Communication or Toxicity: What Is the Effect of Cycloheximide on Leaf-Cutting Ant Workers?
Abstract
:1. Introduction
2. Material and Methods
2.1. Studied Colonies
2.1.1. Preparation of Pellets
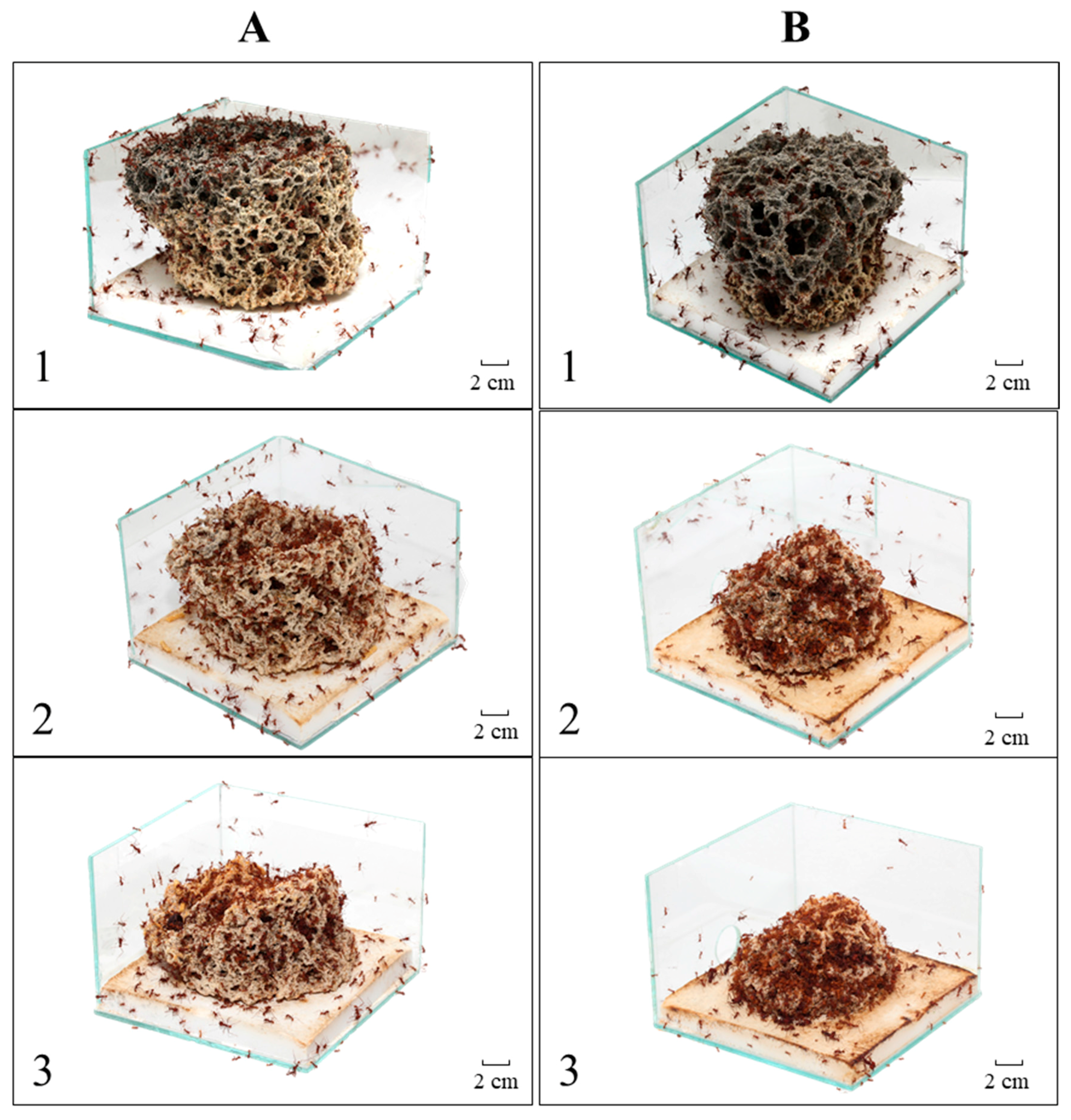
2.1.2. Observation Box
2.1.3. Experiment 1
2.1.4. Experiment 2
2.1.5. Statistical Analysis
3. Results
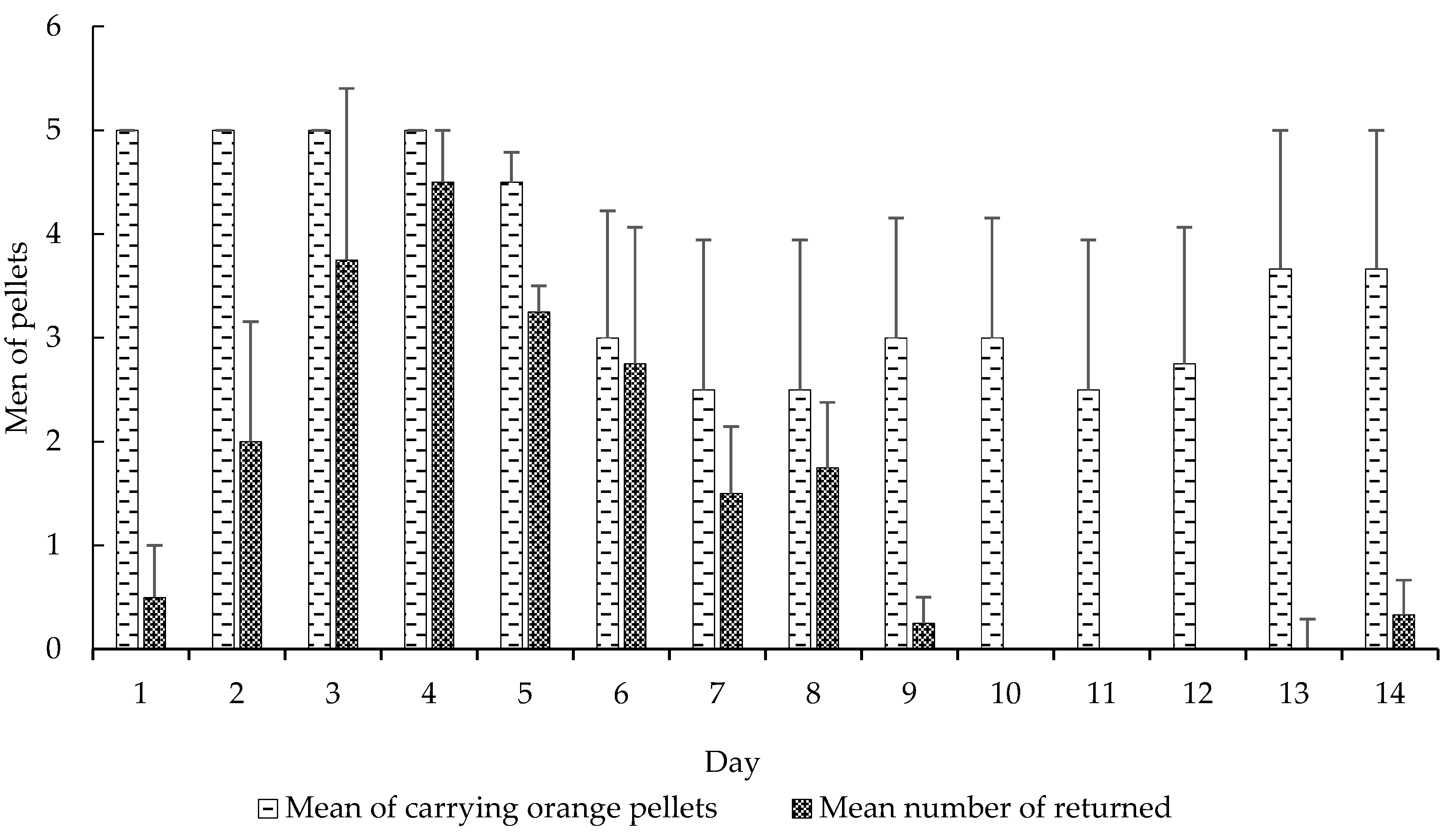
3.1. Experiment 1
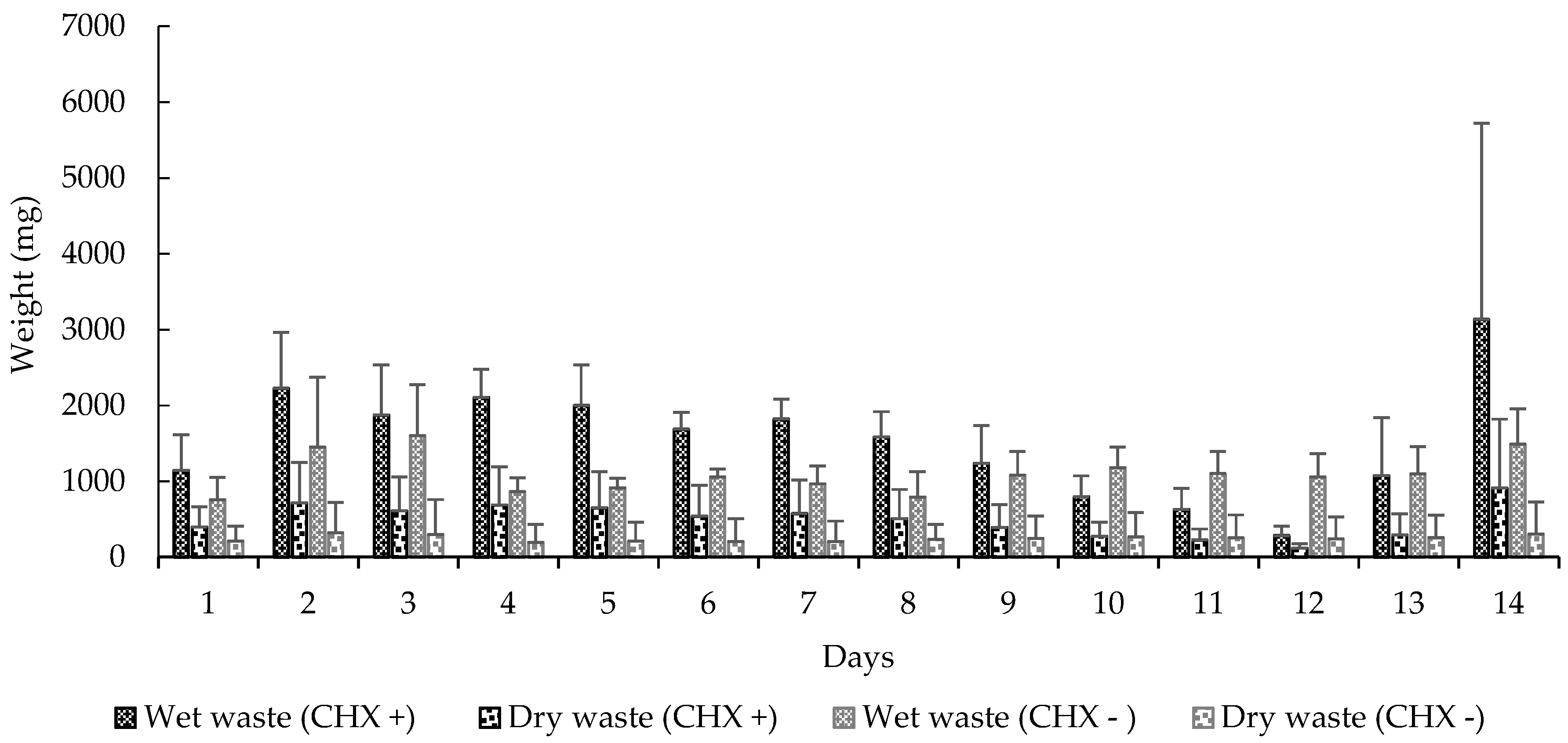
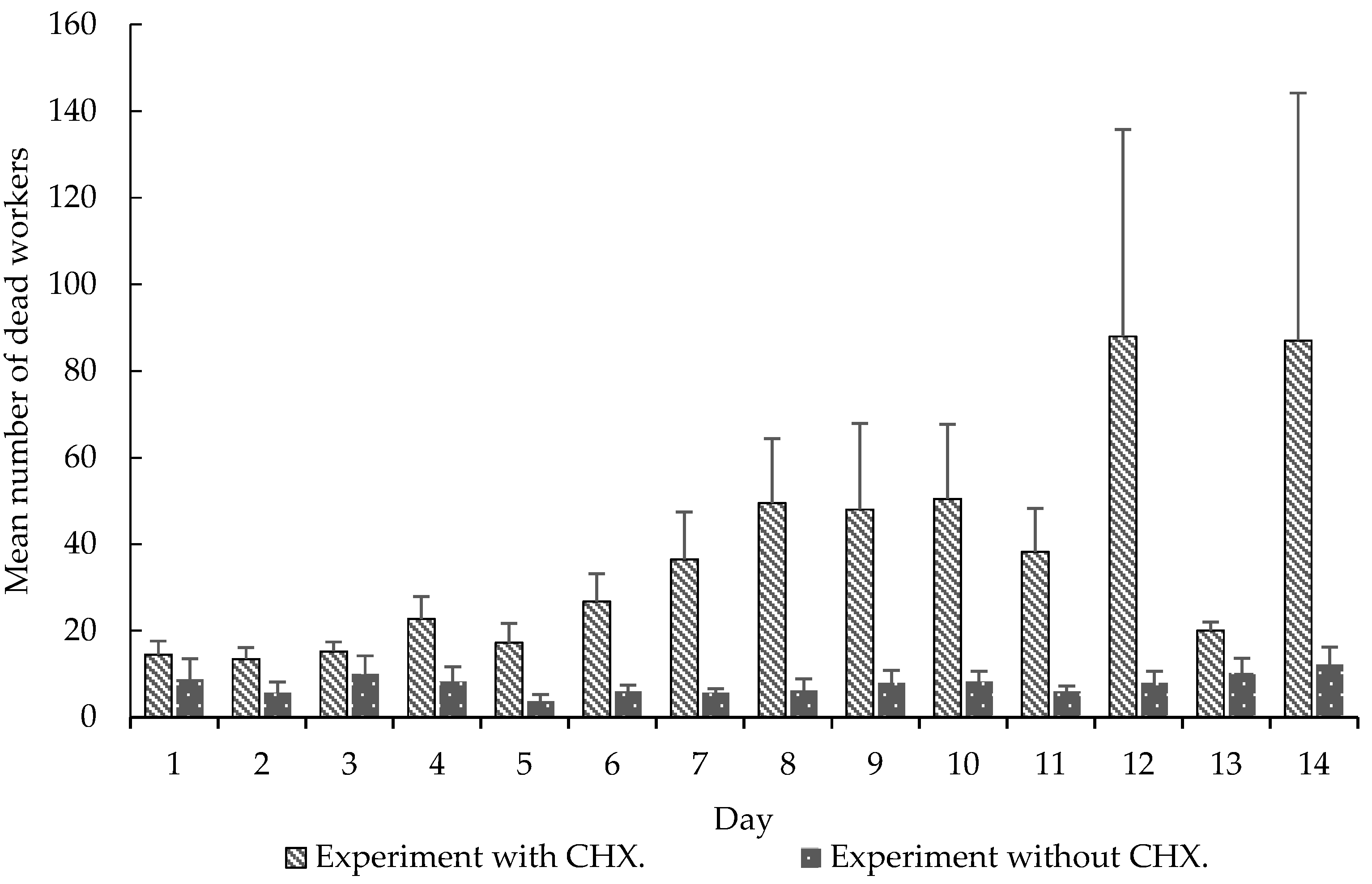
3.2. Experiment 2
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weber, N.A. Gardening Ants, the Attines. Science 1972, 178, 856. [Google Scholar]
- Schultz, T.R.; Mueller, U.G.; Currie, C.R.; Rehner, S.A. Reciprocal Illumination a Comparison of Agriculture in Humans; Oxford University Press: England, UK, 2005. [Google Scholar]
- Weber, N.A. Treatment of substrate by fungus-growing ants. Anat. Rec. 1956, 125, 604–605. [Google Scholar]
- Ridley, P.; Howse, P.E.; Jackson, C.W. Control of the behaviour of leaf-cutting ants by their “symbiotic” fungus. Experientia 1996, 52, 631–635. [Google Scholar] [CrossRef]
- North, R.D.; Jackson, C.W.; Howse, P.E. Communication between the fungus garden and workers of the leaf-cutting ant, Atta sexdens rubropilosa, regarding choice of substrate for the fungus. Physiol. Entomol. 1999, 24, 127–133. [Google Scholar] [CrossRef]
