Comparison between Match and Training Session on Biomarker Responses in Handball Players
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure (Training and Match)
2.2.1. Training
2.2.2. Match
2.3. Sampling and Handling
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Difference in C Dynamics between Handball Training and Match
4.2. Differences in T Dynamics between Handball Training and Match
4.3. Difference in AA Dynamics between Handball Training and Match
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colby, M.J.; Dawson, B.; Heasman, J.; Rogalski, B.; Gabbett, T.J. Accelerometer and GPS-derived running loads and injury risk in elite Australian footballers. J. Strength Cond. Res. 2014, 28, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.J.; Whyte, D.G.; Hartwig, T.B.; Wescombe, H.; Naughton, G.A. The relationship between workloads, physical performance, injury and illness in adolescent male football players. Sports Med. 2014, 44, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Michalsik, L.B.; Aagaard, P. Physical demands in elite team handball: Comparisons between male and female players. J. Sports Med. Phys. Fit. 2015, 55, 878–891. [Google Scholar]
- Šibila, M.; Vuleta, D.; Pori, P. Razlike u volumnu i intenzitetu cikličnih opterećenja između različitih pozicija u napadu. Position-related differences in volume and intensity of large-scale cyclic movements of male players in handball. Kinesiology 2004, 36, 58–68. [Google Scholar]
