Queen Quality and the Impact of Honey Bee Diseases on Queen Health: Potential for Interactions between Two Major Threats to Colony Health
Abstract
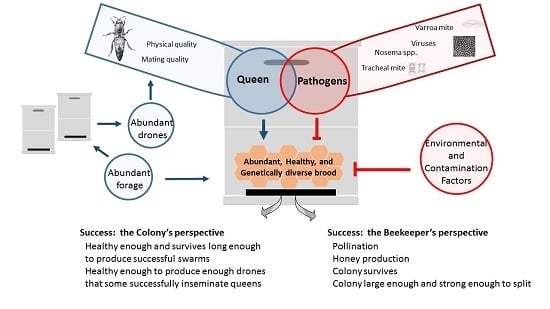
:1. Introduction
2. Honey Bee Queen Reproductive Potential
2.1. Physical Quality
2.1.1. Body Size
2.1.2. Internal Reproductive Organs
2.2. Mating Quality
2.2.1. Mating Number
2.2.2. Insemination Success
3. Parasites and Queen Quality
3.1. Varroa Mites
3.2. Tracheal Mites
3.3. Nosema Species
3.4. Viruses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Breeze, T.D.; Bailey, A.P.; Balcombe, K.G.; Potts, S.G. Pollination services in the UK: How important are honeybees? Agric. Ecosyst. Environ. 2011, 142, 137–143. [Google Scholar] [CrossRef]
- Morse, R.A.; Calderone, N.W. The value of honey bees as pollinators of US crops in 2000. Bee Cult. 2000, 128, 1–15. [Google Scholar]
- Potts, S.; Roberts, S.; Dean, R.; Marris, G.; Brown, M.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15. [Google Scholar] [CrossRef]
- Pirk, C.W.W.; Human, H.; Crewe, R.M.; VanEngelsdorp, D. A survey of managed honey bee colony losses in the Republic of South Africa—2009 to 2011. J. Apic. Res. 2014, 53, 35–42. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, C.; Niu, Q.; Qi, W.; Yuan, C.; Su, S.; Liu, S.; Zhang, Y.; Zhang, X.; Ji, T.; et al. Survey results of honey bee (Apis mellifera) colony losses in China (2010–2013). J. Apic. Res. 2016, 55, 29–37. [Google Scholar] [CrossRef]
- Vanengelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Seitz, N.; Traynor, K.S.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Ellis, J.D.; Rose, R.; Tarpy, D.R.; Sagili, R.R.; Caron, D.M.; et al. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 2015, 54, 292–304. [Google Scholar] [CrossRef]
- Meixner, M.D.; Francis, R.M.; Gajda, A.; Kryger, P.; Andonov, S.; Uzunov, A.; Topolska, G.; Costa, C.; Amiri, E.; Berg, S. Occurrence of parasites and pathogens in honey bee colonies used in a European genotype-environment interactions experiment. J. Apic. Res. 2014, 53, 215–219. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, pests, and economics: Drivers of honey bee colony declines and losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, A.J.; Genersch, E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef]
- Cornman, R.S.; Tarpy, D.R.; Chen, Y.; Jeffreys, L.; Lopez, D.; Pettis, J.S.; van Engelsdorp, D.; Evans, J.D. Pathogen webs in collapsing honey bee colonies. PLoS ONE 2012, 7, e43562. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; Mcgowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [PubMed]
- Boecking, O.; Genersch, E. Varroosis—The ongoing crisis in bee keeping. Journal für Verbraucherschutz und Lebensmittelsicherheit 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-Virus interaction in collapsing honey bee colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef] [PubMed]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.-L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Cordoni, G.; Budge, G. The Acute bee paralysis virus—Kashmir bee virus—Israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010, 103, S30–S47. [Google Scholar] [CrossRef] [PubMed]
- Highfield, A.C.; El Nagar, A.; Mackinder, L.C.M.; Noël, L.M.-L.J.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet, V.; Fürst, M.; Weging, S.; Brown, M.J.F.; Gogol-Döring, A.; Paxton, R.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. B Sci. 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martin-Hernandez, R.; Botias, C.; Bailon, E.G.; Gonzalez-Porto, A.V.; Barrios, L.; del Nozal, M.J.; Bernal, J.L.; Jimenez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martin-Hernandez, R.; Garrido-Bailon, E.; Gonzalez-Porto, A.V.; Garcia-Palencia, P.; Meana, A.; del Nozal, M.J.; Mayo, R.; Bernal, J.L. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 2009, 1, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Meana, A.; Bartolomé, C.; Botías, C.; Martín-Hernández, R. Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep. 2013, 5, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Soroker, V.; Hetzroni, A.; Yakobson, B.; David, D.; David, A.; Voet, H.; Slabezki, Y.; Efrat, H.; Levski, S.; Kamer, Y.; et al. Evaluation of colony losses in Israel in relation to the incidence of pathogens and pests. Apidologie 2011, 42, 192–199. [Google Scholar] [CrossRef]
- Forsgren, E.; Budge, G.E.; Charrière, J.-D.; Hornitzky, M.A.Z. Standard methods for European foulbrood research. J. Apic. Res. 2013. [Google Scholar] [CrossRef]
- Roetschi, A.; Berthoud, H.; Kuhn, R.; Imdorf, A. Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honeybee colonies before and after apiary sanitation. Apidologie 2008, 39, 362–371. [Google Scholar] [CrossRef]