- Herz, H.; Hölldobler, B.; Roces, F. Delayed rejection in a leaf-cutting ant after foraging on plants unsuitable for the symbiotic fungus. Behav. Ecol. 2008, 19, 575–582. [Google Scholar] [CrossRef]
- Arenas, A.; Roces, F. Gardeners and midden workers in leaf-cutting ants learn to avoid plants unsuitable for the fungus at their worksites. Anim. Behav. 2016, 115, 167–174. [Google Scholar] [CrossRef]
- Arenas, A.; Roces, F. Learning through the waste: Olfactory cues from the colony refuse influence plant preferences in foraging leaf-cutting ants. J. Exp. Biol. 2016, 219, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Arenas, A.; Roces, F. Avoidance of plants unsuitable for the symbiotic fungus in leaf-cutting ants: Learning can take place entirely at the colony dump. PLoS ONE 2017, 12, e0171388. [Google Scholar] [CrossRef] [PubMed]
- Saverschek, N.; Herz, H.; Wagner, M.; Roces, F. Avoiding plants unsuitable for the symbiotic fungus: Learning and long-term memory in leaf-cutting ants. Anim. Behav. 2010, 79, 689–698. [Google Scholar] [CrossRef]
- Falibene, A.; Roces, F.; Rössler, W. Long-term avoidance memory formation is associated with a transient increase in mushroom body synaptic complexes in leaf-cutting ants. Front. Behav. Neurosci. 