Recovery Phase Nutrition and Insulin Strategies for a Collegiate Distance Runner with Type 1 Diabetes Mellitus: A Case Study
Abstract
:1. Introduction
2. Athlete Background
3. Methods
3.1. Nutrition Program
3.2. Insulin Dose Adjustment
3.3. Exercise Timing and Strategies
3.4. Data Collection and Analysis
4. Results
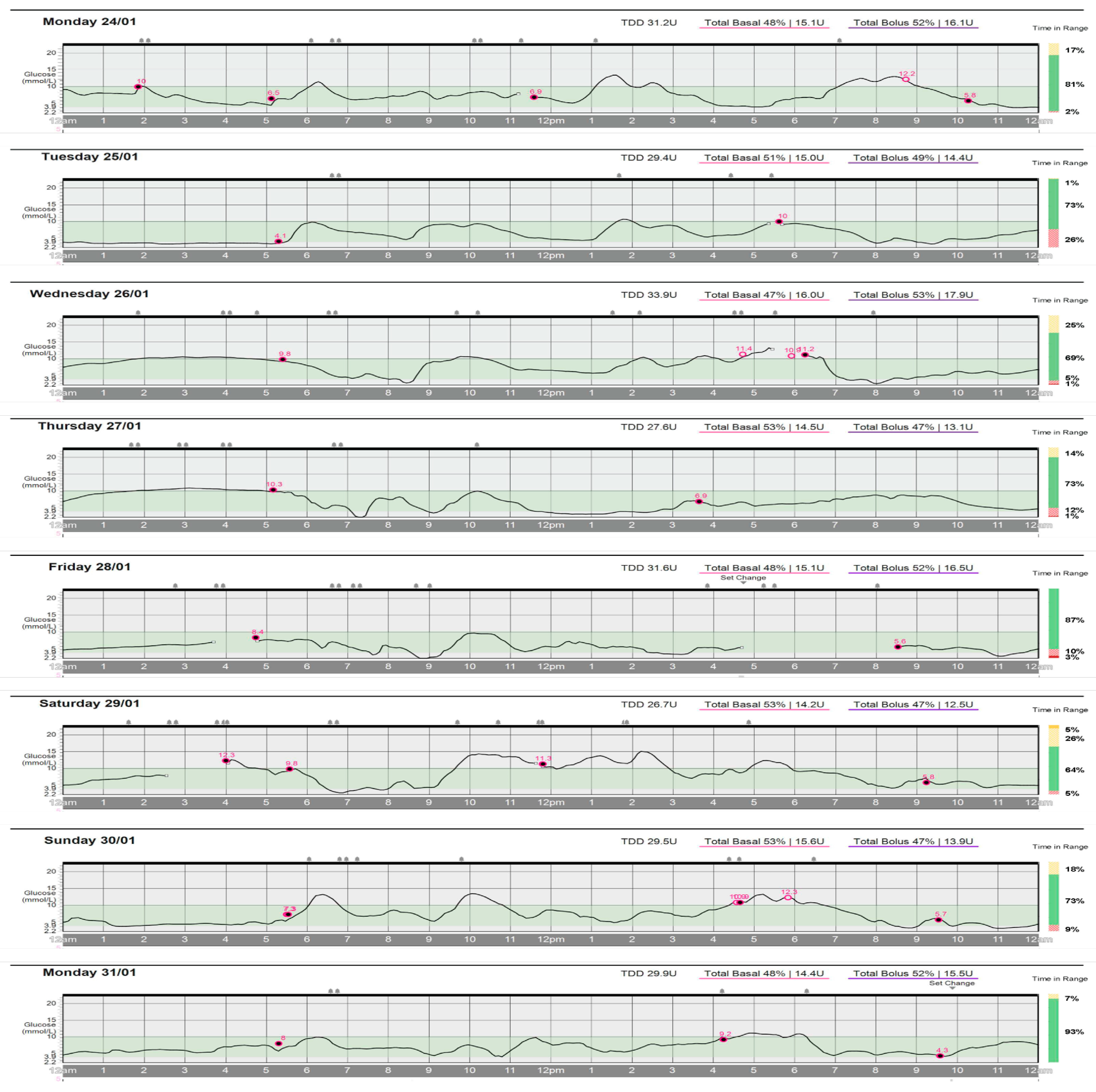
4.1. Daily Glycemic Control
4.2. Reliance on Acute Hypoglycemia Prevention Strategies
4.2.1. Acute Carbohydrate Supplementation
4.2.2. Acute Insulin Reduction
4.2.3. Athletic Performance
4.3. Well-Being
4.4. Recovery Phase Strategies
4.5. Daily Nutrition Program
4.6. 24 h Insulin Adjustments
4.7. Exercise Timing and Strategies
5. Discussion
5.1. Daily Glycemic Control
5.2. Reliance on Acute Hypoglycemia Prevention Strategies
5.2.1. Acute Carbohydrate Supplementation
5.2.2. Acute Insulin Reduction
5.3. Athletic Performance
5.4. Well-Being
5.5. Recovery Phase Strategies
5.5.1. Daily Nutrition Program
5.5.2. 24 h Insulin Adjustments
5.5.3. Exercise Timing and Strategies
5.6. Clinical Implications
5.7. Case Study Limitations
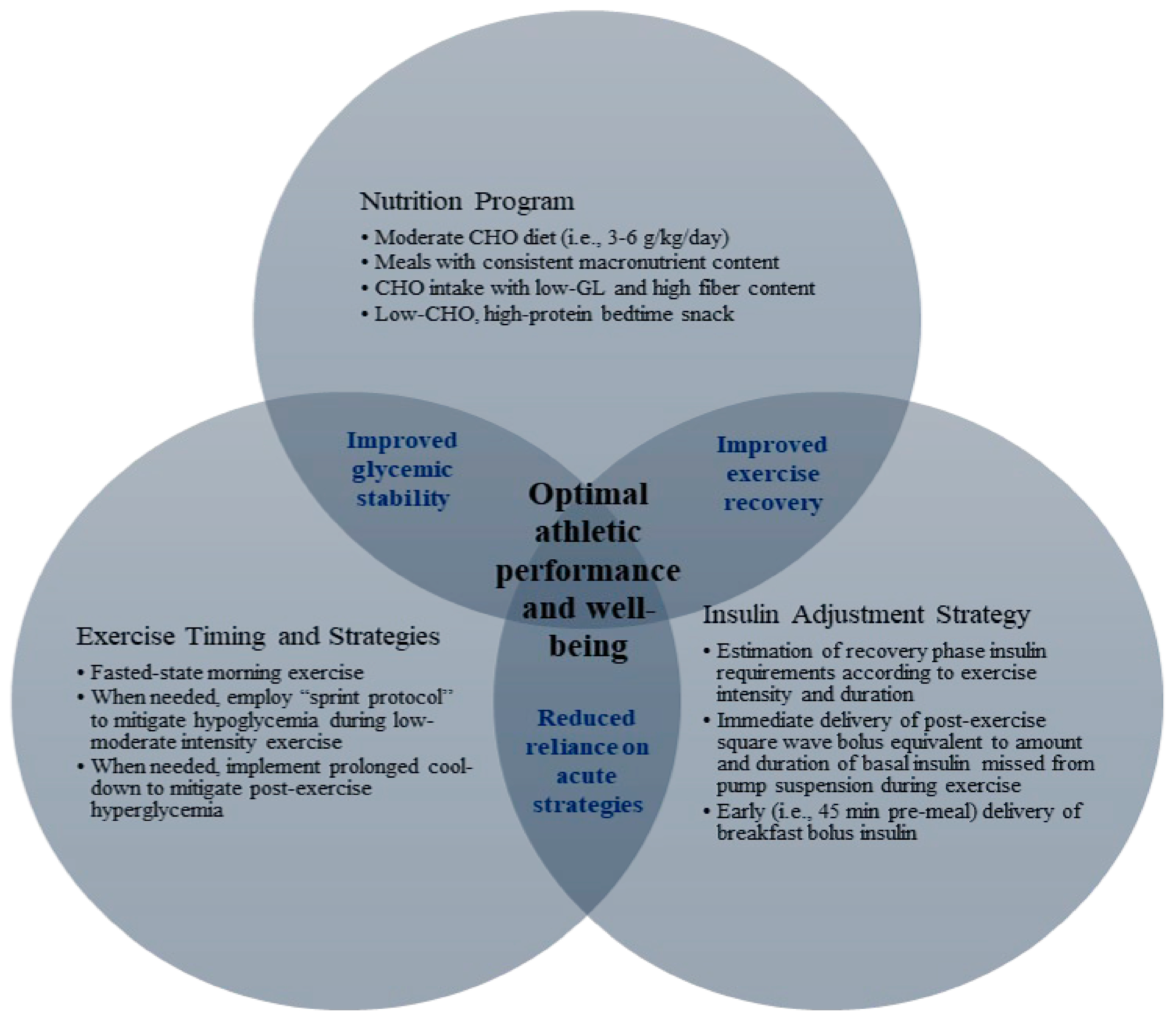
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020, 8, 226–238. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef]
- McCarthy, O.; Pitt, J.; Churm, R.; Dunseath, G.J.; Jones, C.; Bally, L.; Nakas, C.T.; Deere, R.; Eckstein, M.L.; Bain, S.C.; et al. Metabolomic, hormonal and physiological responses to hypoglycemia versus euglycemia during exercise in adults with type 1 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001577. [Google Scholar] [CrossRef]
- Scott, S.N.; Fontana, F.Y.; Cocks, M.; Morton, J.P.; Jeukendrup, A.; Dragulin, R.; Wojtaszewski, J.F.; Jensen, J.; Castol, R.; Riddell, M.C.; et al. Post-exercise recovery for the endurance athlete with type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2021, 9, 304–317. [Google Scholar] [CrossRef]
- Grimm, J.J.; Ybarra, J.; Berné, C.; Muchnick, S.; Golay, A. A new table for prevention of hypoglycaemia during physical activity in type 1 diabetic patients. Diabetes Metab. 2004, 30, 465–470. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; Riddell, M.C. Prevention of exercise-associated dysglycemia: A case study–based approach. Diabetes Spectr. 2015, 28, 55–62. [Google Scholar] [CrossRef]
