Corrosion Behaviour of Mg98.5Nd1Zn0.5 (at. %) Alloy in Phosphate Buffered Saline Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
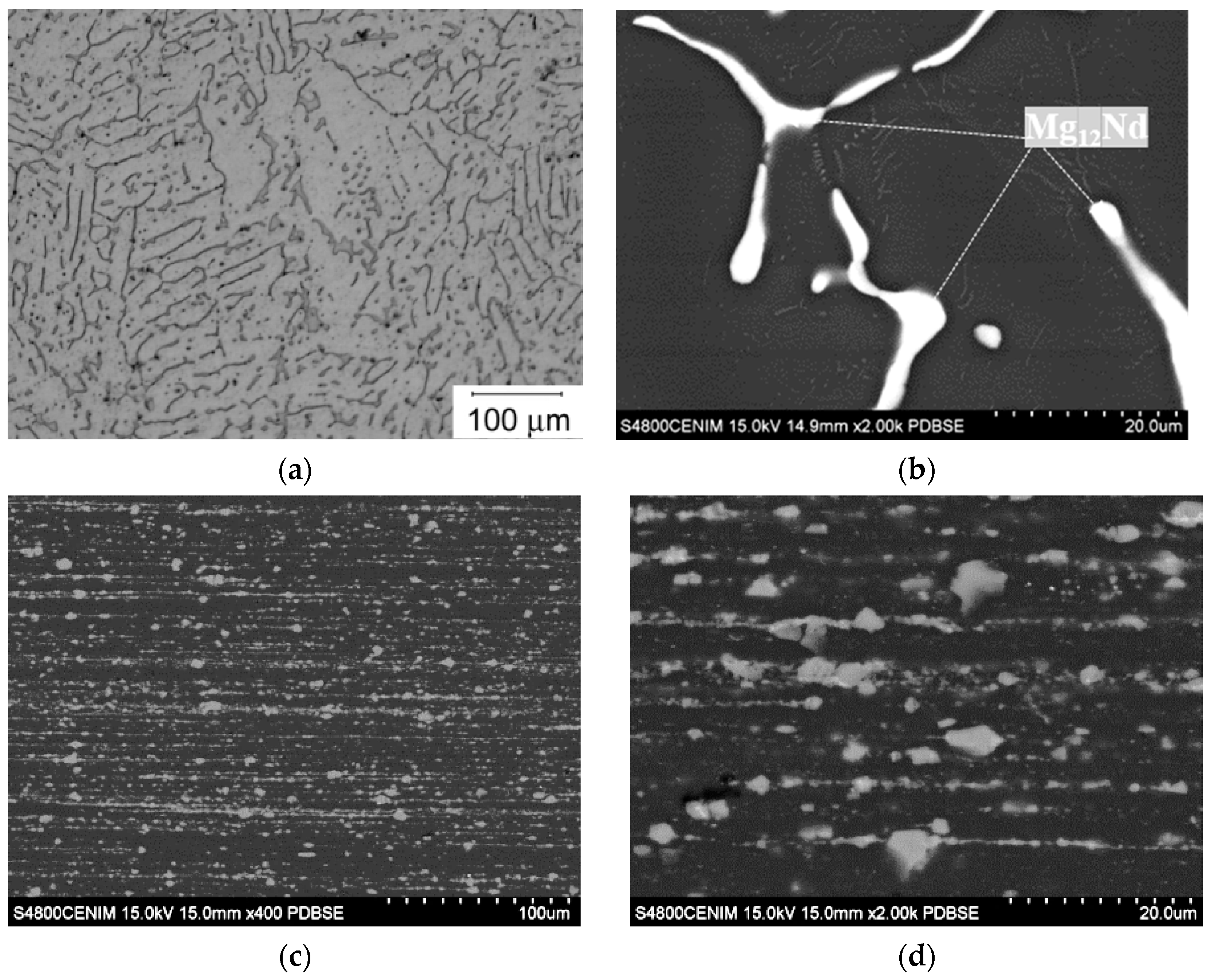
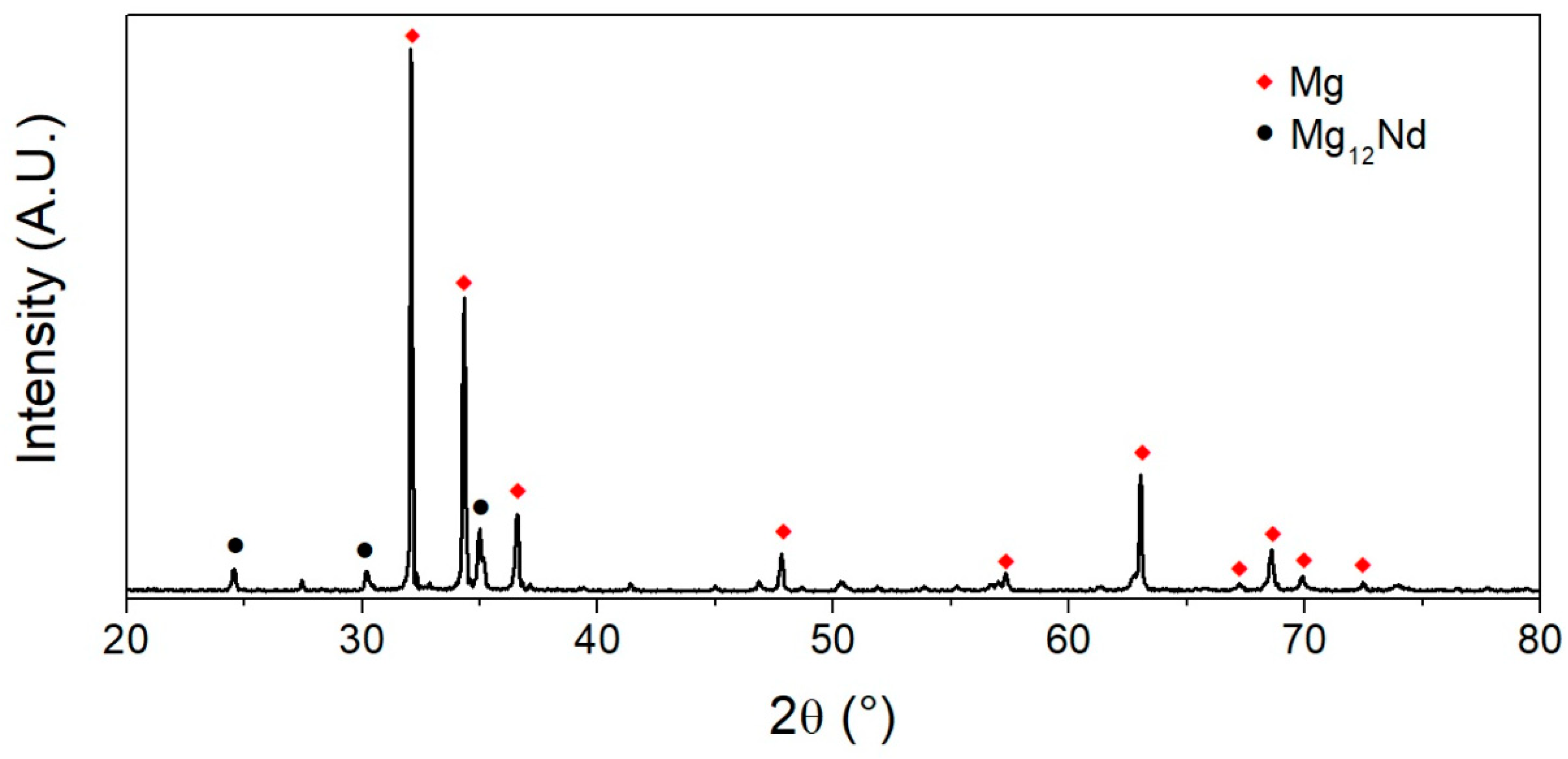
3.1. Microstructural Characterization
3.2. Corrosion Behaviour
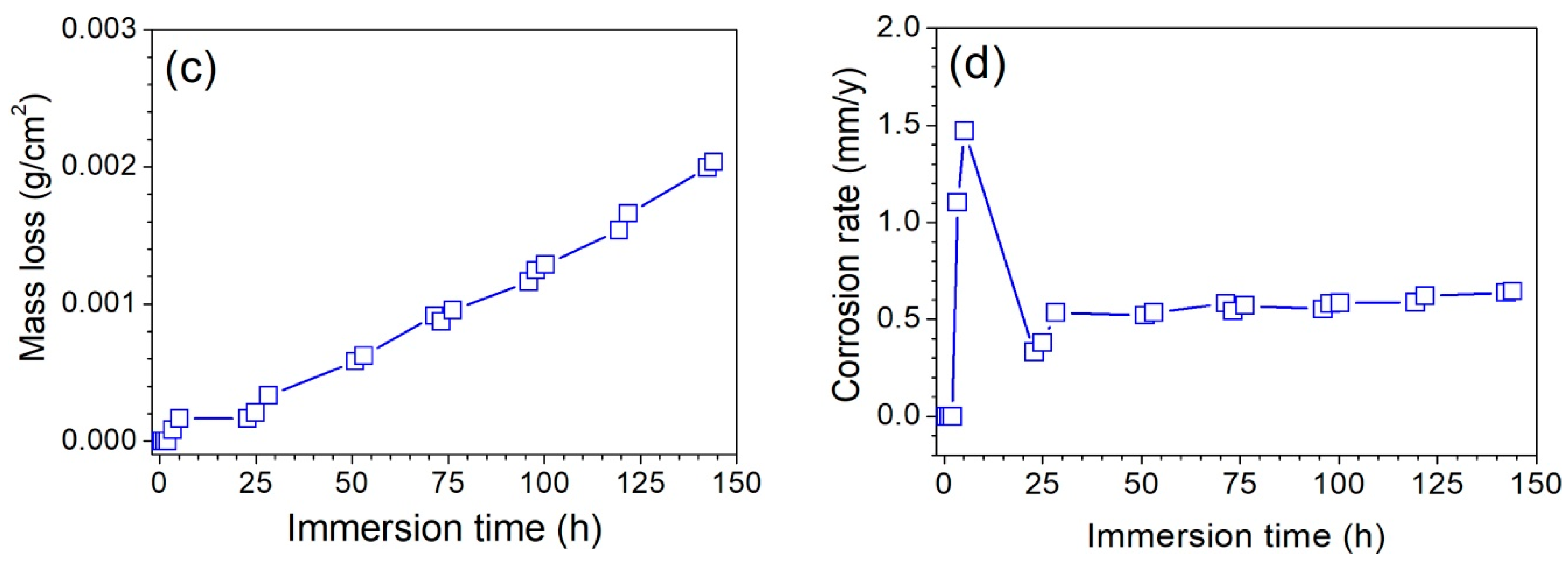
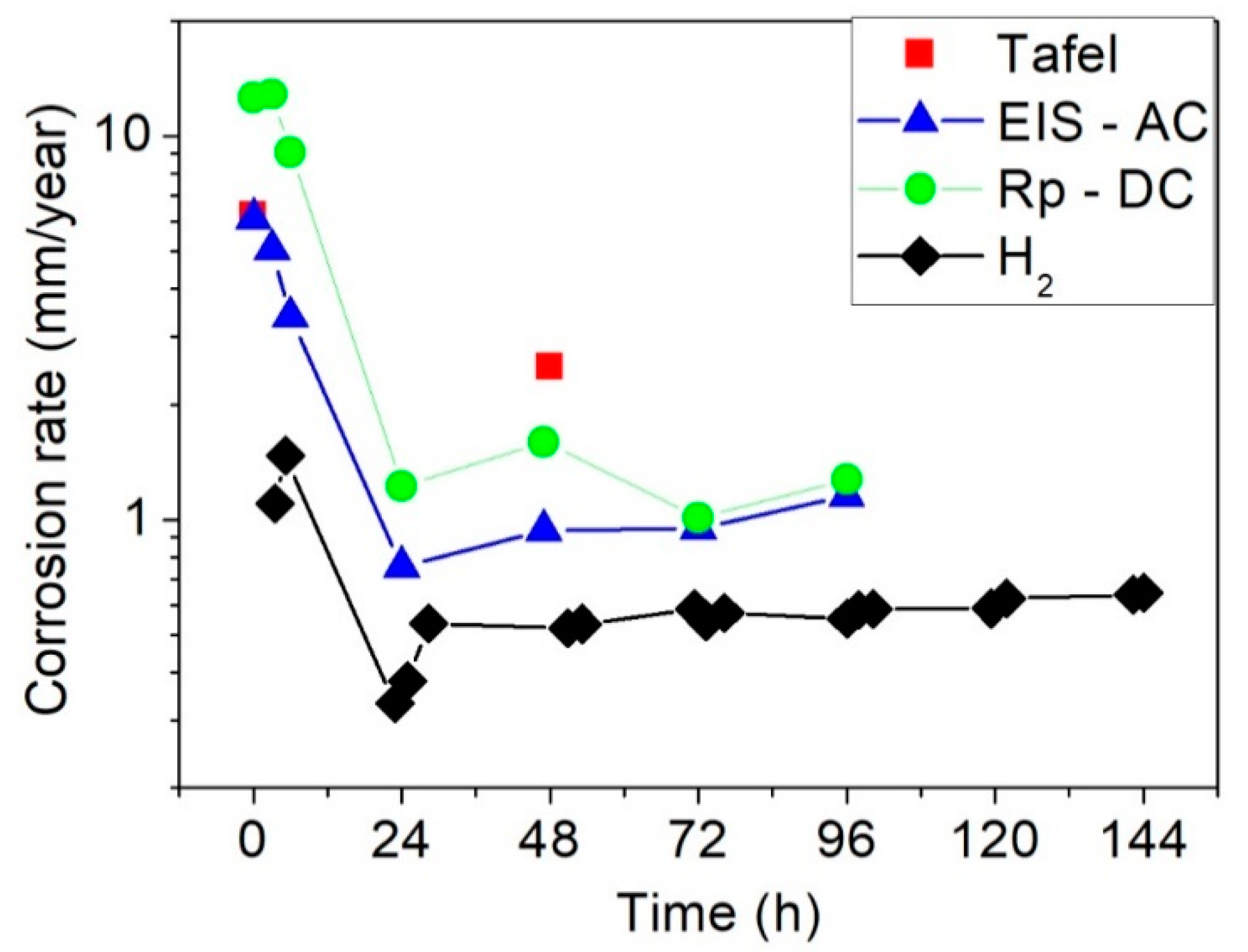
