The Role of Concussion History and Biological Sex on Pupillary Light Reflex Metrics in Adolescent Rugby Players: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection
2.2.1. Demographics/Medical History
2.2.2. Pupillary Light Reflex Metrics
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCrory, P.; Meeuwisse, W.; Dvořák, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef]
- Jon, S.P.; Kathryn, J.S.; Jiri, D.; Osman Hassan, A.; Cheri, B.; Robert, C.C.; Gavin, A.D.; Ruben, J.E.; Michael, M.; Michael, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport–Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695. [Google Scholar] [CrossRef]
- Broglio, S.P.; McAllister, T.; Katz, B.P.; LaPradd, M.; Zhou, W.; McCrea, M.A.; Hoy, A.; Hazzard, J.B.; Kelly, L.A.; DiFiori, J.; et al. The Natural History of Sport-Related Concussion in Collegiate Athletes: Findings from the NCAA-DoD CARE Consortium. Sports Med. 2022, 52, 403–415. [Google Scholar] [CrossRef]
- Rafferty, J.; Ranson, C.; Oatley, G.; Mostafa, M.; Mathema, P.; Crick, T.; Moore, I.S. On average, a professional rugby union player is more likely than not to sustain a concussion after 25 matches. Br. J. Sports Med. 2019, 53, 969. [Google Scholar] [CrossRef]
- Archbold, H.A.; Rankin, A.T.; Webb, M.; Nicholas, R.; Eames, N.W.; Wilson, R.K.; Henderson, L.A.; Heyes, G.J.; Bleakley, C.M. RISUS study: Rugby Injury Surveillance in Ulster Schools. Br. J. Sports Med. 2017, 51, 600–606. [Google Scholar] [CrossRef]
- Zemek, R.; Barrowman, N.; Freedman, S.B.; Gravel, J.; Gagnon, I.; McGahern, C.; Aglipay, M.; Sangha, G.; Boutis, K.; Beer, D.; et al. Clinical Risk Score for Persistent Postconcussion Symptoms among Children with Acute Concussion in the ED. JAMA 2016, 315, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Master, C.; Master, S.; Wiebe, D.; Storey, E.; Lockyer, J.; Podolak, O.; Grady, M. Vision and Vestibular System Dysfunction Predicts Prolonged Concussion Recovery in Children. Clin. J. Sport Med. 2018, 28, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.; Womble, M.N.; Frascoia, C.; Eagle, S.R.; Covassin, T.; Kontos, A.P.; Collins, M.W.; Elbin, R.J. Sex Differences on the Concussion Clinical Profiles Screening in Adolescents With Sport-Related Concussion. J. Athl. Train. 