- Wilkins, S.; Brown, M.A.; Cuthbertson, A.G. The incidence of honey bee pests and diseases in England and Wales. Pest Manag. Sci. 2007, 63, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 2010, 103, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Brodschneider, R.; Moosbeckhofer, R.; Crailsheim, K. Surveys as a tool to record winter losses of honey bee colonies: A two year case study in Austria and South Tyrol. J. Apic. Res. 2010, 49, 23–30. [Google Scholar] [CrossRef]
- Spleen, A.M.; Lengerich, E.J.; Rennich, K.; Caron, D.; Rose, R.; Pettis, J.S.; Henson, M.; Wilkes, J.T.; Wilson, M.; Stitzinger, J.; et al. A national survey of managed honey bee 2011–2012 winter colony losses in the United States: Results from the Bee Informed Partnership. J. Apic. Res. 2013, 52, 44–53. [Google Scholar] [CrossRef]
- Vanengelsdorp, D.; Tarpy, D.R.; Lengerich, E.J.; Pettis, J.S. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev. Vet. Med. 2013, 108, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Vanengelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J. A Survey of honey bee colony losses in the U.S., Fall 2007 to Spring 2008. PLoS ONE 2009, 3, e4071. [Google Scholar] [CrossRef] [PubMed]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: London, UK, 1987. [Google Scholar]
- Tarpy, D.R.; vanEngelsdorp, D.; Pettis, J.S. Genetic diversity affects colony survivorship in commercial honey bee colonies. Naturwissenschaften 2013, 100, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Tarpy, D.R.; Summers, J.; Keller, J.J. Comparison of parasitic mites in Russian-hybrid and Italian honey bee (Hymenoptera: Apidae) colonies across three different locations in North Carolina. J. Econ. Entomol. 2007, 100, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.W. Comparison studies of instrumentally inseminated and naturally mated honey bee queens and factors affecting their performance. Apidologie 2007, 38, 390–410. [Google Scholar] [CrossRef]
- Akyol, E.; Yeninar, H.; Korkmaz, A.; Çakmak, İ. An observation study on the effects of queen age on some characteristics of honey bee colonies. Ital. J. Anim. Sci. 2008, 7, 19–25. [Google Scholar] [CrossRef]
- Kostarelou-Damianidou, M.; Thrasyvoulou, A.; Tselios, D.; Bladenopoulos, K. Brood and honey production of honey bee colonies requeened at various frequencies. J. Apic. Res. 1995, 34, 9–14. [Google Scholar] [CrossRef]
- Rangel, J.; Keller, J.J.; Tarpy, D.R. The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth. Insect Soc. 2013, 60, 65–73. [Google Scholar] [CrossRef]
- Nelson, D.L.; Gary, N.E. Honey productivity of honeybee colonies in relation to body weight, attractiveness and fecundity of the queen. J. Apic. Res. 1983, 22, 209–213. [Google Scholar] [CrossRef]
- Tarpy, D.R. Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc. R. Soc. B Sci. 2003, 270, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hoopingarner, R.; Farrar, C. Genetic control of size in queen honey bees. J. Econ. Entomol. 1959, 52, 547–548. [Google Scholar] [CrossRef]
- Dodologlu, A.; Gene, F. Comparison of some features of queens reared from different honeybee (Apis mellifera L.) genotypes. J. Appl. Anim. Res. 2003, 24, 105–109. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Goodman, R.D.; Allaway, M.A. On the relative importance of queens and workers to honey production. Apidologie 1990, 21, 153–159. [Google Scholar] [CrossRef]
- Kahya, Y.; Gençer, H.V.; Woyke, J. Weight at emergence of honey bee (Apis mellifera caucasica) queens and its effect on live weights at the pre and post mating periods. J. Apic. Res. 2008, 47, 118–125. [Google Scholar] [CrossRef]
- Delaney, D.A.; Keller, J.J.; Caren, J.R.; Tarpy, D.R. The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie 2011, 42, 1–13. [Google Scholar] [CrossRef]
- Hatjina, F.; Bieńkowska, M.; Charistos, L.; Chlebo, R.; Costa, C.; Dražić, M.M.; Filipi, J.; Gregorc, A.; Ivanova, E.N.; Kezić, N.; et al. A review of methods used in some European countries for assessing the quality of honey bee queens through their physical characters and the performance of their colonies. J. Apic. Res. 2014, 53, 337–363. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Keller, J.J.; Caren, J.R.; Delaney, D.A. Assessing the mating ‘health’of commercial honey bee queens. J. Econ. Entomol. 2012, 105, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hatch, S.; Tarpy, R.D.; Fletcher, C.D.J. Worker regulation of emergency queen rearing in honey bee colonies and the resultant variation in queen quality. Insect Soc. 1999, 46, 372–377. [Google Scholar] [CrossRef]
- Jackson, T.J.; Tarpy, R.D.; Fahrbach, E.S. Histological estimates of ovariole number in honey bee queens, Apis mellifera, reveal lack of correlation with other queen quality measures. J. Insect Sci. 2011, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, W.; Bieñkowska, M.G.; Kruk, C. Changes in body weight of honey bee queens during their maturation. J. Apic. Res. 2004, 48, 61–68. [Google Scholar]