- Houlder, S.K.; Yardley, J.E. Continuous Glucose Monitoring and Exercise in Type 1 Diabetes: Past, Present and Future. Biosensors 2018, 8, 73. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef]
- Cockcroft, E.J.; Narendran, P.; Andrews, R.C. Exercise-induced hypoglycaemia in type 1 diabetes. Exp. Physiol. 2020, 105, 590–599. [Google Scholar] [CrossRef]
- Paramalingam, N.; Keating, B.L.; Chetty, T.; Fournier, P.A.; Soon, W.H.K.; O’Dea, J.M.; Roberts, A.G.; Horowitz, M.; Jones, T.W.; Davis, E.A. Protein Ingestion in Reducing the Risk of Late-Onset Post-Exercise Hypoglycemia: A Pilot Study in Adolescents and Youth with Type 1 Diabetes. Nutrients 2023, 15, 543. [Google Scholar] [CrossRef]
- She, R.; Al-Sari, N.H.; Mattila, I.M.; Sejling, A.S.; Pedersen, J.; Legido-Quigley, C.; Pedersen-Bjergaard, U. Decreased branched-chain amino acids and elevated fatty acids during antecedent hypoglycemia in type 1 diabetes. BMJ Open Diabetes Res. Care 2023, 11, e003327. [Google Scholar] [CrossRef]
- Riddell, M.C.; Scott, S.N.; Fournier, P.A.; Colberg, S.R.; Gallen, I.W.; Moser, O.; Stettler, C.; Yardley, J.E.; Zaharieva, D.P.; Adolfsson, P.; et al. The competitive athlete with type 1 diabetes. Diabetologia 2020, 63, 1475–1490. [Google Scholar] [CrossRef]
- Rabasa-Lhoret, R.; Bourque, J.; Ducros, F.; Chiasson, J.-L. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (Ultralente-Lispro). Diabetes Care 2001, 24, 625–630. [Google Scholar] [CrossRef]
- Yardley, J.E.; Iscoe, K.E.; Sigal, R.J.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: An observational study of physically active type 1 diabetes patients. Diabetes Technol. Ther. 2013, 15, 84–88. [Google Scholar] [CrossRef]
- O’Neill, B.T.; Bhardwaj, G.; Penniman, C.M.; Krumpoch, M.T.; Suarez Beltran, P.A.; Klaus, K.; Poro, K.; Li, M.; Pan, H.; Dreyfuss, J.M.; et al. FoxO transcription factors are critical regulators of diabetes-related muscle atrophy. Diabetes 2019, 68, 556–570. [Google Scholar] [CrossRef]
- Bally, L.; Buehler, T.; Dokumaci, A.S.; Boesch, C.; Stettler, C. Hepatic and intramyocellular glycogen stores in adults with type 1 diabetes and healthy controls. Diabetes Res. Clin. Pract. 2015, 109, e1–e3. [Google Scholar] [CrossRef]