- Stajer, V.; Vranes, M.; Ostojic, S.M. Correlation between biomarkers of creatine metabolism and serum indicators of peripheral muscle fatigue during exhaustive exercise in active men. Res. Sports Med. 2020, 28, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Póvoas, S.C.A.; Seabra, A.F.T.; Ascensão, A.A.M.R.; Magalhães, J.; Soares, J.M.C.; Rebelo, A.N.C. Physical and physiological demands of elite team handball. J. Strength Cond. Res. 2012, 26, 3365–3375. [Google Scholar] [CrossRef]
- Gatti, R.; De Palo, E.F. An update: Salivary hormones and physical exercise. Scand. J. Med. Sci. Sports 2011, 21, 157–169. [Google Scholar] [CrossRef]
- Foretić, N.; Nikolovski, Z.; Marić, D.; Perić, R.; Sekulić, D. Analysis of the associations between Salivary cortisol-, alpha-amylase-, and testosterone-responsiveness with the physical contact nature of team handball: A preliminary analysis. J. Sports Med. Phys. Fit. 2022, 27. [Google Scholar] [CrossRef]
- Foretic, N.; Nikolovski, Z.; Peric, I.; Sekulic, D. Testosterone, cortisol and alpha-amylase levels during a handball match; analysis of dynamics and associations. Res. Sports Med. 2020, 28, 360–370. [Google Scholar] [CrossRef]
- Balthazar, C.H.; Garcia, M.C.; Spadari-Bratfisch, R.C. Salivary concentrations of cortisol and testosterone and prediction of performance in a professional triathlon competition. Stress 2012, 15, 495–502. [Google Scholar] [CrossRef]
- Moreira, A.; McGuigan, M.R.; Arruda, A.F.S.; Freitas, C.G.; Aoki, M.S. Monitoring internal load parameters during simulated and official basketball matches. J. Strength Cond. Res. 2012, 26, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.A.; Kivlighan, K.T.; El-Sheikh, M.; Gordis, E.B.; Stroud, L.R. Salivary α-amylase in biobehavioral research: Recent developments and applications. Ann. N. Y. Acad. Sci. 2007, 1098, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Kivlighan, K.T.; Granger, D.A. Salivary