3.2.1. Hydrogen Release
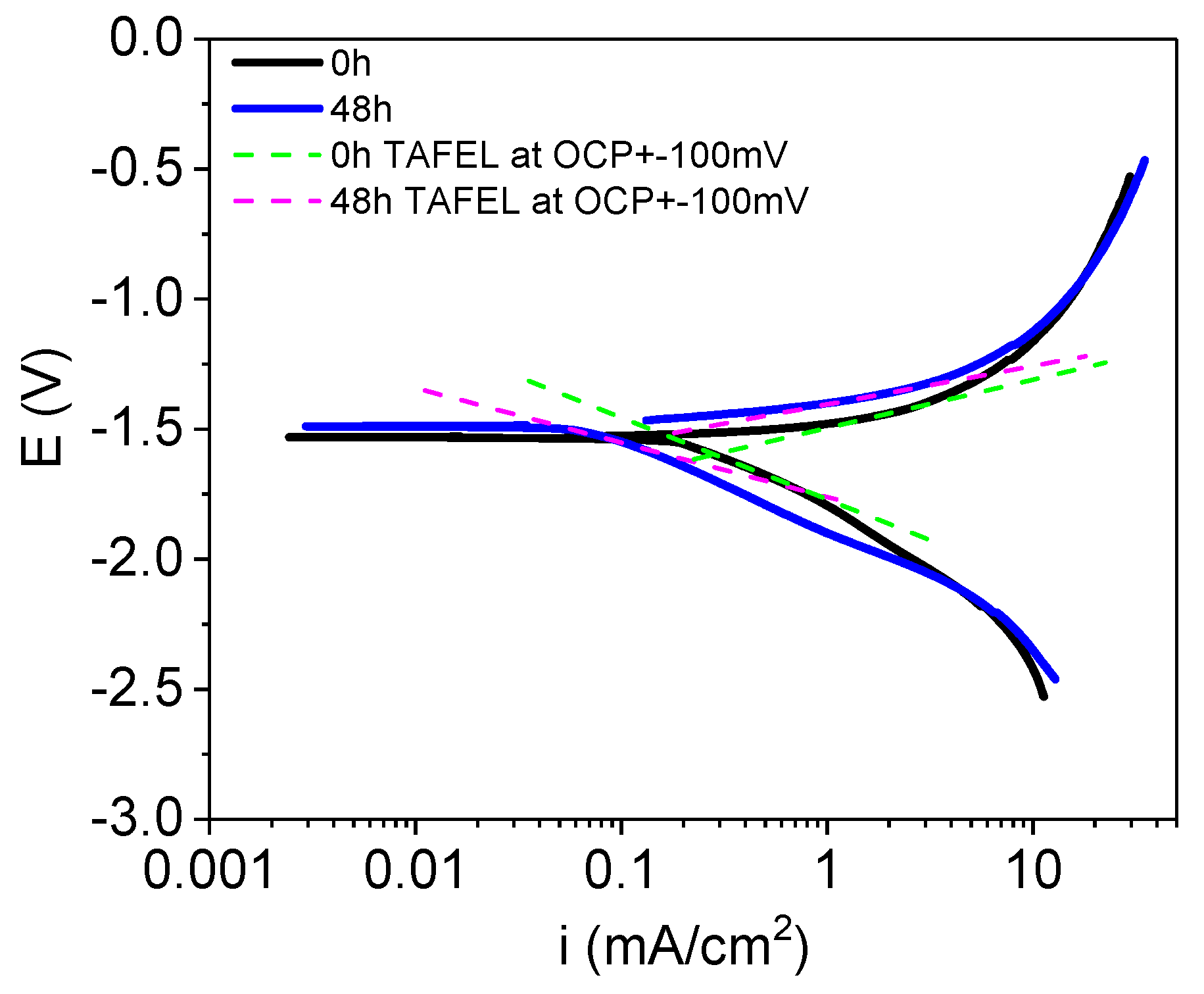
3.2.2. Electrochemical Characterization
4. Discussion
4.1. Microstructure
4.2. Evaluation of Corrosion Rate
4.3. Corrosion Mechanism
5. Conclusions
- The microstructure of the alloy consists of an equiaxed magnesium matrix embedding a high-volume fraction of Mg12Nd particles in the form of arrangements aligned along the extrusion direction. This microstructure renders higher strength and ductility than other Mg-Nd-Zn alloys containing lower zinc and neodymium concentrations.
- Hydrogen release tests and EIS measurements confirms low corrosion rates with values close to 1 mm/y in the steady state after 24 h of immersion. Corrosion rates determined by DC linear polarization tests is at least two times higher than those calculated from hydrogen release tests because of uncertainty for the calculation of Tafel slopes from anodic and cathodic branches.
- The corrosion rate of the EZ51 alloy is similar to that reported for other ternary Mg-Nd-Zn alloys containing lower volume fractions of second phases.
- The low corrosion rate is associated with deposition of dense continuous magnesium-rich phosphates over the Mg(OH)2 layer initially formed. Low corrosion rates allow the sealing of any defect/crack generated in the corrosion scale in the course of the corrosion process.