- Janež, A.; Guja, C.; Mitrakou, A.; Lalic, N.; Tankova, T.; Czupryniak, L.; Tabák, A.G.; Prazny, M.; Martinka, E.; Smircic-Duvnjak, L. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: A Narrative Review. Diabetes Ther. 2020, 11, 387–409. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Burke, L.M. Ketogenic low-CHO, high-fat diet: The future of elite endurance sport? J. Physiol. 2021, 599, 819–843. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, S.; Jendle, J.; Adolfsson, P. Carbohydrate Loading Followed by High Carbohydrate Intake During Prolonged Physical Exercise and Its Impact on Glucose Control in Individuals With Diabetes Type 1-An Exploratory Study. Front Endocrinol 2019, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Murillo, S.; Brugnara, L.; del Campo, E.; Yagüe, I.; Dueñas, B.; Novials, A. Carbohydrate management in athletes with type 1 diabetes in a 10 km run competition. Int. J. Sports Med. 2015, 36, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.N.; Anderson, L.; Morton, J.P.; Wagenmakers, A.J.; Riddell, M.C. Carbohydrate restriction in type 1 diabetes: A realistic therapy for improved glycaemic control and athletic performance? Nutrients 2019, 11, 1022. [Google Scholar] [CrossRef]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef]
- Colberg, S.R. Nutrition and exercise performance in adults with type 1 diabetes. Can. J. Diabetes 2020, 44, 750–758. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Paterson, M.; Bell, K.J.; O’Connell, S.M.; Smart, C.E.; Shafat, A.; King, B. The role of dietary protein and fat in glycaemic control in type 1 diabetes: Implications for intensive diabetes management. Curr. Diab Rep. 2015, 15, 61. [Google Scholar] [CrossRef]
- Campbell, M.D.; Walker, M.; Bracken, R.M.; Turner, D.; Stevenson, E.J.; Gonzalez, J.T.; Shaw, J.A.; West, D.J. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: A randomized controlled trial. BMJ Open Diabetes Res. Care 2015, 3, e000085. [Google Scholar] [CrossRef]
- Yasawy, M.I. The unexpected truth about dates and hypoglycemia. J. Fam. Community Med. 2016, 23, 115–118. [Google Scholar] [CrossRef]
