D.L. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef] [PubMed]
- Mondet, F.; de Miranda, J.R.; Kretzschmar, A.; Le Conte, Y.; Mercer, A.R. On the Front Line: Quantitative Virus Dynamics in Honeybee (Apis mellifera L.) Colonies along a New Expansion Front of the Parasite Varroa destructor. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, G.E.; Chejanovsky, N.; Chen, Y.P.; Gauthier, L.; Genersch, E.; de Graaf, D.C.; et al. Standard methods for virus research in Apis mellifera. J. Apic. Res. 2013. [Google Scholar] [CrossRef]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010, 103, S62–S72. [Google Scholar] [CrossRef] [PubMed]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Neumann, P.; Härtel, S. Removal of small hive beetle (Aethina tumida) eggs and larvae by African honeybee colonies (Apis mellifera scutellata). Apidologie 2004, 35, 31–36. [Google Scholar] [CrossRef]
- Starks, P.T.; Blackie, C.A.; Seeley, T.D. Fever in honeybee colonies. Naturwissenschaften 2000, 87, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Pirk, C.W.W.; Hepburn, H.R.; Solbrig, A.J.; Ratnieks, F.L.W.; Elzen, P.J.; Baxter, J.R. Social encapsulation of beetle parasites by Cape honeybee colonies (Apis mellifera capensis Esch.). Naturwissenschaften 2001, 88, 214–216. [Google Scholar] [PubMed]
- Page, P.; Lin, Z.; Buawangpong, N.; Zheng, H.; Hu, F.; Neumann, P.; Chantawannakul, P.; Dietemann, V. Social apoptosis in honey bee superorganisms. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Bluthgen, N. A sticky affair: Resin collection by bornean stingless bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Roubik, D.W. Stingless bee nesting biology. Apidologie 2006, 37, 124–143. [Google Scholar] [CrossRef]
- Castella, G.; Chapuisat, M.; Christe, P. Prophylaxis with resin in wood ants. Anim. Behav. 2008, 75, 1591–1596. [Google Scholar] [CrossRef]
- Chapuisat, M.; Oppliger, A.; Magliano, P.; Christe, P. Wood ants use resin to protect themselves against pathogens. Proc. R. Soc. B Biol. Sci. 2007, 274, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Langenheim, J. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany; Timber Press: Portland, OR, USA; Cambridge, UK, 2003. [Google Scholar]
- Ghisalberti, E.L. Propolis: A review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Greenaway, W.; Scaysbrook, T.; Whatley, F.R. The composition and plant origin of propolis: A report of work at Oxford. Bee World 1990, 71, 107–118. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis—Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Coll, F.V.; Jorda, R.E. The composition, active components and bacteriostatic activity of propolis in dietics. J. Am. Oil Chem. Soc. 1994, 71, 529–532. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Massaro, C.F.; Smyth, T.J.; Smyth, W.F.; Heard, T.; Leonhardt, S.D.; Katouli, M.; Wallace, H.M.; Brooks, P. Phloroglucinols from anti-microbial deposit-resins of australian stingless bees (Tetragonula carbonaria). Phytother. Res. 2015, 29, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Stratis, E.; Chinou, I. Volatile constituents of propolis from various regions of Greece—Antimicrobial activity. Food Chem. 2007, 103, 375–380. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Bankova, V.; Ignatova, A.; Popov, S. Antibacterial activity of propolis, some of Its components and their analogs. Pharmazie 1993, 48, 785–786. [Google Scholar] [PubMed]
- Bankova, V.; Christov, R.; Popov, S.; Pureb, O.; Bocari, G. Volatile constituents of propolis. Z. Naturforsch. C J. Biosci. 1994, 49, 6–10. [Google Scholar]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28:1–28:8. [Google Scholar] [CrossRef] [PubMed]