α-amylase response to competition: Relation to gender, previous experience, and attitudes. Psychoneuroendocrinology 2006, 31, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Koibuchi, E.; Suzuki, Y. Exercise upregulates salivary amylase in humans (Review). Exp. Ther. Med. 2014, 7, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Nater, U.M.; Rohleder, N.; Schlotz, W.; Ehlert, U.; Kirschbaum, C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 2007, 32, 392–401. [Google Scholar] [CrossRef]
- Moreira, A.; Freitas, C.G.; Nakamura, F.Y.; Drago, G.; Drago, M.; Aoki, M.S. Effect of Match Importance on Salivary Cortisol and Immunoglobulin A Responses in Elite Young Volleyball Players. J. Strength Cond. Res. 2013, 27, 202–207. [Google Scholar] [CrossRef]
- Haneishi, K.; Fry, A.C.; Moore, C.A.; Schilling, B.K.; Li, Y.; Fry, M.D. Cortisol and stress responses during a game and practice in female collegiate soccer players. J. Strength Cond. Res. 2007, 21, 583. [Google Scholar]
- Gonzalez-Bono, E.; Salvador, A.; Serrano, M.A.; Ricarte, J. Testosterone, cortisol, and mood in a sports team competition. Horm. Behav. 1999, 35, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Bruzda-Zwiech, A.; Konieczka, M.; Hilt, A.; Daszkowska, M.; Grzegorczyk, J.; Szczepańska, J. Salivary cortisol, alpha-amylase and immunoglobulin a responses to a morning session of basketball or volleyball training in boys aged 14–18 years. J. Biol. Regul. Homeost. Agents 2017, 31, 105–110. [Google Scholar]
- Elloumi, M.; Maso, F.; Michaux, O.; Robert, A.; Lac, G. Behaviour of saliva cortisol [C], testosterone [T] and the T/C ratio during a rugby match and during the post-competition recovery days. Eur. J. Appl. Physiol. 2003, 90, 23–28. [Google Scholar] [CrossRef]