2015, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saverschek, N.; Roces, F. Foraging leafcutter ants: Olfactory memory underlies delayed avoidance of plants unsuitable for the symbiotic fungus. Anim. Behav. 2011, 82, 453–458. [Google Scholar] [CrossRef]
- Akahane, R.; Amakawa, T. Stable and unstable phase of memory in classically conditioned fly, Phormia regina: Effects of nitrogen gas anaesthesia and cycloheximide injection. J. Insect Physiol. 1983, 29, 331–337. [Google Scholar] [CrossRef]
- Flyg, B.C.; Kenne, K.; Boman, H.G. Phageresistant mutants with a decreased resistance to cecropia immunity and a dtxreased virulence to drosophila. J. Gen. Microbiol. 1980, 120, 173–181. [Google Scholar] [PubMed]
- Matsumoto, Y.; Hirashima, D.; Terao, K.; Mizunami, M. Roles of NO Signaling in long-term memory formation in visual learning in an insect. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, S.E.; Choi, M.K.; Truman, J.W. Inhibitory effects of actinomycin D and cycloheximide on neuronal death in adult Manduca sexta. Dev. Neurobiol. 1994, 25, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, H.; Leach, C.E.; Lynn, D.E. Effects of Cycloheximide on Cellular Elongation in a Manduca sexta Cell Line. Dev. Genes Evol. 1981, 190, 60–61. [Google Scholar]