- Bally, L.; Kempf, P.; Zueger, T.; Speck, C.; Pasi, N.; Ciller, C.; Feller, K.; Loher, H.; Rosset, R.; Wilhelm, M.; et al. Metabolic effects of glucose-fructose co-ingestion compared to glucose alone during exercise in type 1 diabetes. Nutrients 2017, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Dietrich, M.; McCarthy, O.; Bracken, R.M.; Eckstein, M.L. Bolus insulin dose depends on previous-day race intensity during 5 days of professional road-cycle racing in athletes with type 1 diabetes: A prospective observational study. Diabetes Obes. Metab. 2020, 22, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care In Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S73–S84. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J. Daniels’ Running Formula; Human Kinetics: Champaign, IL, USA, 2022. [Google Scholar]
- Liguory, G.; Feito, Y.; Fountaine, C.; Roy, B.A. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Philadelphia, PA, USA, 2022; pp. 147–150. [Google Scholar]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020, 50, 1729–1756. [Google Scholar] [CrossRef]
- Lindmeyer, A.M.; Meier, J.J.; Nauck, M.A. Patients with type 1 diabetes treated with insulin pumps need widely heterogeneous basal rate profiles ranging from negligible to pronounced diurnal variability. J. Diabetes Sci. Technol. 2020, 15, 1262–1272. [Google Scholar] [CrossRef]
- Nauck, M.A.; Lindmeyer, A.M.; Mathieu, C.; Meier, J.J. Twenty-four hour fasting (basal rate) tests to achieve custom-tailored, hour-by-hour basal insulin infusion rates in patients with type 1 diabetes using insulin pumps (CSII). J. Diabetes Sci. Technol. 2019, 15, 360–370. [Google Scholar] [CrossRef]
- Scott, S.; Kempf, P.; Bally, L.; Stettler, C. Carbohydrate intake in the context of exercise in people with type 1 diabetes. Nutrients 2019, 11, 3017. [Google Scholar] [CrossRef]
- Gomez, A.M.; Gomez, C.; Aschner, P.; Veloza, A.; Munoz, O.; Rubio, C.; Vallejo, S. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J. Diabetes Sci. Technol. 2015, 9, 619–624. [Google Scholar] [CrossRef]
- Yardley, J.E.; Sigal, R.J. Exercise Strategies for Hypoglycemia Prevention in Individuals with Type 1 Diabetes. Diabetes Spectr. 2015, 28, 32–38. [Google Scholar] [CrossRef]
- Curvey, R.M.G.; Reese, R.J.; Cormier, M.L. Student-Athlete Well-Being Scale. NCAA.org. 2019. Available online: https://www.ncaa.org/about/resources/research/student-athlete-well-being-scale (accessed on 21 July 2022).
- Robinson, D.J.; Coons, M.; Haensel, H.; Vallis, M.; Yale, J.F. Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes and Mental Health. Can. J. Diabetes 2023, 47, 308–344. [Google Scholar] [CrossRef]