- Chatzinikolaou, A.; Christoforidis, C.; Avloniti, A.; Draganidis, D.; Jamurtas, A.Z.; Stampoulis, T.; Ermidis, G.; Sovatzidis, A.; Papassotiriou, I.; Kambas, A. A microcycle of inflammation following a team handball game. J. Strength Cond. Res. 2014, 28, 1981–1994. [Google Scholar] [CrossRef]
- Filaire, E.; Duche, P.; Lac, G.; Robert, A. Saliva cortisol, physical exercise and training: Influences of swimming and handball on cortisol concentrations in women. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 274–278. [Google Scholar] [CrossRef]
- Mariscal, G.; Vera, P.; Platero, J.L.; Bodí, F.; de la Rubia Ortí, J.E.; Barrios, C. Changes in different salivary biomarkers related to physiologic stress in elite handball players: The case of females. Sci. Rep. 2019, 9, 19554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karcher, C.; Buchheit, M. On-court demands of elite handball, with special reference to playing positions. Sports Med. 2014, 44, 797–814. [Google Scholar] [CrossRef] [PubMed]
- van Paridon, K.N.; Timmis, M.A.; Nevison, C.M.; Bristow, M. The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport Exerc. Med. 2017, 3, e000261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, R.A.; Beltran, O.A.B.; Zapata, D.M.; Silva, E.; Freitas, W.Z.; Junior, R.V.; da Silva, F.F.; Higino, W.P. Heart rate variability, salivary cortisol and competitive state anxiety responses during pre-competition and pre-training moments. Biol. Sport 2019, 36, 39–46. [Google Scholar] [CrossRef]