- Soltani-Mazouni, N.; Soltani, N. Protein synthesis in the fat body of Tenebrio molitor (L.) during oocyte maturation: Effect of diflubenzuron, cycloheximide and starvation. J. Stored Prod. Res. 1995, 31, 117–122. [Google Scholar] [CrossRef]
- Nouri, N.; Fallon, A.M. Pleiotropic changes in cycloheximide-resistant insect cell clones. In Vitro Cell. Dev. Biol. 1987, 23, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, S.; Kaatz, H.H.; Menzel, R. Inhibition of brain protein synthesis by cycloheximide does not affect formation of long-term memory in honeybees after olfactory conditioning. J. Neurosci. 1993, 13, 1379–1386. [Google Scholar] [PubMed]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introctory Guide; Cambridge University Press: New York, NY, USA, 1986. [Google Scholar]
- Camargo, R.S.; Forti, L.C.; De Matos, C.A.O.; Lopes, J.F.; De Andrade, A.P.P.; Ramos, V.M. Post-selection and return of foraged material by Acromyrmex subterraneus brunneus (Hymenoptera: Formicidae). Sociobiology 2003, 42, 93–102. [Google Scholar]
- Camargo, R.S.; Forti, L.C.; De Matos, C.A.O.; Lopes, J.F.; De Andrade, A.P.P. Physical resistance as a criterion in the selection of foraging material by Acromyrmex subterraneus brunneus Forel, 1911 (Hym., Formicidae). J. Appl. Entomol. 2004, 128, 329–331. [Google Scholar] [CrossRef]