2022, 58, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.; Johnston, W.; Bleakley, C.M.; Davies, R.J.; Rankin, A.T.; Webb, M.; Caulfield, B.C.; Archbold, H.A.P. Concussion History and Balance Performance in Adolescent Rugby Union Players. Am. J. Sports Med. 2021, 49, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.L.; Yarbrough, M.B.; Perez, J.; Evans, K.; Buckley, T. Sport-related concussion induces transient cardiovascular autonomic dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R575–R584. [Google Scholar] [CrossRef]
- Pertab, J.L.; Merkley, T.L.; Cramond, A.J.; Cramond, K.; Paxton, H.; Wu, T. Concussion and the autonomic nervous system: An introduction to the field and the results of a systematic review. NeuroRehabilitation 2018, 42, 397–427. [Google Scholar] [CrossRef] [PubMed]
- Esterov, D.; Greenwald, B.D. Autonomic Dysfunction after Mild Traumatic Brain Injury. Brain Sci. 2017, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Filipe, J.A.; Falcao-Reis, F.; Castro-Correia, J.; Barros, H. Assessment of autonomic function in high level athletes by pupillometry. Auton. Neurosci. 2003, 104, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Rickmann, A.; Waizel, M.; Kazerounian, S.; Szurman, P.; Wilhelm, H.; Boden, K.T. Digital Pupillometry in Normal Subjects. Neuro-Ophthalmology 2016, 41, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, K.J.; Joshi, N.R.; Truong, J.Q. Understanding the effects of mild traumatic brain injury on the pupillary light reflex. Concussion 2017, 2, CNC36. [Google Scholar] [CrossRef] [PubMed]
- Capó-Aponte, J.E.; Beltran, T.A.; Walsh, D.V.; Cole, W.R.; Dumayas, J.Y. Validation of Visual Objective Biomarkers for Acute Concussion. Mil. Med. 2018, 183, 9–17. [Google Scholar] [CrossRef]
- Carrick, F.A.-O.; Azzolino, S.F.; Hunfalvay, M.; Pagnacco, G.; Oggero, E.; D’Arcy, R.C.N.; Abdulrahman, M.A.-O.; Sugaya, K.A.-O. The Pupillary Light Reflex as a Biomarker of Concussion. Life 2021, 11, 1104. [Google Scholar] [CrossRef]
- Boev, A.N.; Fountas, K.N.; Karampelas, I.; Boev, C.; Machinis, T.G.; Feltes, C.; Okosun, I.; Dimopoulos, V.; Troup, C. Quantitative pupillometry: Normative data in healthy pediatric volunteers. J. Neurosurg. 2005, 103, 496–500. [Google Scholar] [CrossRef]
- Winston, M.; Zhou, A.; Rand, C.M.; Dunne, E.C.; Warner, J.J.; Volpe, L.J.; Pigneri, B.A.; Simon, D.; Bielawiec, T.; Gordon, S.C.; et al. Pupillometry measures of autonomic nervous system regulation with advancing age in a healthy pediatric cohort. Clin. Auton. Res. 2020, 30, 43–51. [Google Scholar] [CrossRef]