- Souglis, A.; Bogdanis, G.C.; Giannopoulou, I.; Papadopoulos, C.; Apostolidis, N. Comparison of Inflammatory Responses and Muscle Damage Indices Following a Soccer, Basketball, Volleyball and Handball Game at an Elite Competitive Level. Res. Sports Med. 2015, 23, 59–72. [Google Scholar] [CrossRef]
- Živanović, N.; Palić, R.; Stanković, V.; Ćirić, M.; Andrašić, S. Effects of speed endurance test on the levels of cortisol and testosterone in handball players. Gymnasium 2011, 12, 31–34. [Google Scholar]
- Barnes, M.J.; Mundel, T.; Stannard, S.R. The effects of acute alcohol consumption on recovery from a simulated rugby match. J. Sports Sci. 2012, 30, 295–304. [Google Scholar] [CrossRef]
- Cunniffe, B.; Hore, A.J.; Whitcombe, D.M.; Jones, K.P.; Baker, J.S.; Davies, B. Time course of changes in immuneoendocrine markers following an international rugby game. Eur. J. Appl. Physiol. 2010, 108, 113–122. [Google Scholar] [CrossRef]
- Gaviglio, C.M.; Osborne, M.; Kelly, V.G.; Kilduff, L.P.; Cook, C.J. Salivary testosterone and cortisol responses to four different rugby training exercise protocols. Eur. J. Sport Sci. 2015, 15, 497–504. [Google Scholar] [CrossRef]
- Aguilar, R.; Jiménez, M.; Alvero-Cruz, J.R. Testosterone, cortisol and anxiety in elite field hockey players. Physiol. Behav. 2013, 119, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Geniole, S.N.; Bird, B.M.; Ruddick, E.L.; Carré, J.M. Effects of competition outcome on testosterone concentrations in humans: An updated meta-analysis. Horm. Behav. 2017, 92, 37–50. [Google Scholar] [CrossRef]
- Ponce-González, J.G.; Olmedillas, H.; Calleja-González, J.; Guerra, B.; Sanchis-Moysi, J. Physical fitness, adiposity and testosterone concentrations are associated to playing position in professional basketballers. Nutr. Hosp. 2015, 31, 2624–2632. [Google Scholar]