- Camargo, S.; Camargo, S.; Puccini, C.; Forti, L.C.; Alberto, C.; Matos, O. Behaviors in fungus garden cultivation: Routes of contamination of leaf cutting ant workers with fat-soluble tracer dye. Int. J. Agric. Innov. Res. 2017, 5, 555–560. [Google Scholar]
- Silva, L.C.; Camargo, R.S.; Forti, L.C.; Matos, C.A.O.; Travaglini, R.V. Do Atta sexdens rubropilosa workers prepare leaves and bait pellets in similar ways to their symbiotic fungus? Sociobiology 2015, 62, 484–493. [Google Scholar]
- Garrett, R.W.; Carlson, K.A.; Goggans, M.S.; Nesson, M.H.; Shepard, C.A.; Schofield, R.M.S. Leaf processing behaviour in Atta leafcutter ants: 90% of leaf cutting takes place inside the nest, and ants select pieces that require less cutting. R. Soc. Open Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- De Britto, J.S.; Forti, L.C.; de Oliveira, M.A.; Zanetti, R.; Wilcken, C.F.; Zanuncio, J.C.; Loeck, A.E.; Caldato, N.; Nagamoto, N.S.; Lemes, P.G.; et al. Use of alternatives to PFOS, its salts and PFOSF for the control of leaf-cutting ants Atta and Acromyrmex. Int. J. Res. Environ. Stud. 2016, 3, 11–92. [Google Scholar]
- Lopes, J.F.S.; Forti, L.C.; Boaretto, M.A.; Camargo, R.S.; Andrade, A.P.; Ramos, V.M.; Nagamoto, N.S. Devolution rates of grass by Atta capiguara (Hymenoptera, Formicidae) in field conditions. Pasturas Trop. 2003, 25, 42–45. [Google Scholar]