- Akyol, E.; Yeninar, H.; Kaftanoglu, O. Live weight of queen honey bees (Apis Mellifera L.) predicts reproductive characteristics. J. Kans. Entomol. Soc. 2008, 81, 92–100. [Google Scholar] [CrossRef]
- Woyke, J. Correlations between the age at which honeybee brood was grafted, characteristics of the resultant queens, and results of insemination. J. Apic. Res. 1971, 10, 45–55. [Google Scholar] [CrossRef]
- Collins, A.M.; Pettis, J.S. Correlation of queen size and spermathecal contents and effects of miticide exposure during development. Apidologie 2013, 44, 351–356. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Hatch, S.; Fletcher, D.J.C. The influence of queen age and quality during queen replacement in honeybee colonies. Anim. Behav. 2000, 59, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Gençer, H.V.; Shah, S.Q.; Firatli, Ç. Effects of supplemental feeding of queen rearing colonies and larval age on the acceptance of grafted larvae and queen traits. Pak. J. Biol. Sci. 2000, 3, 1319–1322. [Google Scholar]
- Koç, A.U.; Karacaoglu, M. Effects of rearing season on the quality of queen honeybees (Apis mellifera L.) raised under the conditions of aegean region. Mellifera 2004, 4, 34–37. [Google Scholar]
- Masry, S.; El-Wahab, T.A.; Hassona, N.M. Origin, weight at emergence of virgin honey bee queens and its effect on acceptance during introduction. Acad. J. Entomol. 2015, 8, 174–182. [Google Scholar]
- Harano, K.-I.; Sasaki, M.; Sasaki, K. Effects of reproductive state on rhythmicity, locomotor activity and body weight in European honeybee, Apis mellifera (Hymenoptera, Apini) queens. Sociobiology 2007, 50, 189–200. [Google Scholar]
- Hayworth, M.K.; Johnson, N.G.; Wilhelm, M.E.; Gove, R.P.; Metheny, J.D.; Rueppell, O. Added weights lead to reduced flight behavior and mating success in polyandrous honey bee queens (Apis mellifera). Ethology 2009, 115, 698–706. [Google Scholar] [CrossRef]
- Shehata, S.M.; Townsend, G.F.; Shuel, R.W. Seasonal physiological changes in queen and worker honeybees. J. Apic. Res. 1981, 20, 69–78. [Google Scholar] [CrossRef]
- Szabo, T.I.; Mills, P.F.; Heikel, D.T. Effects of honeybee queen weight and air temperature on the initiation of oviposition. J. Apic. Res. 1987, 26, 73–78. [Google Scholar]
- Szabo, T.I. Relationship between weight of honey-bee queens (Apis mellifera L.) at emergence and at the cessation of egg laying. Am. Bee J. 1973, 13, 127–135. [Google Scholar]
- Yadava, R.P.S.; Smith, M.V. Aggressive behavior of Apis mellifera L. workers towards introduced queens. III. Relationship between the attractiveness of the queen and worker aggression. Can. J. Zool. 1971, 49, 1359–1362. [Google Scholar] [CrossRef]
- Moretto, G.; Guerra, J.; Kalvelage, H.; Espindola, E. Maternal influence on the acceptance of virgin queens introduced into Africanized honey bee (Apis mellifera) colonies. Genet. Mol. Res. 2004, 3, 441–445. [Google Scholar] [PubMed]
- Szabo, T.I.; Townsend, G.F. Behavioural studies on queen introduction in the honeybee 1. effect of the age of workers (from a colony with a laying queen) on their behaviour towards an introduced virgin queen. J. Apic. Res. 1974, 13, 19–25. [Google Scholar] [CrossRef]
- Szabo, T.I. Behavioural studies of queen introduction in the honeybee 6. multiple queen introduction. J. Apic. Res. 1977, 16, 65–83. [Google Scholar] [CrossRef]
- Richard, F.J.; Tarpy, D.R.; Grozinger, C.M. Effects of insemination quantity on honey bee queen physiology. PLoS ONE 2007, 2, e980. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Westcott, L.C.; Winston, M.L. Balling behaviour in the honey bee in response to exogenous queen mandibular gland pheromone. J. Apic. Res. 1998, 37, 125–131. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Keller, J.J.; Caren, J.R.; Delaney, D.A. Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens. Insect Soc. 2011, 58, 569–574. [Google Scholar] [CrossRef]
- Skowronek, W.; Kruk, C.; Klopot, J. Factors affecting oviposition of artificially inseminated honeybee queens. J. Apic. Res. 2002, 2, 85–95. [Google Scholar]
- Gregorc, A.; Smodiš Škerl, M.I. Characteristics of honey bee (Apis Mellifera Carnica Pollman, 1879) queens reared in Slovenian commercial breeding stations. J. Apic. Sci. 2015, 59, 5–12. [Google Scholar] [CrossRef]
- Gilley, D.C.; Tarpy, D.R.; Land, B.B. Effect of queen quality on interactions between workers and dueling queens in honeybee (Apis mellifera L.) colonies. Behav. Ecol. Sociobiol. 2003, 55, 190–196. [Google Scholar] [CrossRef]