- Bonvehi, J.S.; Gutierrez, A.L. The antimicrobial effects of propolis collected in different regions in the Basque Country (Northern Spain). World J. Microbiol. Biotechnol. 2012, 28, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Unlu, G.; Silici, S.; Unlu, M. Composition and in vitro antimicrobial activity of Populus buds and poplar-type propolis. World J. Microbiol. Biotechnol. 2008, 24, 1011–1017. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Silici, S.; Koc, N.A.; Ayangil, D.; Cankaya, S. Antifungal activities of propolis collected by different races of honeybees against yeasts isolated from patients with superficial mycoses. J. Pharmacol. Sci. 2005, 99, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Gekker, G.; Hu, S.X.; Spivak, M.; Lokensgard, J.R.; Peterson, P.K. Anti-HIV-1 activity of propolis in CD4(+) lymphocyte and microglial cell cultures. J. Ethnopharmacol. 2005, 102, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Dimov, V.; Ivanovska, N.; Manolova, N.; Bankova, V.; Nikolov, N.; Popov, S. Immunomodulatory action of propolis—Influence on antiinfectious protection and macrophage function. Apidologie 1991, 22, 155–162. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Bastos, E.; Simone, M.; Jorge, D.M.; Soares, A.E.E.; Spivak, M. In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J. Invertebr. Pathol. 2008, 97, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.B.; Brinkman, D.; Spivak, M.; Gardner, G.; Cohen, J.D. Regional variation in composition and antimicrobial activity of US propolis against Paenibacillus larvae and Ascosphaera apis. J. Invertebr. Pathol. 2015, 124, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.D.; Spivak, M. Increased Resin Collection after Parasite Challenge: A Case of Self-Medication in Honey Bees? PLoS ONE 2012, 7, e34601. [Google Scholar] [CrossRef] [PubMed]
- Garedew, A.; Lamprecht, I.; Schmolz, E.; Schricker, B. The varroacidal action of propolis: A laboratory assay. Apidologie 2002, 33, 41–50. [Google Scholar] [CrossRef]
- Damiani, N.; Daniel Maggi, M.; Brenda Gende, L.; Faverin, C.; Javier Eguaras, M.; Augusto Marcangeli, J. Evaluation of the toxicity of a propolis extract on Varroa destructor (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae). J. Apic. Res. 2010, 49, 257–264. [Google Scholar] [CrossRef]
- Nicodemo, D.; Malheiros, E.B.; De Jong, D.; Nogueira Couto, R.H. Increased brood viability and longer lifespan of honeybees selected for propolis production. Apidologie 2013, 45, 269–275. [Google Scholar] [CrossRef]
- Nicodemo, D.; De Jong, D.; Couto, R.H.N.; Malheiros, B. Honey bee lines selected for high propolis production also have superior hygienic behavior and increased honey and pollen stores. Genet. Mol. Res. 2013, 12, 6931–6938. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Retschnig, G.; Williams, G.R.; Odemer, R.; Boltin, J.; Di Poto, C.; Mehmann, M.M.; Retschnig, P.; Winiger, P.; Rosenkranz, P.; Neumann, P. Effects, but no interactions, of ubiquitous pesticide and parasite stressors on honey bee (Apis mellifera) lifespan and behaviour in a colony environment. Environ. Microbiol. 2015, 17, 4322–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Hino, A.; Tsutsumi, A.; Park, Y.K.; Watanabe, W.; Kurokawa, M. Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antivir. Chem. Chemother. 2008, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Amoros, M.; Sauvager, F.; Girre, L.; Cormier, M. In vitro antiviral activity of propolis. Apidologie 1992, 23, 231–240. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Dietemann, V.; Ellis, J.D.; Neumann, P. COLOSS BEEBOOK; International Bee Research Association: Cardiff, UK, 2013; Volume II. [Google Scholar]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apic. Res. 2013. [Google Scholar] [CrossRef]
- Dainat, B.; Kuhn, R.; Cherix, D.; Neumann, P. A scientific note on the ant pitfall for quantitative diagnosis of Varroa destructor. Apidologie 2011, 42, 740–742. [Google Scholar] [CrossRef]
- Branco, M.R.; Kidd, N.A.C.; Pickard, R.S. A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie 2006, 37, 452–461. [Google Scholar] [CrossRef]
- Delaplane, K.S.; van der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Evans, J.D.; Schwarz, R.S.; Chen, Y.P.; Budge, G.; Cornman, R.S.; De la Rua, P.; de Miranda, J.R.; Foret, S.; Foster, L.; Gauthier, L.; et al. Standard methods for molecular research in Apis mellifera. J. Apic. Res. 2013. [Google Scholar] [CrossRef]
- Locke, B.; Forsgren, E.; Fries, I.; de Miranda, J.R. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 2012, 78, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Isanu, V. Anti-herpes simplex virus effect of an aqueous extract of propolis. Isr. Med. Assoc. J. 2002, 4, 923–927. [Google Scholar] [PubMed]
- Hepburn, R.H.; Radloff, S.E. Honeybees of Africa; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001. [Google Scholar] [CrossRef]