- Knapp, J.J.; Howse, P.E.; Kermarrec, A. Factors controlling foraging patterns in the leaf-cutting ant Acromyrmex octospinosus (Reich). In Applied Myrmecology: A World Perspective; Westview Press: Boulder, CO, USA, 1990; pp. 382–409. [Google Scholar]
| Mean of Behaviors | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behaviors | 1 Day | 2 Day | 3 Day | 4 Day | 5 Day | 6 Day | 7 Day | 8 Day | 9 Day | 10 Day | 11 Day | 12 Day | 13 Day | 14 Day |
| 1 | 2.5 ± 1.0 | 34.8 ± 16.6 | 83 ± 31.6 | 51.3 ± 6.0 | 64.5 ± 28.8 | 29.3 ± 21.0 | 16.8 ± 15.1 | 42.8 ± 30.4 | 40 ± 31.1 | 56 ± 43.7 | 11.8 ± 6.8 | 6.0 ± 3.5 | 5.7 ± 2.5 | 5.7 ± 1.8 |
| 2 | 268.75 ± 29.0 | 389.3 ± 63.6 | 543.8 ± 112.3 | 601.3 ± 88.3 | 438.5 ± 60.6 | 314.8 ± 136.3 | 163.8 ± 95.2 | 262.5 ± 154.6 | 463 ± 216.5 | 397.8 ± 168.8 | 303.8 ± 101.0 | 290.8 ± 67.4 | 308 ± 72.6 | 258.3 ± 101.2 |
| 3 | 13.75 ± 2.3 | 7 ± 3.5 | 4.8 ± 1.7 | 4.5 ± 2.6 | 1.3 ± 0.9 | 0.0 | 0.0 | 0.0 | 0.3 ± 0.3 | 0.3 ± 0.3 | 0.3 ± 0.3 | 0.8 ± 0.5 | 1.3 ± 0.8 | 0.7 ± 0.6 |
| 4 | 39.75 ± 7.0 | 38 ± 26.0 | 18 ± 14.3 | 7.5 ± 4.5 | 0.3 ± 0.3 | 0.5 ± 0.5 | 0.3 ± 0.3 | 1.3 ± 1.3 | 6.3 ± 3.7 | 28.5 ± 17.0 | 48.5 ± 28.1 | 32.8 ± 19.6 | 74.7 ± 35.6 | 67.7 ± 29.3 |