- Patricio, K.; Cruz-Landim, C. Mating influence in the ovary differentiation in adult queens of Apis mellifera L. (Hymenoptera, Apidae). Braz. J. Biol. 2002, 62, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Kocher, S.D.; Richard, F.-J.; Tarpy, D.R.; Grozinger, C.M. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera). BMC Genom. 2008, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Niño, E.L.; Tarpy, D.R.; Grozinger, C.M. Differential effects of insemination volume and substance on reproductive changes in honey bee queens (Apis mellifera L.). Insect Mol. Biol. 2013, 22, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Carreck, N.L.; Andree, M.; Brent, C.S.; Cox-Foster, D.; Dade, H.A.; Ellis, J.D.; Hatjina, F.; vanEnglesdorp, D. Standard methods for Apis mellifera anatomy and dissection. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef]
- Dedej, S.; Hartfelder, K.; Aumeier, P.; Rosenkranz, P.; Engels, W. Caste determination is a sequential process: Effect of larval age at grafting on ovariole number, hind leg size and cephalic volatiles in the honey bee (Apis mellifera carnica). J. Apic. Res. 1998, 37, 183–190. [Google Scholar] [CrossRef]
- Al-Lawati, H.; Kamp, G.; Bienefeld, K. Characteristics of the spermathecal contents of old and young honeybee queens. J. Insect Physiol. 2009, 55, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.B.; Neumann, P.; Moritz, R.F.A. Genetic variance of mating frequency in the honeybee (Apis mellifera L.). Insect Soc. 2005, 52, 1–5. [Google Scholar] [CrossRef]
- Koeniger, G.; Koeniger, N.; Ellis, J.; Connor, L.J. Mating Biology of Honey Bees (Apis mellifera); Wicwas Press: Kalamazoo, MI, USA, 2014. [Google Scholar]
- Tarpy, D.R.; Nielsen, D.I. Sampling error, effective paternity, and estimating the genetic structure of honey bee colonies (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2002, 95, 513–528. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Nielsen, R.; Nielsen, D.I. A scientific note on the revised estimates of effective paternity frequency in Apis. Insect Soc. 2004, 51, 203–204. [Google Scholar] [CrossRef]
- Schlüns, H.; Moritz, R.F.A.; Neumann, P.; Kryger, P.; Koeniger, G. Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim. Behav. 2005, 70, 125–131. [Google Scholar] [CrossRef]
- Neumann, P.; Moritz, R.F.A.; van Praagh, J. Queen mating frequency in different types of honey bee mating apiaries. J. Apic. Res. 1999, 38, 11–18. [Google Scholar] [CrossRef]
- Boomsma, J.J.; Ratnieks, F.L.W. Paternity in eusocial Hymenoptera. Philos. Trans. R. Soc. B 1996, 351, 947–975. [Google Scholar] [CrossRef]
- Palmer, K.A.; Oldroyd, B.P. Evolution of multiple mating in the genus Apis. Apidologie 2000, 31, 235–248. [Google Scholar] [CrossRef]
- Amiri, E.; Meixner, M.D.; Kryger, P. Deformed wing virus can be transmitted during natural mating in honey bees and infect the queens. Sci. Rep. 2016, 6, 33065. [Google Scholar] [CrossRef] [PubMed]
- Rueppell, O.; Johnson, N.; Rychtář, J. Variance-based selection may explain general mating patterns in social insects. Biol. Lett. 2008, 4, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Seeley, T.D. Extreme polyandry improves a honey bee colony’s ability to track dynamic foraging opportunities via greater activity of inspecting bees. Apidologie 2014, 45, 347–363. [Google Scholar] [CrossRef]
- Kolmes, S.A.; Winston, M.L.; Fergusson, L.A. The division of labor among worker honey bees (Hymenoptera: Apidae): The effects of multiple patrilines. J. Kans. Entomol. Soc. 1989, 62, 80–95. [Google Scholar]
- Jones, J.C.; Myerscough, M.R.; Graham, S.; Oldroyd, B.P. Honey bee nest thermoregulation: Diversity promotes stability. Science 2004, 305, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, B.P.; Fewell, J.H. Genetic diversity promotes homeostasis in insect colonies. Trend Ecol. Evol. 2007, 22, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Burke, K.M.; Seeley, T.D. Genetic diversity within honeybee colonies increases signal production by waggle-dancing foragers. Proc. R. Soc. B Sci. 2008, 275, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Seeley, T.D. Does a polyandrous honeybee queen improve through patriline diversity the activity of her colony’s scouting foragers? Behav. Ecol. Sociobiol. 2011, 65, 799–811. [Google Scholar] [CrossRef]
- Carr-Markell, M.K.; McDonald, K.M.; Mattila, H.R. Intracolonial genetic diversity increases chemical signaling by waggle-dancing honey bees, Apis mellifera. Insect Soc. 2013, 60, 485–496. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Seeley, T.D. Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 2006, 93, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Tarpy, D.R. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. B Sci. 2007, 274, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.A.; Oldroyd, B.P. Evidence for intra-colonial genetic variance in resistance to American foulbrood of honey bees (Apis mellifera): Further support for the parasite/pathogen hypothesis for the evolution of polyandry. Naturwissenschaften 2003, 90, 265–268. [Google Scholar] [PubMed]