- Aderman, M.J.; Meister, M.R.; Roach, M.H.; Dengler, B.A.; Ross, J.D.; Malvasi, S.R.; Cameron, K.L. Normative Values for Pupillary Light Reflex Metrics Among Healthy Service Academy Cadets. Mil. Med. 2023, usad271. [Google Scholar] [CrossRef]
- Master, C.L.; Podolak, O.E.; Ciuffreda, K.J.; Metzger, K.B.; Joshi, N.R.; McDonald, C.C.; Margulies, S.S.; Grady, M.F.; Arbogast, K.B. Utility of Pupillary Light Reflex Metrics as a Physiologic Biomarker for Adolescent Sport-Related Concussion. JAMA Ophthalmol. 2020, 138, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Connor Shane, M.; Mark, M.; Alan, R.; Chris, B. Multisystem recovery after sport-related concussion in adolescent rugby players: A prospective study protocol. BMJ Open 2023, 13, e073677. [Google Scholar] [CrossRef]
- Archbold, P.; Rankin, A.; Webb, M.; Davies, R.; Nicholas, R.; Eames, N.; Wilson, R.; Vincent, J.; McKeever, D.; Duddy, K.; et al. Injury patterns in U15 rugby players in Ulster Schools: A Rugby Injury Surveillance (RISUS) study. Transl. Sports Med. 2021, 4, 524–533. [Google Scholar] [CrossRef]
- Fuller, C.W.; Molloy, M.G.; Bagate, C.; Bahr, R.; Brooks, J.H.; Donson, H.; Kemp, S.P.T.; McCrory, P.; McIntosh, A.S.; Meeuwisse, W.H.; et al. Consensus statement on injury definitions and data collection procedures for studies of injuries in rugby union. Br. J. Sports Med. 2007, 41, 328–331. [Google Scholar] [CrossRef]
- Schmidt, J.D.; Register-Mihalik, J.K.; Mihalik, J.P.; Kerr, Z.Y.; Guskiewicz, K.M. Identifying Impairments after concussion: Normative data versus individualized baselines. Med. Sci. Sports Exerc. 2012, 44, 1621–1628. [Google Scholar] [CrossRef]
- Mayer, A.R.; Wertz, C.; Ryman, S.G.; Storey, E.P.; Park, G.; Phillips, J.; Dodd, A.B.; Oglesbee, S.; Campbell, R.; Yeo, R.A.; et al. Neurosensory Deficits Vary as a Function of Point of Care in Pediatric Mild Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1178–1184. [Google Scholar] [CrossRef]
- Lovell, M.R.; Iverson, G.L.; Collins, M.W.; Podell, K.; Johnston, K.M.; Pardini, D.; Pardini, J.; Norwig, J.; Maroon, J.C. Measurement of symptoms following sports-related concussion: Reliability and normative data for the post-concussion scale. Appl. Neuropsychol. 2006, 13, 166–174. [Google Scholar] [CrossRef]