| 5 | 30 ± 3.7 | 27.3 ± 16.0 | 16.8 ± 12.8 | 10.5 ± 6.3 | 0.0 | 0.0 | 1.0 ± 1.1 | 0.8 ± 0.8 | 5 ± 3.0 | 34.3 ± 22.0 | 43 ± 25.5 | 36.3 ± 0.5 | 57.7 ± 25.0 | 73.3 ± 31.8 |
| 6 | 47 ± 12.8 | 5 ± 3.0 | 11.8 ± 9.6 | 4.8 ± 3.3 | 0.0 | 0.0 | 0.5 ± 0.5 | 0.3 ± 0.3 | 0.3 ± 0.3 | 7.3 ± 5.4 | 7 ± 4.4 | 9.3 ± 6.6 | 16.7 ± 7.2 | 12.3 ± 5.3 |
| 7 | 266.25 ± 65.4 | 36.3 ± 28.3 | 21.8 ± 12.6 | 10.5 ± 6.1 | 0.0 | 0.0 | 1.5 ± 1.5 | 0.5 ± 0.5 | 0.0 | 3.3 ± 2.1 | 100.3 ± 60.2 | 83 ± 4.2 | 136 ± 62.0 | 111.7 ± 54.5 |
| 8 | 11.25 ± 2.9 | 3.8 ± 2.4 | 4 ± 2.6 | 2.3 ± 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 ± 1.8 | 4.8 ± 2.8 | 11.3 ± 5.8 | 8.7 ± 3.9 |
| 9 | 226.75 ± 76.6 | 245 ± 75.8 | 287.3 ± 82.8 | 377 ± 109.4 | 473.3 ± 135.6 | 517.3 ± 201.4 | 590.3 ± 162.9 | 593 ± 127.5 | 505 ± 169.6 | 609 ± 57.7 | 591.8 ± 84.9 | 545.3 ± 122.8 | 441 ± 53.9 | 526 ± 132.0 |
| 10 | 37.25 ± 6.7 | 42.3 ± 11.8 | 45.3 ± 11.9 | 60.3 ± 8.2 | 40.3 ± 5.2 | 71 ± 64.6 | 43.8 ± 2 | 38 ± 7.1 | 32.3 ± 6.1 | 16.8 ± 1.1 | 10.8 ± 3.8 | 7 ± 3.8 | 25.7 ±11.0 | 43 ± 13.0 |
| Day | Mean Number of Pellets | Mean Time (min) Spent | ||
|---|---|---|---|---|
| CHX + | CHX − | CHX + | CHX − | |
| 1 | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.5 ± 0.5 | 5.0 ± 0.0 |
| 2 | 5.0 ± 0.0 | 5.0 ± 0.0 | 14.3 ± 8.6 | 5.0 ± 0.0 |
| 3 | 5.0 ± 0.0 | 5.0 ± 0.0 | 24.8 ± 12.2 | 5.0 ± 0.0 |
| 4 | 3.8 ± 1.3 | 5.0 ± 0.0 | 21.5 ± 8.5 | 5.0 ± 0.0 |