- Mattila, H.R.; Seeley, T.D. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 2007, 317, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Woyke, J. Dynamics of entry of spermatozoa into the spermatheca of instrumentally inseminated queen honeybees. J. Apic. Res. 1983, 22, 150–154. [Google Scholar] [CrossRef]
- Woyke, J. Natural and artificial insemination of queen honeybees. Bee World 1962, 43, 21–25. [Google Scholar] [CrossRef]
- Camazine, S.; Cakmak, I.; Cramp, K.; Finley, J.; Fisher, J.; Frazier, M.; Rozo, A. How healthy are commercially-produced US honey bee queens? Am. Bee J. 1998, 138, 677–680. [Google Scholar]
- Lodesani, M.; Balduzzi, D.; Galli, A. A study on spermatozoa viability over time in honey bee (Apis mellifera ligustica) queen spermathecae. J. Apic. Res. 2004, 43, 27–28. [Google Scholar] [CrossRef]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yañez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 2015, 5, 14621. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, S.P.A.; Boomsma, J.J.; Baer, B. Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. J. Insect Physiol. 2009, 55, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Zareie, R.; Eubel, H.; Millar, A.H.; Baer, B. Long-term survival of high quality sperm: Insights into the sperm proteome of the honeybee Apis mellifera. J. Proteome Res. 2013, 12, 5180–5188. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Eubel, H.; Millar, A.H.; Baer, B. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J. Insect Physiol. 2011, 57, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Rice, N.; Joselow, K.; vanEngelsdorp, D.; Chaimanee, V. Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PLoS ONE 2016, 11, e0147220. [Google Scholar]
- Rueppell, O.; Aumer, D.; Moritz, R.F.A. Ties between ageing plasticity and reproductive physiology in honey bees (Apis mellifera) reveal a positive relation between fecundity and longevity as consequence of advanced social evolution. Curr. Opin. Insect Sci. 2016, 16, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Meixner, M.; Büchler, R.; Kryger, P. Chronic bee paralysis virus in honeybee queens: Evaluating susceptibility and infection routes. Viruses 2014, 6, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Kulincevic, J.M.; Rothenbuhler, W.C. The effects of artificial infection with Chronic bee paralysis virus on queens from strains of honeybee resistant or susceptible to Hairless-black syndrome. J. Apic. Res. 1989, 28, 79–80. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Zheng, H.; Cao, L.; Niu, D.; Yu, D.; Sun, Y.; Hu, S.; Hu, F. Transcriptome comparison between honey bee queen- and worker-destined larvae. Insect Biochem. Mol. 2012, 42, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Traver, B.E.; Fell, R.D. Low natural levels of Nosema ceranae in Apis mellifera queens. J. Invertebr. Pathol. 2012, 110, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Czekonska, K. The influence of Nosema apis on young honeybee queens and transmission of the disease from queens to workers. Apidologie 2000, 31, 701–706. [Google Scholar] [CrossRef]
- Webster, T.C.; Pomper, K.W.; Hunt, G.; Thacker, E.M.; Jones, S.C. Nosema apis infection in worker and queen Apis mellifera. Apidologie 2004, 35, 49–54. [Google Scholar] [CrossRef]
- Porporato, M.; Grillone, G.; Patetta, A.; Manino, A.; Laurino, D. Survey of the health status of some honey bee queens in Italy. J. Apic. Res. 2015, 59, 27–36. [Google Scholar] [CrossRef]
- Burgett, M.; Kitprasert, C. Tracheal mite infestation of queen honey bees. J. Apic. Res. 1992, 31, 110–111. [Google Scholar] [CrossRef]
- Villa, J.D.; Danka, R.G. Caste, sex and strain of honey bees (Apis mellifera) affect infestation with tracheal mites (Acarapis woodi). Exp. Appl. Acarol. 2005, 37, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Dietz, A.; Eischen, F.A. Incidence rates of Acarapis woodi (Rennie) in queen honey bees of various ages. Apidologie 1989, 20, 69–75. [Google Scholar] [CrossRef]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Patterns of viral infection in honey bee queens. J. Gen. Virol. 2013, 94, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Ravallec, M.; Tournaire, M.; Cousserans, F.; Bergoin, M.; Dainat, B.; de Miranda, J.R. Viruses associated with ovarian degeneration in Apis mellifera L. queens. PLoS ONE 2011, 6, e16217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pettis, J.S.; Feldlaufer, M.F. Detection of multiple viruses in queens of the honey bee Apis mellifera L. J. Invertebr. Pathol. 2005, 90, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J.; Spivak, M.; Visscher, P.K.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y.; et al. Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 2014, 10, e1004261. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Bakonyi, T. Viral infections in queen bees (Apis mellifera carnica) from rearing apiaries. Acta Vet. Brno 2012, 81, 15–19. [Google Scholar] [CrossRef]