- Póvoas, S.C.A.; Ascensão, A.A.M.R.; Magalhães, J.; Seabra, A.F.; Krustrup, P.; Soares, J.M.C.; Rebelo, A.N.C. Physiological demands of elite team handball with special reference to playing position. J. Strength Cond. Res. 2014, 28, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Springham, M.; Williams, S.; Waldron, M.; McLellan, C.; Newton, R.U. Summated training and match load predictors of salivary immunoglobulin-A, alpha-amylase, testosterone, cortisol and T:C profile changes in elite-level professional football players: A longitudinal analysis. Eur. J. Sport Sci. 2022, 22, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Bere, T.; Alonso, J.-M.; Wangensteen, A.; Bakken, A.; Eirale, C.; Dijkstra, H.P.; Ahmed, H.; Bahr, R.; Popovic, N. Injury and illness surveillance during the 24th Men’s Handball World Championship 2015 in Qatar. Br. J. Sports Med. 2015, 49, 1151. [Google Scholar] [CrossRef] [PubMed]
- Gleason, E.D.; Fuxjager, M.J.; Oyegbile, T.O.; Marler, C.A. Testosterone release and social context: When it occurs and why. Front. Neuroendocrinol. 2009, 30, 460–469. [Google Scholar] [CrossRef]
- Kuzawa, C.W.; Georgiev, A.V.; McDade, T.W.; Bechayda, S.A.; Gettler, L.T. Is There a Testosterone Awakening Response in Humans? Adapt. Hum. Behav. Physiol. 2016, 2, 166–183. [Google Scholar] [CrossRef] [Green Version]
- De Pero, R.; Minganti, C.; Cibelli, G.; Cortis, C.; Piacentini, M.F. The Stress of Competing: Cortisol and Amylase Response to Training and Competition. J. Funct. Morphol. Kinesiol. 2021, 6, 5. [Google Scholar] [CrossRef]