- Wisting, L.; Skrivarhaug, T.; Dahl-Jørgensen, K.; Rø, Ø. Prevalence of disturbed eating behavior and associated symptoms of anxiety and depression among adult males and females with type 1 diabetes. J. Eat. Disord. 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.C.; Corcoran, M.H.; Crawley, J.T.; Hornsby, W.G., Jr.; Peer, K.S.; Philbin, R.D.; Riddell, M.C. National Athletic Trainers’ Association position statement: Management of the athlete with type 1 diabetes mellitus. J. Athl. Train. 2007, 42, 536–545. [Google Scholar] [PubMed]
- Yardley, J.E.; Colberg, S.R. Update on Management of Type 1 Diabetes and Type 2 Diabetes in Athletes. Curr. Sports Med. Rep. 2017, 16, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Bergenstal, R.M.; Cheng, P.; Kollman, C.; Carlson, A.L.; Johnson, M.L.; Rodbard, D. The relationships between time in range, hyperglycemia metrics, and HbA1c. J. Diabetes Sci. Technol. 2019, 13, 614–626. [Google Scholar] [CrossRef]
- Runge, A.S.; Kennedy, L.; Brown, A.S.; Dove, A.E.; Levine, B.J.; Koontz, S.P.; Iyengar, V.S.; Odeh, S.A.; Close, K.L.; Hirsch, I.B.; et al. Does time-in-range matter? perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin. Diabetes 2018, 36, 112–119. [Google Scholar] [CrossRef]
- Yardley, J.E. Fasting may alter blood glucose responses to high-intensity interval exercise in adults with type 1 diabetes: A randomized, acute crossover study. Can. J. Diabetes 2020, 44, 727–733. [Google Scholar] [CrossRef]



| Day | ED (min) | EI (%VO2max) | Description |
|---|---|---|---|
| 1 | 100 | 60% | long run |
| 2 | 60 | 65% | easy run |
| 3 | 60 | 60% | easy run |
| 4 | 75 | 55%, 75% | tempo run a |
| 5 | 60 | 55%, 85% | light pre-race workout b |
| 6 | 105 | 60%, 45%, 85% | morning easy run, afternoon race c |
| 7 | 50 | 50% | recovery jog |
| 8 | 100 | > 60% | long run |
| Carbohydrate a | Protein b | Fat c | |
|---|---|---|---|
| Macronutrient Servings | 1/3 cup oats, dry | 1 ounce poultry or beef | 2 TBSP nuts/seeds |
| 1/3 cup brown rice, cooked | 1 ounce fish | 1 TBSP nut/seed butter | |
| 1/2 cup legumes, cooked | 1 large egg | 1 TBSP butter | |
| 1/2 cup quinoa, cooked | 1 cup milk, 1% | 1 TBSP olive oil | |
| 1 cup milk, 1% | 1 cup chocolate milk, 1% | 1/2 avocado, medium | |
| 1/2 cup chocolate milk, 1% | 1/3 cup cottage cheese, 1% | ||
| 1/2 potato, medium | 1/3 cup Greek yogurt, 1% | ||