- Liu, T.P. Oocytes degeneration in the queen honey bee after infection by Nosema apis. Tissue Cell 1992, 24, 131–138. [Google Scholar] [CrossRef]
- Alaux, C.; Folschweiller, M.; McDonnell, C.; Beslay, D.; Cousin, M.; Dussaubat, C.; Brunet, J.-L.; Le Conte, Y. Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J. Invertebr. Pathol. 2011, 106, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Yañez, O.; Jaffé, R.; Jarosch, A.; Fries, I.; Moritz, R.F.A.; Paxton, R.J.; Miranda, J.R. Deformed wing virus and drone mating flights in the honey bee (Apis mellifera): Implications for sexual transmission of a major honey bee virus. Apidologie 2012, 43, 17–30. [Google Scholar] [CrossRef]
- Yue, C.; Schroder, M.; Bienefeld, K.; Genersch, E. Detection of viral sequences in semen of honeybees (Apis mellifera): Evidence for vertical transmission of viruses through drones. J. Invertebr. Pathol. 2006, 92, 105–108. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Fries, I. Venereal and vertical transmission of Deformed wing virus in honeybees (Apis mellifera L.). J. Invertebr. Pathol. 2008, 98, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ravoet, J.; De Smet, L.; Wenseleers, T.; de Graaf, D.C. Vertical transmission of honey bee viruses in a Belgian queen breeding program. BMC Vet. Res. 2015, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Schroder, M.; Gisder, S.; Genersch, E. Vertical-transmission routes for deformed wing virus of honeybees (Apis mellifera). J. Gen. Virol. 2007, 88, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Mutinelli, F. The spread of pathogens through trade in honey bees and their products (including queen bees and semen): Overview and recent developments. Rev. Sci. Tech. Int. Epiz. 2011, 30, 257–271. [Google Scholar] [CrossRef]
- Büchler, R.; Andonov, S.; Bienefeld, K.; Costa, C.; Hatjina, F.; Kezic, N.; Kryger, P.; Spivak, M.; Uzunov, A.; Wilde, J. Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res. 2013, 52, 1–30. [Google Scholar] [CrossRef]
- Anderson, D.L.; Trueman, J.W. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Schwarz, R.S. Bees brought to their knees: Microbes affecting honey bee health. Trends Microbiol. 2011, 19, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Walker, P.L.; Martin, S.J.; Gunn, A. The transmission of Deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 1999, 73, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mockel, N.; Gisder, S.; Genersch, E. Horizontal transmission of Deformed wing virus: Pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J. Gen. Virol. 2011, 92, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F.; Evans, J.D.; Chen, Y. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Erban, T.; Harant, K.; Hubalek, M.; Vitamvas, P.; Kamler, M.; Poltronieri, P.; Tyl, J.; Markovic, M.; Titera, D. In-depth proteomic analysis of Varroa destructor: Detection of DWV-complex, ABPV, VdMLV and honeybee proteins in the mite. Sci. Rep. 2015, 5, 13907. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yang, X.; Cox-Foster, D.; Cui, L. The role of varroa mites in infections of Kashmir bee virus (KBV) and Deformed wing virus (DWV) in honey bees. Virology 2005, 342, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Duay, P.; De Jong, D.; Engels, W. Weight loss in drone pupae (Apis mellifera) multiply infested by Varroa destructor mites. Apidologie 2003, 34, 61–66. [Google Scholar] [CrossRef]
- Duay, P.; De Jong, D.; Engels, W. Decreased flight performance and sperm production in drones of the honey bee (Apis mellifera) slightly infested by Varroa destructor mites during pupal development. Genet. Mol. Res. 2002, 1, 227–232. [Google Scholar] [PubMed]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S. Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie 1990, 21, 193–199. [Google Scholar] [CrossRef]
- Calderone, N.W.; Lin, S.; Kuenen, L.P.S. Differential infestation of honey bee, Apis mellifera, worker and queen brood by the parasitic mite Varroa destructor. Apidologie 2002, 33, 389–398. [Google Scholar] [CrossRef]
- Le Conte, Y.; Arnold, G.; Trouiller, J.; Masson, C.; Chappe, B.; Ourisson, G. Attraction of the parasitic mite Varroa to the drone larvae of honey bees by simple aliphatic esters. Science 1989, 245, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Calderone, N.W.; Kuenen, L.P.S. Differential tending of worker and drone larvae of the honey bee, Apis mellifera, during the 60 h prior to cell capping. Apidologie 2003, 34, 543–552. [Google Scholar] [CrossRef]