- Damiani, N.; Fernandez, N.J.; Maldonado, L.M.; Alvarez, A.R.; Eguaras, M.J.; Marcangeli, J.A. Bioactivity of propolis from different geographical origins on Varroa destructor (Acari: Varroidae). Parasitol. Res. 2010, 107, 31–37. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Masri, L.; Cremer, S. Individual and social immunisation in insects. Trends Immunol. 2014, 35, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J. Social immunity and the evolution of group-living in insects. Philos. Trans. R. Soc. B Biol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Q.; Cui, L.W.; Ostiguy, N.; Cox-Foster, D. Intricate transmission routes and interactions between picorna-like viruses (Kashmir bee virus and Sacbrood virus) with the honeybee host and the parasitic Varroa mite. J. Gen. Virol. 2005, 86, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, C.L.; Missima, F.; Pagliarone, A.C.; Bachiega, T.F.; Bufalo, M.C.; Araujo, J.P.; Sforcin, J.M. Propolis Immunomodulatory action in Vivo on Toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phytother. Res. 2010, 24, 1141–1146. [Google Scholar] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A virulent strain of Deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vtro, transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.G.; Nunes, S.; Cruz, C.; Duarte, J.; Antunes, M.D.; Cavaco, A.M.; Mendes, M.D.; Lima, A.S.; Pedro, L.G.; Barroso, J.G.; et al. Propolis volatiles characterisation from acaricide-treated and -untreated beehives maintained at Algarve (Portugal). Nat. Prod. Res. 2013, 27, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.W.; Negri, G.; Meira, R.; Message, D.; Salatino, A. Plant origin of green propolis: Bee behavior, plant anatomy and chemistry. Evid.-Based Complement. Altern. Med. 2005, 2, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Reyes, M.; Le Conte, Y.; Bankova, V. Propolis chemical composition and honeybee resistance against Varroa destructor. Nat. Prod. Res. 2014, 28, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Locke, B. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 2016, 47, 467–482. [Google Scholar] [CrossRef]
- Pirk, C.W.W.; Human, H.; Crewe, R.M.; van Engelsdorp, D. A survey of managed honey bee colony losses in the Republic of South Africa-2009 to 2011. J. Apic. Res. 2014, 53, 35–42. [Google Scholar] [CrossRef]
| Assay | Primers | Sequence (5′–3′) | Reference |
|---|---|---|---|
| BQCV | BQCV-qF7893 | AGTGGCGGAGATGTATGC | Locke et al. [95] |
| BQCV-qB8150 | GGAGGTGAAGTGGCTATATC | ||
| DWV | DWV-F8668 | TTCATTAAAGCCACCTGGAACATC | Forsgren et al. [96] |
| DWV-B8757 | TTTCCTCATTAACTGTGTCGTTGA | ||
| IAPV | IAPV-F6627 | CCATGCCTGGCGATTCAC | de Miranda et al. [97] |
| KIABPV-B6707 | CTGAATAATACTGTGCGTATC | ||
| SBV | SBV-qF3164 | TTGGAACTACGCATTCTCTG | Locke et al. [95] |
| SBV-qB3461 | GCTCTAACCTCGCATCAAC | ||
| β-actin (A.m.) | Am-actin2-qF | CGTGCCGATAGTATTCTTG | Locke et al. [95] |
| Am-actin2-qB | CTTCGTCACCAACATAGG |
| Response Variables | Explanatory Variables | df | p |
|---|---|---|---|
| DWV | Treatment × Varroa | 7 | 0.027 |
| Treatment + Varroa | 6 | 0.108 | |
| Treatment | 5 | 0.263 | |
| Varroa | 5 | 0.282 | |
| SBV | Treatment × Varroa | 7 | 0.065 |
| Treatment + Varroa | 6 | 0.219 | |
| Treatment | 5 | 0.086 | |
| Varroa | 5 | 0.623 |
| Explanatory Variables | p | R2m (%) | R2c (%) |
|---|---|---|---|
| Treatment × DWV | 0.022 | 32 | 72 |
| DWV | 0.203 | 2 | 76 |
| Treatment × Varroa | 0.176 | 24 | 71 |
| Varroa | 0.706 | 0.5 | 68 |
| Treatment × Colony strength | 0.475 | 18 | 72 |
| Colony strength | 0.127 | <0.01 | 72 |
| Treatment | 0.886 | 17 | 71 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drescher, N.; Klein, A.-M.; Neumann, P.; Yañez, O.; Leonhardt, S.D. Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa destructor and Viruses. Insects 2017, 8, 15. https://doi.org/10.3390/insects8010015
Drescher N, Klein A-M, Neumann P, Yañez O, Leonhardt SD. Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa destructor and Viruses. Insects. 2017; 8(1):15. https://doi.org/10.3390/insects8010015
Chicago/Turabian StyleDrescher, Nora, Alexandra-Maria Klein, Peter Neumann, Orlando Yañez, and Sara D. Leonhardt. 2017. "Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa destructor and Viruses" Insects 8, no. 1: 15. https://doi.org/10.3390/insects8010015