- Moran, R.N.; Covassin, T.; Elbin, R.J.; Gould, D.; Nogle, S. Reliability and Normative Reference Values for the Vestibular/Ocular Motor Screening (VOMS) Tool in Youth Athletes. Am. J. Sports Med. 2018, 46, 1475–1480. [Google Scholar] [CrossRef]
- Ishikawa, M. Clinical factors affecting pupillary light reflex parameters: A single-centre, cross-sectional study. Ophthalmic Physiol. Opt. 2021, 41, 952–960. [Google Scholar] [CrossRef]
- Lynch, G.A.-O. Using Pupillometry to Assess the Atypical Pupillary Light Reflex and LC-NE System in ASD. Behav. Sci. 2018, 8, 108. [Google Scholar] [CrossRef]
- Sanchis-Gimeno, J.A.; Sanchez-Zuriaga, D.; Martinez-Soriano, F. White-to-white corneal diameter, pupil diameter, central corneal thickness and thinnest corneal thickness values of emmetropic subjects. Surg. Radiol. Anat. 2012, 34, 167–170. [Google Scholar] [CrossRef]
- Fan, X.; Yao, G. Modeling transient pupillary light reflex induced by a short light flash. IEEE Trans. Biomed. Eng. 2010, 58, 36–42. [Google Scholar]
- Kahneman, D.; Tursky, B.; Shapiro, D.; Crider, A. Pupillary, heart rate, and skin resistance changes during a mental task. J. Exp. Psychol. 1969, 79, 164. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef]
- Winn, B.; Whitaker, D.; Elliott, D.B.; Phillips, N.J. Factors affecting light-adapted pupil size in normal human subjects. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1132–1137. [Google Scholar]
- Bradley, M.M.; Miccoli, L.; Escrig, M.A.; Lang, P.J. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 2008, 45, 602–607. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Ciuffreda, K.J. Pupillary responses to light in chronic non-blast-induced mTBI. Brain Inj. 2015, 29, 1420–1425. [Google Scholar] [CrossRef]
- Kiel, M.A.-O.X.; Grabitz, S.D.; Hopf, S.; Koeck, T.; Wild, P.S.; Schmidtmann, I.; Lackner, K.J.; Münzel, T.; Beutel, M.E.; Pfeiffer, N.; et al. Distribution of Pupil Size and Associated Factors: Results from the Population-Based Gutenberg Health Study. J. Ophthalmol. 2022, 2022, 9520512. [Google Scholar] [CrossRef]
- Sharma, S.; Baskaran, M.; Rukmini, A.V.; Nongpiur, M.E.; Htoon, H.; Cheng, C.Y.; Perera, S.A.; Gooley, J.J.; Aung, T.; Milea, D. Factors influencing the pupillary light reflex in healthy individuals. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1353–1359. [Google Scholar] [CrossRef]

| Initial/Max. Pupil Diameter | Steady State of the Pupil Size before the Light Stimulus |
|---|---|
| End/Min. Pupil Diameter | Pupil size after the maximum constriction in response to the light stimulus |