- Nazzi, F.; Bortolomeazzi, R.; Della Vedova, G.; Del Piccolo, F.; Annoscia, D.; Milani, N. Octanoic acid confers to royal jelly varroa-repellent properties. Naturwissenschaften 2009, 96, 309–314. [Google Scholar] [CrossRef]
- Harizanis, P.C. Infestation of queen cells by the mite Varroa jacobsoni. Apidologie 1991, 22, 533–538. [Google Scholar] [CrossRef]
- Rennie, J. Isle of wight disease in hive bees—Acarine disease: The organism associated with the disease—Tarsonemus woodi, n. sp. Trans. R. Soc. Edinb. 1921, 52, 768–779. [Google Scholar] [CrossRef]
- Sammataro, D.; de Guzman, L.D.; George, S.; Ochoa, R.; Otis, G. Standard methods for tracheal mite research. J. Apic. Res. 2013, 52, 1–20. [Google Scholar] [CrossRef]
- Harrison, J.F.; Camazine, S.; Marden, J.H.; Kirkton, S.D.; Rozo, A.; Yang, X. Mite not make it home: Tracheal mites reduce the safety margin for oxygen delivery of flying honeybees. J. Exp. Biol. 2001, 204, 805–814. [Google Scholar]
- Eischen, F.A.; Cardoso-Tamez, D.; Wilson, W.T.; Dietz, A. Honey production of honey bee colonies infested with Acarapis woodi (Rennie). Apidologie 1989, 20, 1–8. [Google Scholar] [CrossRef]
- Eischen, F.A. Overwintering performance of honey bee colonies heavily infested with Acarapis woodi (Rennie). Apidologie 1987, 18, 293–304. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- De Graaf, D.C.; Raes, H.; Sabbe, G.; Derycke, P.H.; Jacobs, F.J. Early development of Nosema apis (Microspora, Nosematidae) in the midgut epithelium of the honeybee (Apis mellifera). J. Invertebr. Pathol. 1994, 63, 74–81. [Google Scholar] [CrossRef]
- Natsopoulou, M.E.; McMahon, D.P.; Doublet, V.; Bryden, J.; Paxton, R.J. Interspecific competition in honeybee intracellular gut parasites is asymmetric and favours the spread of an emerging infectious disease. Proc. R. Soc. B Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martin-Hernandez, R.; Garcia-Palencia, P.; Marin, P.; Meana, A. Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera). Environ. Microbiol. Rep. 2009, 1, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.C.; Thacker, E.M.; Pomper, K.; Lowe, J.; Hunt, G. Nosema apis infection in honey bee (Apis mellifera) queens. J. Apic. Res. 2008, 47, 53–57. [Google Scholar] [CrossRef]
- Peng, Y.; Grassl, J.; Millar, A.H.; Baer, B. Seminal fluid of honeybees contains multiple mechanisms to combat infections of the sexually transmitted pathogen Nosema apis. Proc. R. Soc. B Sci. 2016, 283, 20151785. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.E.; Evison, S.E.F.; Baer, B.; Hughes, W.O.H. The cost of promiscuity: Sexual transmission of Nosema microsporidian parasites in polyandrous honey bees. Sci. Rep. 2015, 5, 10982. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-F.; Solter, L.F. Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J. Invertebr. Pathol. 2013, 113, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martin, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Antunez, K.; Martin-Hernandez, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.H. Studies on the effect of infection with Nosema apis on the physiology of the queen honey bee. Q. J. Microsc. Sci. 1951, 92, 225–231. [Google Scholar]
- Loskotova, J.; Peroutka, M.; Vesely, V. Nosema disease of honeybee queens (Apis mellifica L.). Apidologie 1980, 11, 153–161. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, G.E.; Chejanovsky, N.; Chen, Y.; Gauthier, L.; Genersch, E.; de Graaf, D.C.; et al. Standard methods for virus research in Apis mellifera. J. Apic. Res. 2013, 52, 1–56. [Google Scholar] [CrossRef]
- Ball, B.V.; Bailey, L. Viruses. In Honey Bee Pests, Predators, and Diseases; Morse, R.F.K., Ed.; AI Root Company: Medina, OH, USA, 1997; pp. 11–32. [Google Scholar]
- Chen, Y.; Siede, R. Honey bee viruses. In Advances in Virus Research; Maramorosch, K., Shabalina, S.A., Murphy, F.A., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2007; Volume 70, pp. 33–80. [Google Scholar]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Lavi, S.; Mozes-Koch, R.; Gantman, Y.; Peretz, Y.; Edelbaum, O.; Tanne, E.; Sela, I. Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honeybees in Israel: Evidence for diversity due to intra- and inter-species recombination. J. Gen. Virol. 2007, 88, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Fievet, J.; Tentcheva, D.; Gauthier, L.; de Miranda, J.; Cousserans, F.; Colin, M.E.; Bergoin, M. Localization of Deformed wing virus infection in queen and drone Apis mellifera L. Virol. J. 2006, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pettis, J.S.; Collins, A.; Feldlaufer, M.F. Prevalence and transmission of honeybee viruses. Appl. Environ. Microbiol. 2006, 72, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cox-Foster, D. Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitology 2007, 134, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Rogers, R.E.L.; Kalkstein, A.L.; Taylor, B.A.; Shutler, D.; Ostiguy, N. Deformed wing virus in western honey bees (Apis mellifera) from Atlantic Canada and the first description of an overtly-infected emerging queen. J. Invertebr. Pathol. 2009, 101, 77–79. [Google Scholar] [CrossRef]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. Two viruses from adult honey bees (Apis mellifera Linnaeus). Virology 1963, 21, 390–395. [Google Scholar] [CrossRef]