| 1/2 sweet potato, medium | 1/4 cup feta crumbles | ||
| 1/4 cup low-fat granola | 1/4 cup shredded cheese | ||
| 1 cup berries | 1 ounce sliced cheese | ||
| 1/2 piece of fruit | |||
| 2 TBSP dried fruit | |||
| 1 medjool date | |||
| 1 TBSP honey or maple syrup | |||
| Non-starchy vegetables | |||
| 2 cups leafy greens | |||
| 1 cup raw vegetables |
| Calculation | Example a | |
|---|---|---|
| −%TDD | [%VO2max × (0.75 b × ED)] ÷ 100 = -%TDD | [0.65 × (0.75 × 60)] ÷ 100 = 0.29 (i.e., −29%) |
| TBR | 1 − (-%TDD) = TBR | 1 − 0.29 = 0.71 (i.e., 71%) |
| ICR | 450 c ÷ (TDDB × TBR) = ICR consequent d | 450 ÷ (50 × 0.71) = 12.67 (i.e., 1:13) |
| CF | 94 e ÷ (TDDB × TBR) = CF | 94 ÷ (50 × 0.71) = 2.65 (i.e., 2.7) |
| Carbohydrate Intake (g) | Exercise | Insulin Adjustments | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | Daily | Pre-Exercise | During Exercise | Duration (min) | %VO2max | TBR | ICR | CF | %TIR |
| 1 | 240 | 30 | - | 100 | 60 | 55 | 1:16 | 3.4 | 81 |
| 2 | 240 | 30 | - | 60 | 65 | 65 | 1:13 | 2.7 | 73 |
| 3 | 225 | - | - | 60 | 60 | 70 | 1:13 | 2.6 | 69 |
| 4 | 175 | - | - | 75 | 55, 75 | 55 | 1:15 | 3.2 | 73 |
| 5 | 230 | - | - | 60 | 55, 85 | 70 | 1:12 | 2.6 | 87 |
| 6 | 210 | - | - | 105 | 60, 45, 85 | 60, 55 | 1:16 | 3.4 | 64 |
| 7 | 240 | 20 | - | 50 | 50 | 70, 80 | 1:13 | 2.7 | 73 |
| 8 | 240 | 20 | - | 100 | >60 | 55 | 1:16 | 3.4 | 93 |
| Mean | 225 | 12.5 | 0 | ~75 | >60 | ~77 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeder, A.E.; Rosenkranz, R.R.; Yarrow, L.K.; Haub, M.D.; Rosenkranz, S.K. Recovery Phase Nutrition and Insulin Strategies for a Collegiate Distance Runner with Type 1 Diabetes Mellitus: A Case Study. Sports 2023, 11, 214. https://doi.org/10.3390/sports11110214
Schroeder AE, Rosenkranz RR, Yarrow LK, Haub MD, Rosenkranz SK. Recovery Phase Nutrition and Insulin Strategies for a Collegiate Distance Runner with Type 1 Diabetes Mellitus: A Case Study. Sports. 2023; 11(11):214. https://doi.org/10.3390/sports11110214
Chicago/Turabian StyleSchroeder, Amie E., Richard R. Rosenkranz, Linda K. Yarrow, Mark D. Haub, and Sara K. Rosenkranz. 2023. "Recovery Phase Nutrition and Insulin Strategies for a Collegiate Distance Runner with Type 1 Diabetes Mellitus: A Case Study" Sports 11, no. 11: 214. https://doi.org/10.3390/sports11110214