- Hudson, J.; Davison, G.; Robinson, P. Psychophysiological and stress responses to competition in team sport coaches: An exploratory study. Scand. J. Med. Sci. Sports 2013, 23, e279–e285. [Google Scholar] [CrossRef] [PubMed]
- Kamarauskas, P.; Conte, D. The effect of basketball matches on salivary markers: A systematic review. Biol. Sport 2022, 39, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Deneen, W.P.; Jones, A.B. Cortisol and Alpha-amylase changes during an Ultra-Running Event. Int. J. Exerc. Sci. 2017, 10, 531–540. [Google Scholar] [PubMed]

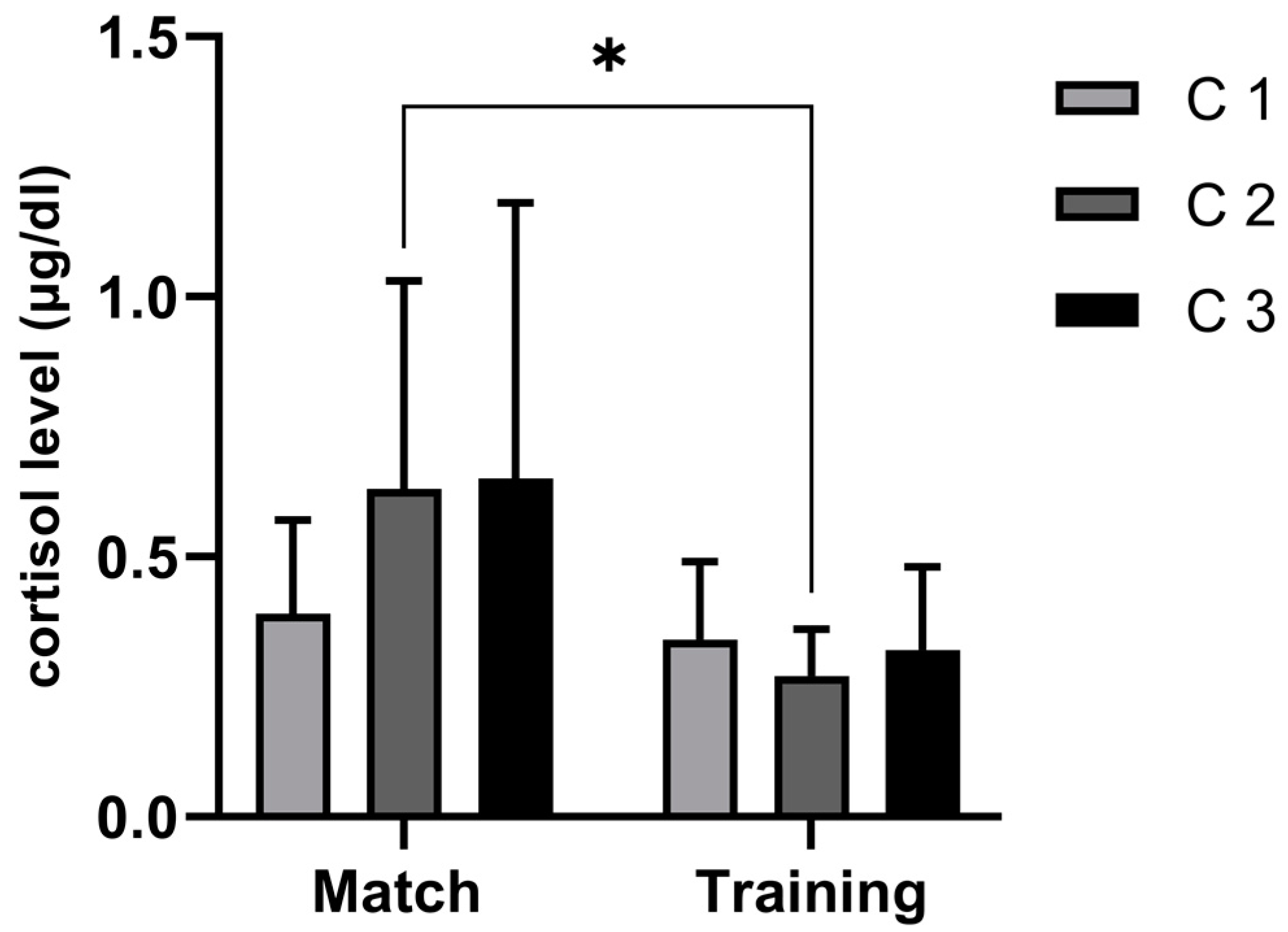
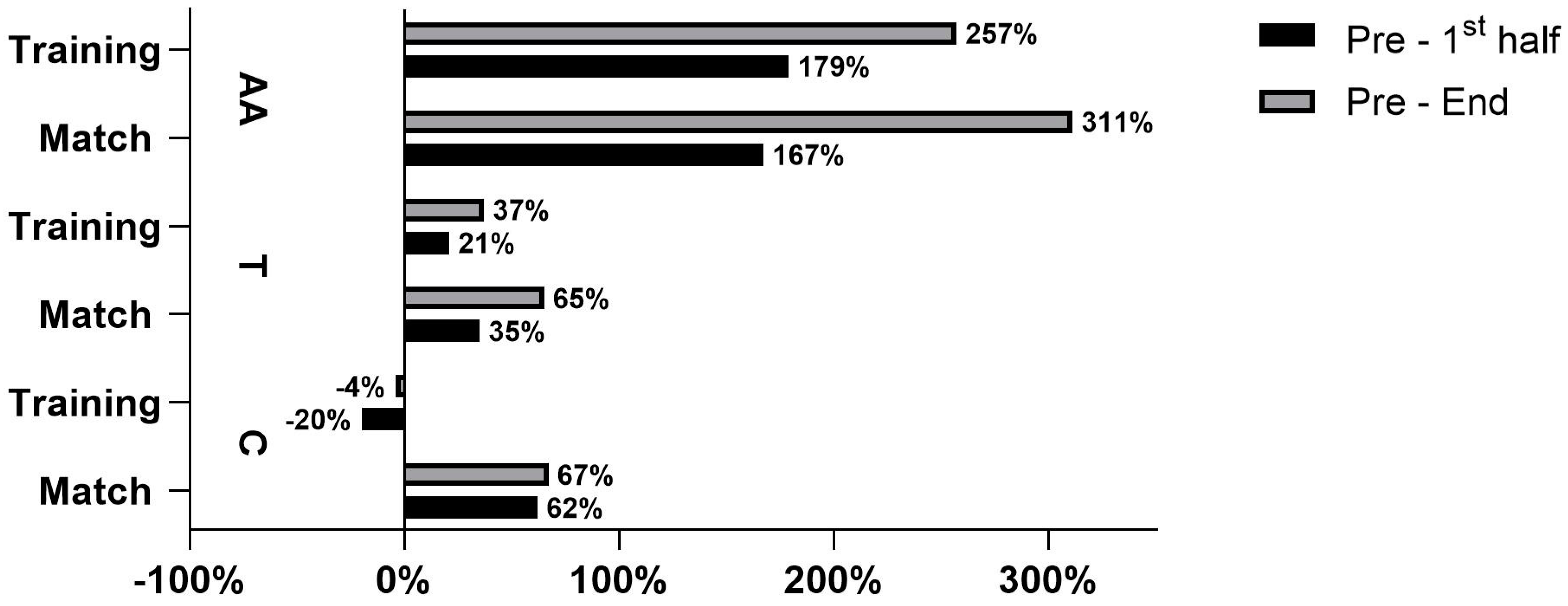
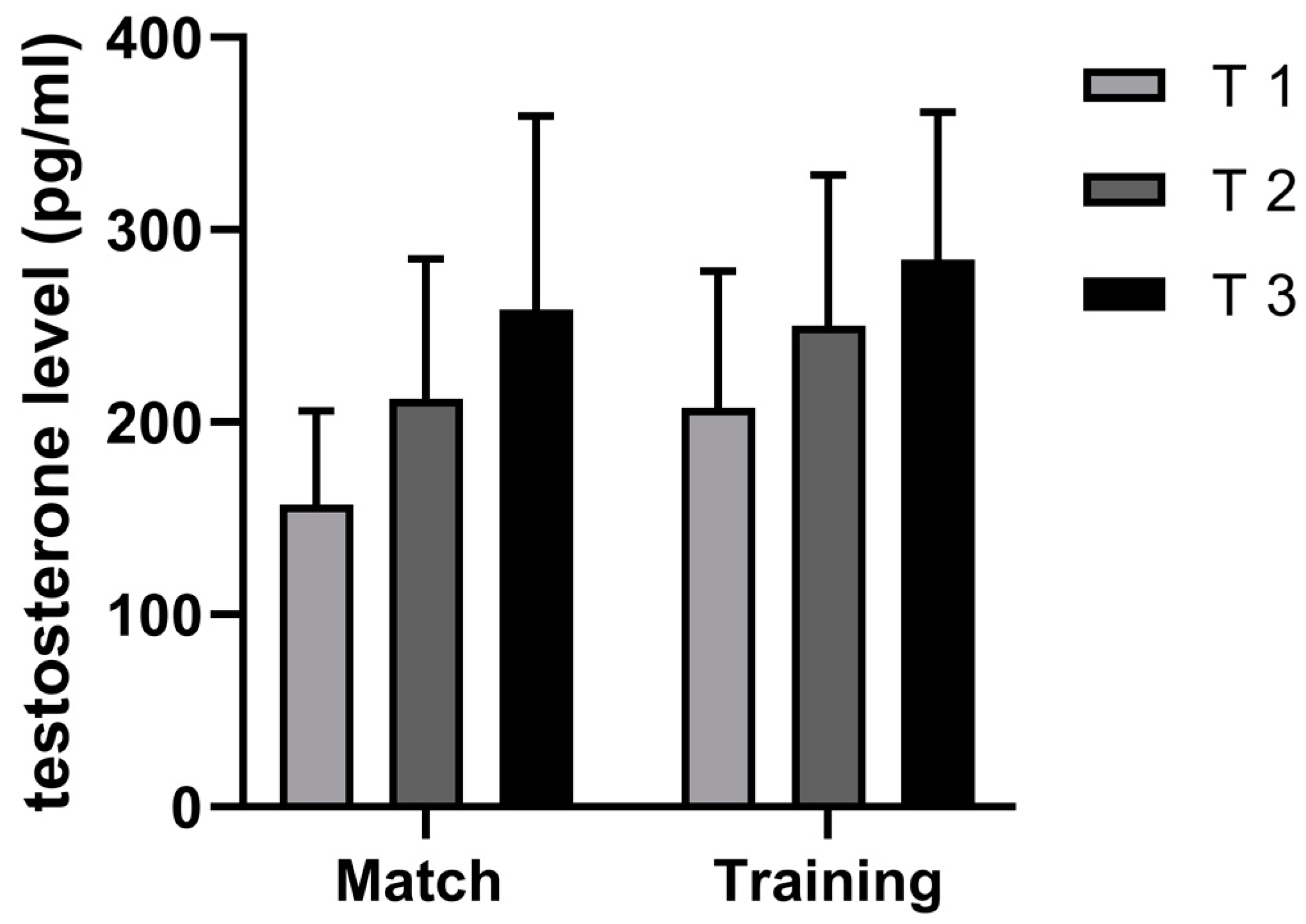
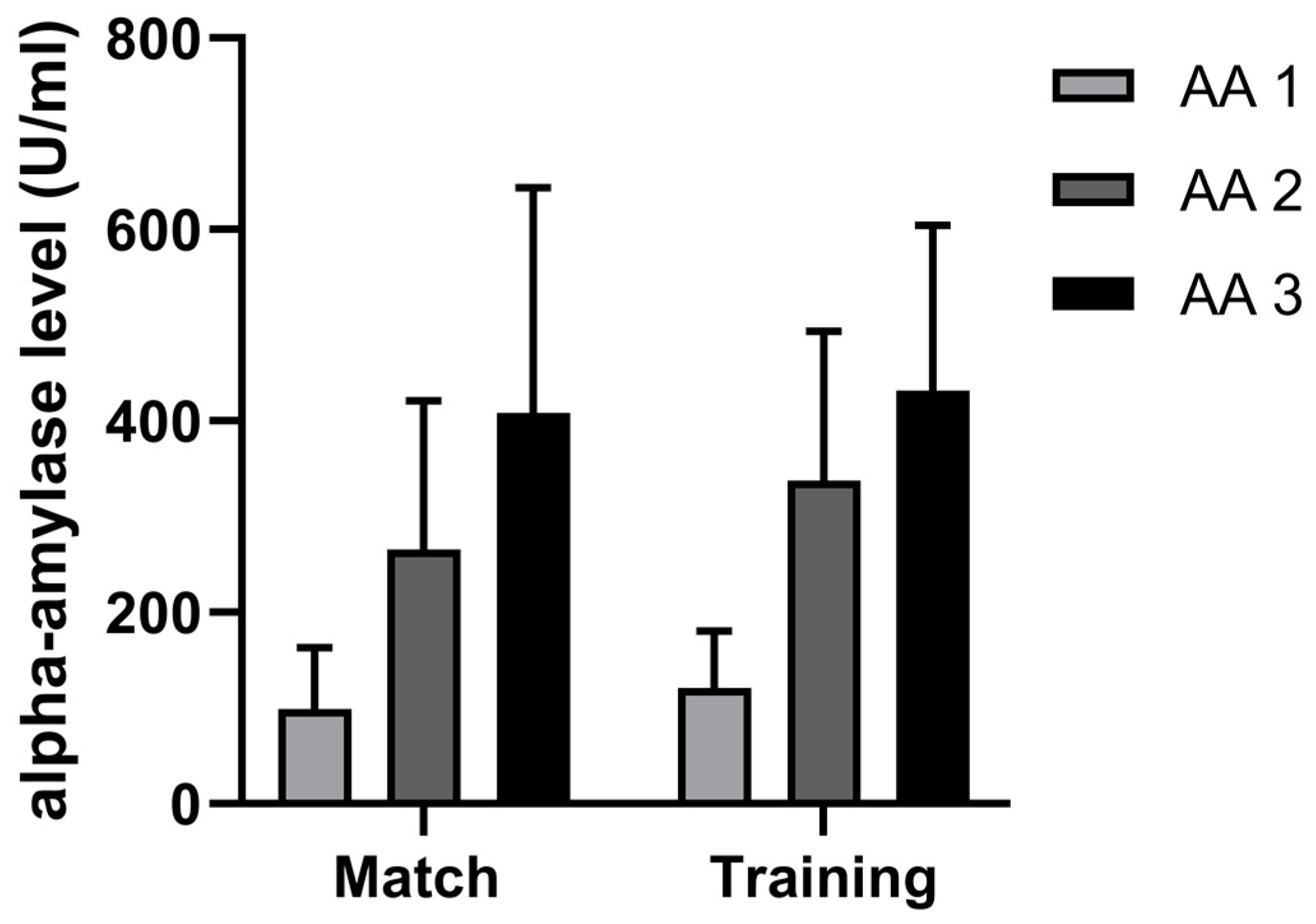
| Variables | Mean | Min | Max | SD | ANOVA | ES | Skew. | Kurt. | Max D | K-S |
|---|---|---|---|---|---|---|---|---|---|---|
| C 1 m | 0.39 | 0.14 | 0.66 | 0.18 | 0.46 | 0.15 | 0.06 | −1.19 | 0.15 | p > 20 |
| C 1 t | 0.34 | 0.23 | 0.75 | 0.15 | 2.52 | 7.01 | 0.29 | p > 20 | ||
| C 2 m | 0.63 | 0.29 | 1.51 | 0.40 | 0.14 | 0.53 * | 1.66 | 1.88 | 0.31 | p > 20 |
| C 2 t | 0.27 | 0.16 | 0.42 | 0.09 | 0.71 | −0.17 | 0.19 | p > 20 | ||
| C 3 m | 0.65 | 0.23 | 1.78 | 0.53 | 0.05 ¥ | 0.39 | 1.52 | 1.26 | 0.29 | p > 20 |
| C 3 t | 0.32 | 0.17 | 0.63 | 0.16 | 0.94 | −0.11 | 0.16 | p > 20 | ||
| T 1 m | 157.06 | 98.84 | 235.89 | 48.93 | 0.00 ¥ | −0.38 | 0.32 | −1.53 | 0.20 | p > 20 |
| T 1 t | 207.44 | 141.33 | 354.53 | 71.02 | 1.33 | 0.92 | 0.29 | p > 20 | ||
| T 2 m | 212.02 | 139.09 | 340.19 | 72.75 | 0.17 | −0.24 | 0.83 | −0.65 | 0.21 | p > 20 |
| T 2 t | 250.12 | 145.10 | 402.72 | 78.43 | 0.83 | 0.52 | 0.24 | p > 20 | ||
| T 3 m | 258.43 | 106.90 | 420.81 | 100.70 | 0.43 | −0.14 | 0.03 | −1.15 | 0.19 | p > 20 |