- Ribiere, M.; Lallemand, P.; Iscache, A.L.; Schurr, F.; Celle, O.; Blanchard, P.; Olivier, V.; Faucon, J.P. Spread of infectious Chronic bee paralysis virus by honeybee (Apis mellifera L.) feces. Appl. Environ. Microbiol. 2007, 73, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Ribiere, M.; Olivier, V.; Blanchard, P. Chronic bee paralysis: A disease and a virus like no other? J. Invertebr. Pathol. 2010, 103, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ribière, M.; Faucon, J.P.; Pépin, M. Detection of Chronic honey bee (Apis mellifera L.) paralysis virus infection: Application to a field survey. Apidologie 2000, 31, 567–577. [Google Scholar] [CrossRef]
- Bailey, L. The multiplication and spread of sacbrood virus of bees. Ann. Appl. Biol. 1969, 63, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Woods, R.D. Two more small RNA viruses from honey bees and further observations on Sacbrood and Acute bee-paralysis viruses. J. Gen. Virol. 1977, 37, 175–182. [Google Scholar] [CrossRef]
- Bailey, L.; Ball, B.V.; Perry, J.N. Association of viruses with two protozoal pathogens of the honey bee. Ann. Appl. Biol. 1983, 103, 13–20. [Google Scholar] [CrossRef]
- Retschnig, G.; Williams, G.R.; Mehmann, M.M.; Yañez, O.; de Miranda, J.R.; Neumann, P. Sex-specific differences in pathogen susceptibility in honey bees (Apis mellifera). PLoS ONE 2014, 9, e85261. [Google Scholar] [CrossRef] [PubMed]
- Doublet, V.; Labarussias, M.; de Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, P.; Stevanovic, J.; Cirkovic, D.; Radojicic, S.; Lakic, N.; Stanisic, L.; Stanimirovic, Z. Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. J. Apic. Res. 2014, 53, 545–554. [Google Scholar] [CrossRef]
- Rangel, J.; Böröczky, K.; Schal, C.; Tarpy, D.R. Honey bee (Apis mellifera) queen reproductive potential affects queen mandibular gland pheromone composition and worker retinue response. PLoS ONE 2016, 11, e0156027. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín-Hernández, R.; Días, J.; García-Palencia, P.; Matabuena, M.; Juarranz, Á.; Barrios, L.; Meana, A.; Nanetti, A.; Higes, M. The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera Iberiensis) colonies. Environ. Microbiol. 2012, 14, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Richards, J.; Mattila, H.R. From molecules to societies: Mechanisms regulating swarming behavior in honey bees (Apis spp.). Apidologie 2014, 45, 327–346. [Google Scholar] [CrossRef]
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA 2012, 109, 11002–11007. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.K.; Moran, N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Tarpy, D.R.; Mattila, H.R.; Newton, I.L.G. Development of the honey bee gut microbiome throughout the queen-rearing process. Appl. Environ. Microbiol. 2015, 81, 3182–3191. [Google Scholar] [CrossRef] [PubMed]
- Kapheim, K.M.; Rao, V.D.; Yeoman, C.J.; Wilson, B.A.; White, B.A.; Goldenfeld, N.; Robinson, G.E. Caste-specific differences in hindgut microbial communities of honey bees (Apis mellifera). PLoS ONE 2015, 10, e0123911. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.W.; Sheppard, W.S.; Tarpy, D.R. Status of breeding practices and genetic diversity in domestic U.S. honey bee. In Honey Bee Colony Health; CRC Press: Boca Raton, FL, USA, 2011; pp. 25–36. [Google Scholar]
- Büchler, R.; Costa, C.; Hatjina, F.; Andonov, S.; Meixner, M.D.; Conte, Y.L.; Uzunov, A.; Berg, S.; Bienkowska, M.; Bouga, M.; et al. The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J. Apic. Res. 2014, 53, 205–214. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiri, E.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Queen Quality and the Impact of Honey Bee Diseases on Queen Health: Potential for Interactions between Two Major Threats to Colony Health. Insects 2017, 8, 48. https://doi.org/10.3390/insects8020048
Amiri E, Strand MK, Rueppell O, Tarpy DR. Queen Quality and the Impact of Honey Bee Diseases on Queen Health: Potential for Interactions between Two Major Threats to Colony Health. Insects. 2017; 8(2):48. https://doi.org/10.3390/insects8020048
Chicago/Turabian StyleAmiri, Esmaeil, Micheline K. Strand, Olav Rueppell, and David R. Tarpy. 2017. "Queen Quality and the Impact of Honey Bee Diseases on Queen Health: Potential for Interactions between Two Major Threats to Colony Health" Insects 8, no. 2: 48. https://doi.org/10.3390/insects8020048