| Latency | Time to maximum constriction in response to the light stimulus |
| Average Constriction Velocity | Average speed of pupil constriction in response to the light stimulus |
| Maximum Constriction Velocity | Maximum speed of pupil constriction in response to the light stimulus |
| Average Dilation Velocity | Average speed of pupil dilation after light stimulus |
| T75 | Time for pupil re-dilation from min. diameter to 75% max. diameter |
| PLR Metrics | No Concussion Hx | Concussion Hx | p Value | ||||
|---|---|---|---|---|---|---|---|
| Male (n = 59) | Female (n = 27) | Male (n = 54) | Female (n = 9) | Sex | Concussion History | Sex * Concussion History | |
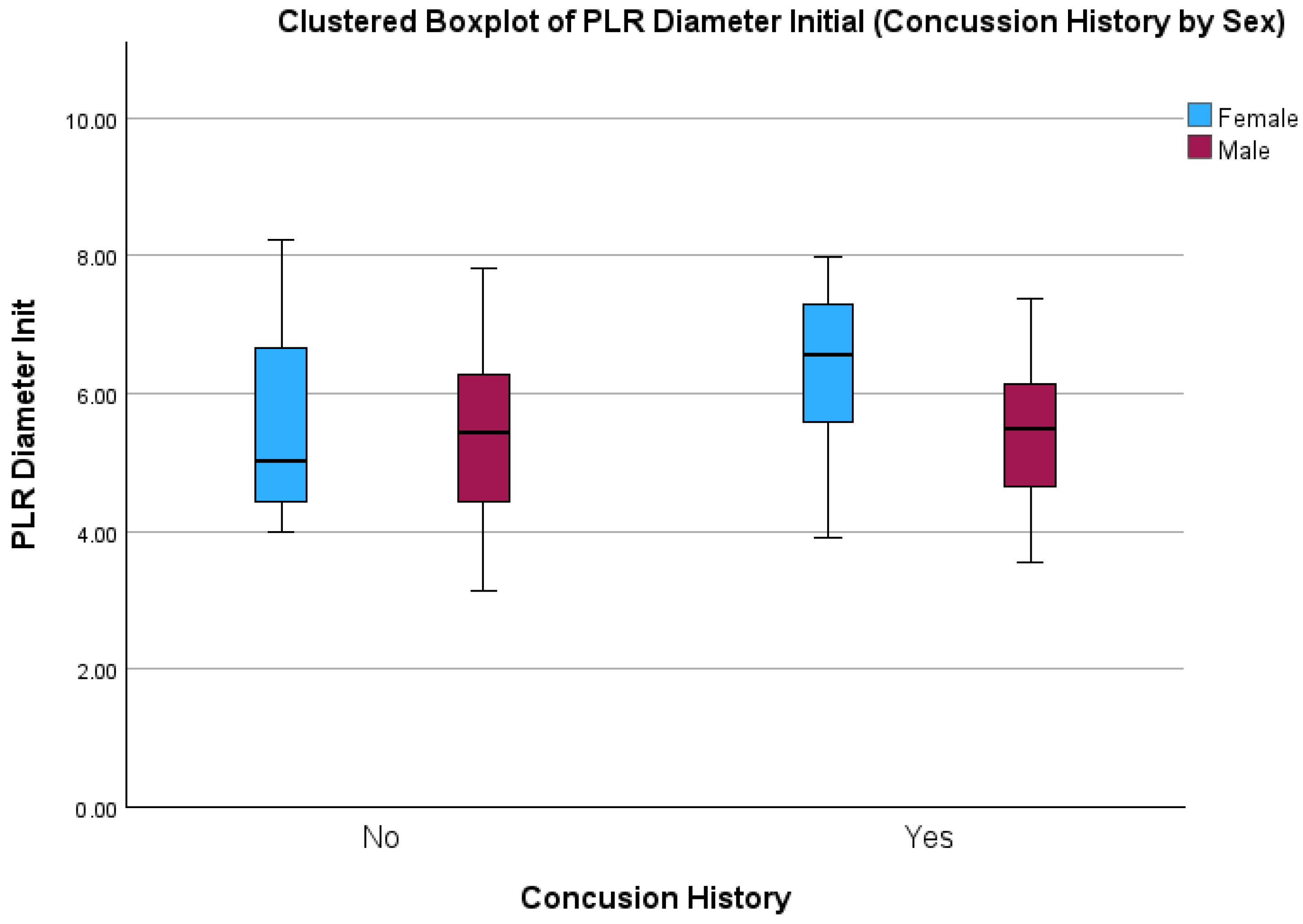
| Initial Pupil Diameter (mm) | 5.35 (±1.13) | 5.51 (±1.26) | 5.40 (±0.92) | 6.22 (±1.42) | 0.04 * | 0.11 | 0.17 |
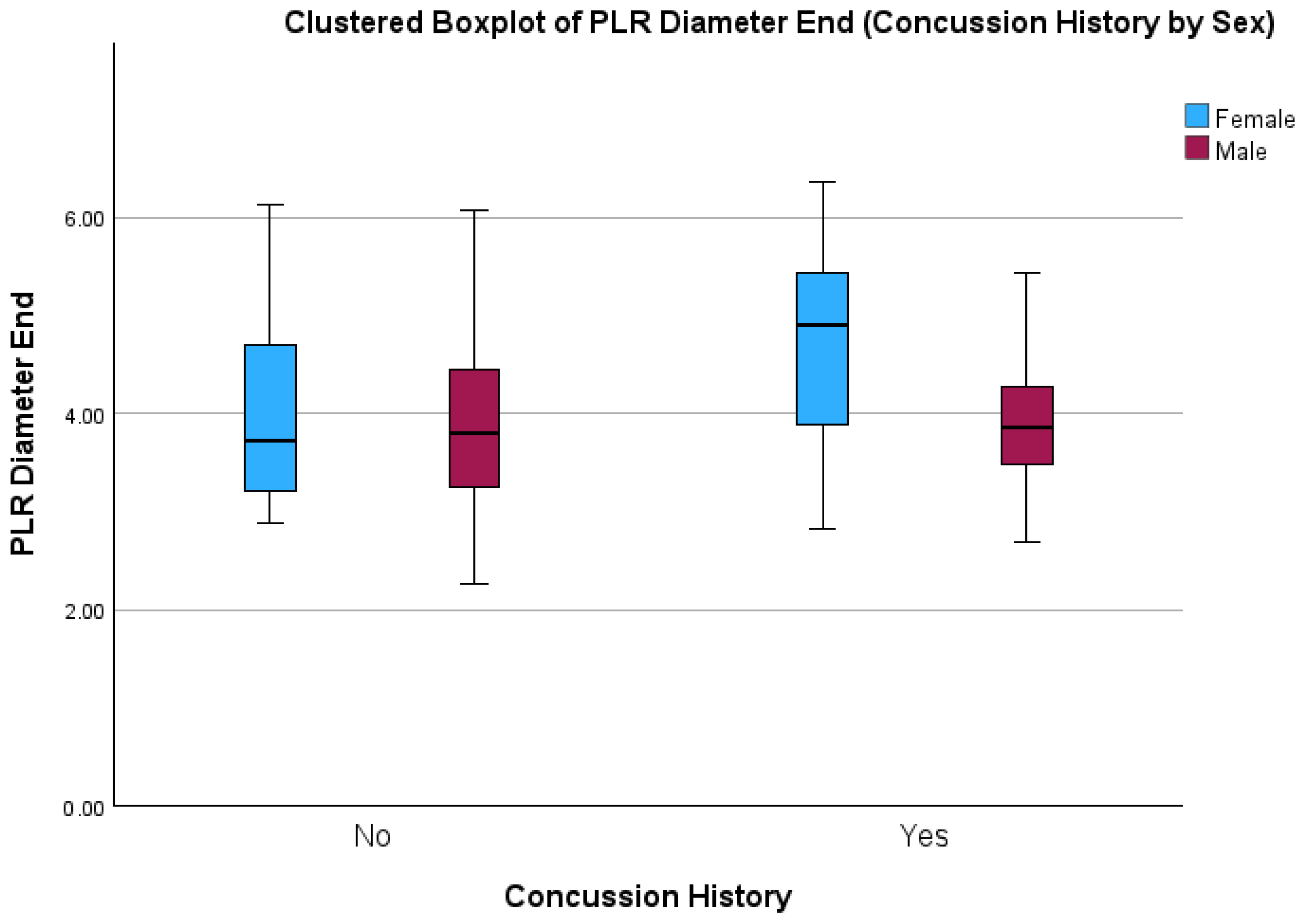
| End Pupil Diameter (mm) | 3.87 (±0.88) | 3.96 (±0.91) | 3.91 (±0.63) | 4.64 (±1.20) | 0.02 * | 0.05 | 0.08 |
| Latency (sec) | 0.21 (±0.02) | 0.21 (±0.02) | 0.22 (±0.02) | 0.21 (±0.02) | 0.30 | 0.50 | 0.90 |
| Average Constriction Velocity (mm/sec) | 3.78 (±0.63) | 3.79 (±0.54) | 3.75 (±0.67) | 3.92 (±0.45) | 0.50 | 0.72 | 0.55 |
| Max Constriction Velocity (mm/sec) | 4.86 (±0.96) | 4.87 (±0.74) | 4.72 (±0.9) | 4.99 (±0.71) | 0.48 | 0.96 | 0.49 |
| Average Dilation velocity (mm/sec) | 1.37 (±0.24) | 1.39 (±0.21) | 1.32 (±0.23) | 1.41 (±0.20) | 0.30 | 0.76 | 0.49 |
| T75 (sec) | 1.36 (±0.56) | 1.38 (±0.65) | 1.36 (±0.57) | 1.68 (±0.69) | 0.18 | 0.23 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKee, C.; Matthews, M.; Rankin, A.; Bleakley, C. The Role of Concussion History and Biological Sex on Pupillary Light Reflex Metrics in Adolescent Rugby Players: A Cross-Sectional Study. Sports 2024, 12, 56. https://doi.org/10.3390/sports12020056
McKee C, Matthews M, Rankin A, Bleakley C. The Role of Concussion History and Biological Sex on Pupillary Light Reflex Metrics in Adolescent Rugby Players: A Cross-Sectional Study. Sports. 2024; 12(2):56. https://doi.org/10.3390/sports12020056
Chicago/Turabian StyleMcKee, Connor, Mark Matthews, Alan Rankin, and Chris Bleakley. 2024. "The Role of Concussion History and Biological Sex on Pupillary Light Reflex Metrics in Adolescent Rugby Players: A Cross-Sectional Study" Sports 12, no. 2: 56. https://doi.org/10.3390/sports12020056