| T 3 t | 284.57 | 181.92 | 430.36 | 76.62 | 0.37 | 0.03 | 0.12 | p > 20 | ||
| AA 1 m | 99.29 | 37.26 | 225.17 | 63.55 | 0.37 | −0.17 | 1.11 | 0.02 | 0.26 | p > 20 |
| AA 1 t | 121.09 | 43.92 | 215.33 | 59.08 | 0.18 | −1.27 | 0.14 | p > 20 | ||
| AA 2 m | 265.35 | 96.60 | 609.22 | 155.83 | 0.14 | −0.23 | 1.38 | 2.38 | 0.21 | p > 20 |
| AA 2 t | 337.55 | 144.09 | 667.18 | 156.06 | 0.86 | 0.95 | 0.16 | p > 20 | ||
| AA 3 m | 408.53 | 114.50 | 853.79 | 235.38 | 0.77 | −0.06 | 0.61 | −0.24 | 0.18 | p > 20 |
| AA 3 t | 431.94 | 134.55 | 688.64 | 172.06 | −0.25 | −0.74 | 0.17 | p > 20 | ||
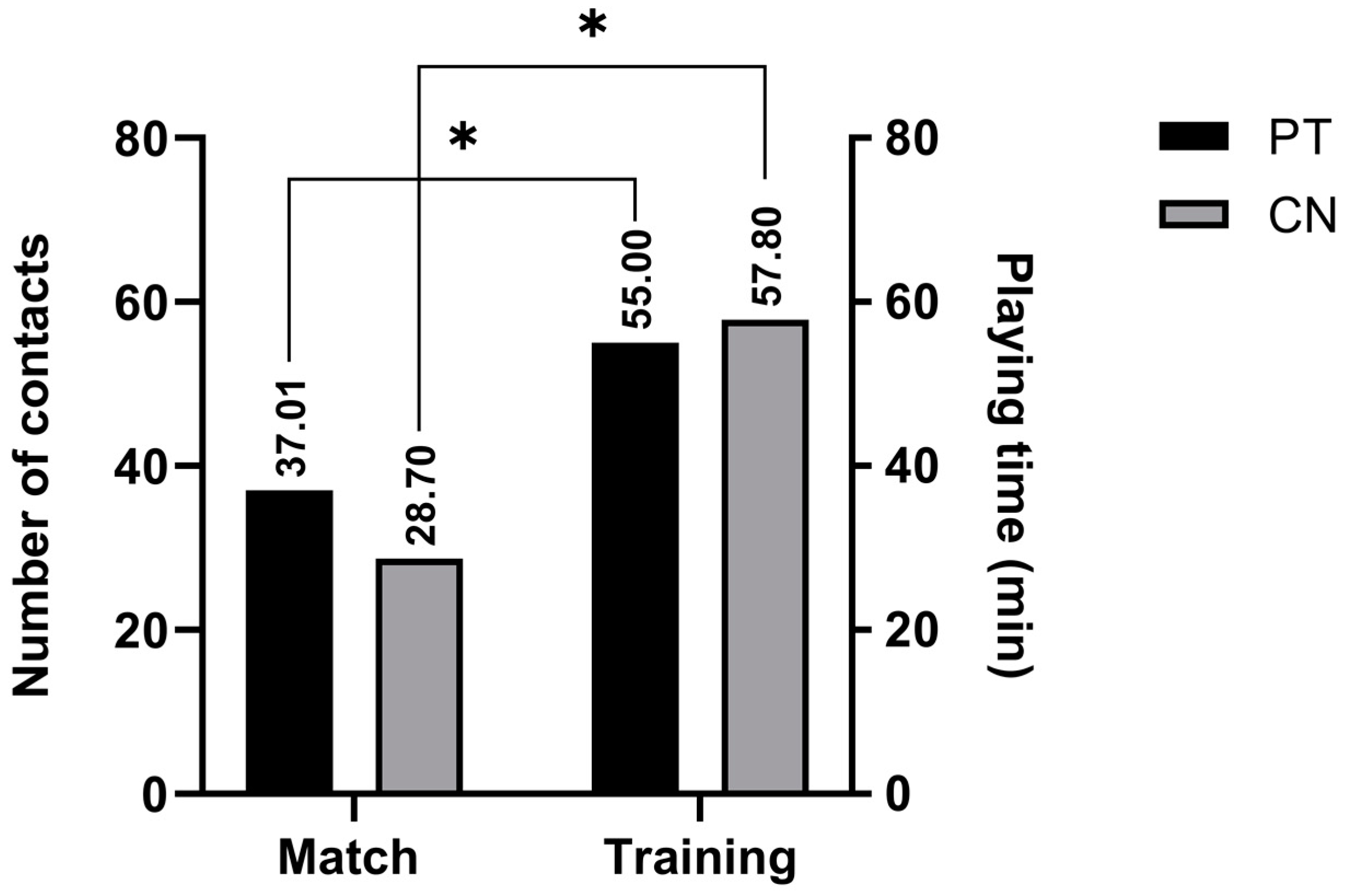
| PT m | 37.01 | 14.17 | 53.08 | 11.86 | 0.00 ¥ | −0.73 * | −0.51 | 0.29 | 0.17 | p > 20 |
| PT t | 55.00 | 55.00 | 55.00 | 0.00 | - | - | 1.00 | p < 01 | ||
| Total CN m | 28.70 | 4.00 | 65.00 | 20.69 | 0.00 ¥ | −0.69 * | 0.43 | −1.05 | 0.17 | p > 20 |
| Total CN t | 57.80 | 50.00 | 64.00 | 5.33 | −0.26 | −1.86 | 0.22 | p > 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolovski, Z.; Foretić, N.; Vrdoljak, D.; Marić, D.; Perić, M. Comparison between Match and Training Session on Biomarker Responses in Handball Players. Sports 2023, 11, 83. https://doi.org/10.3390/sports11040083
Nikolovski Z, Foretić N, Vrdoljak D, Marić D, Perić M. Comparison between Match and Training Session on Biomarker Responses in Handball Players. Sports. 2023; 11(4):83. https://doi.org/10.3390/sports11040083
Chicago/Turabian StyleNikolovski, Zoran, Nikola Foretić, Dario Vrdoljak, Dora Marić, and Mia Perić. 2023. "Comparison between Match and Training Session on Biomarker Responses in Handball Players" Sports 11, no. 4: 83. https://doi.org/10.3390/sports11040083