| 5 | 3.8 ± 0.9 | 5.0 ± 0.0 | 246.5 ± 134.8 | 5.0 ± 0.0 |
| 6 | 3.0 ± 1.2 | 5.0 ± 0.0 | 260.3 ± 127.3 | 5.0 ± 0.0 |
| 7 | 2.5 ± 1.4 | 5.0 ± 0.0 | 266.8 ± 123.2 | 5.0 ± 0.0 |
| 8 | 3.8 ± 1.3 | 5.0 ± 0.0 | 138.5 ± 114.9 | 5.0 ± 0.0 |
| 9 | 3.0 ± 1.2 | 5.0 ± 0.0 | 252 ± 131.6 | 5.0 ± 0.0 |
| 10 | 2.5 ± 1.4 | 5.0 ± 0.0 | 124 ± 118.7 | 5.0 ± 0.0 |
| 11 | 2.5 ± 1.4 | 5.0 ± 0.0 | 125.8 ± 118.1 | 5.0 ± 0.0 |
| 12 | 2.8 ± 1.3 | 5.0 ± 0.0 | 125.8 ± 118.1 | 5.0 ± 0.0 |
| 13 | 3.7 ± 1.3 | 5.0 ± 0.0 | 165 ± 157.5 | 5.0 ± 0.0 |
| 14 | 3.7 ± 1.3 | 5.0 ± 0.0 | 165 ± 157.5 | 5.0 ± 0.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade Sousa, K.K.; Da Silva Camargo, R.; Forti, L.C. Communication or Toxicity: What Is the Effect of Cycloheximide on Leaf-Cutting Ant Workers? Insects 2017, 8, 126. https://doi.org/10.3390/insects8040126
Andrade Sousa KK, Da Silva Camargo R, Forti LC. Communication or Toxicity: What Is the Effect of Cycloheximide on Leaf-Cutting Ant Workers? Insects. 2017; 8(4):126. https://doi.org/10.3390/insects8040126
Chicago/Turabian StyleAndrade Sousa, Kátia Kaelly, Roberto Da Silva Camargo, and Luiz Carlos Forti. 2017. "Communication or Toxicity: What Is the Effect of Cycloheximide on Leaf-Cutting Ant Workers?" Insects 8, no. 4: 126. https://doi.org/10.3390/insects8040126