- Neodymium additions seem to reduce more effectively microgalvanic coupling potential among the intermetallic second phases and the magnesium matrix, as reported in commercial alloys modified with neodymium additions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W.; et al. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2015, 11, 554–562. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.; Fechner, D.; Störmer, M.; Blawert, C.; Witte, F.; Vogt, C.; Drücker, H.; Willumeit, R.; Kainer, K.U.; et al. Magnesium alloys as implant materials—Principles of property design for Mg-RE alloys. Acta Biomater. 2010, 6, 1714–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S.; Cohen, S. The relation between microstructure and corrosion behaviour of Mg–Y–RE–Zr alloys. J. Alloys Compd. 2007, 431, 269–276. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Pérez, P.; Onofre, E.; Cabeza, S.; Llorente, I.; del Valle, J.A.; García-Alonso, M.C.; Adeva, P.; Escudero, M.L. Corrosion behaviour of Mg–Zn–Y–Mischmetal alloys in phosphate buffer saline solution. Corros. Sci. 2013, 69, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, J.; Qiu, X.; Zhang, D.; Tian, Z.; Niu, X. Effect of Nd on the microstructure, mechanical properties and corrosion behaviour of die-cast Mg–4Al-based alloy. J. Alloys Compd. 2008, 464, 556–564. [Google Scholar] [CrossRef]
- Wang, B.; Guan, S.; Wang, J.; Wang, L.; Zhu, S. Effects of Nd on microstructures and properties of extruded Mg–2Zn–0.46Y–xNd alloys for stent application. Mater. Sci Eng. B Adv. 2011, 176, 1673–1678. [Google Scholar] [CrossRef]
- Mao, L.; Yuan, G.; Wang, S.; Niu, J.; Wu, G.; Ding, W. A novel biodegradable Mg–Nd–Zn–Zr alloy with uniform corrosion behaviour in artificial plasma. Mater. Lett. 2012, 88, 1–4. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, G.; Niu, J.; Fu, P.; Ding, W. Microstructure, mechanical properties, biocorrosion behaviour, and cytotoxicity of as-extruded Mg–Nd–Zn–Zr alloy with different extrusion ratios. J. Mech. Behav. Biomed. Mater. 2012, 9, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Horn, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsugawa, I.; Kamado, S.; Kojima, Y.; Ninomiya, R.; Kubota, K. Corrosion of magnesium alloys containing rare earth elements. Corros. Rev. 1998, 16, 139–157. [Google Scholar] [CrossRef]
- Elkaiam, L.; Hakimi, O.; Goldman, J.; Aghion, E. The effect of Nd on mechanical properties and corrosion performance of biodegradable Mg-5%Zn alloy. Metals 2018, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Elkaiam, L.; Hakimi, O.; Yosafovich-Doitch, G.; Ovadia, S.; Aghion, E. In Vivo evaluation of Mg-5%Zn–2%Nd alloy as an innovative biodegradable implant material. Ann. Biomed. Eng. 2020, 48, 380–392. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; et al. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials 2019, 221, 119414. [Google Scholar] [CrossRef]
- Kong, X.; Wang, L.; Li, G.; Qu, X.; Niu, J.; Tang, T.; Dai, K.; Yuan, G.; Hao, Y. Mg-based bone implants show promising osteoinductivity and controllable degradation: A long-term study in a goat femoral condyle fracture model. Mater. Sci. Eng. C 2018, 86, 42–47. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, W.; Huang, H.; Pei, J.; Qu, H.; Yuan, G.; Li, Y. The degradation and transport mechanism of a Mg-Nd-Zn-Zr stent in rabbit common carotid artery: A 20-month study. Acta Biomater. 2018, 69, 372–384. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Jiang, G.; Guo, J.; Li, Q.; Li, B.; Wang, Q.; Cheng, M.; He, G.; Zhang, X. The improvement of corrosion resistance, biocompatibility and osteogenesis of the novel porous Mg-Nd-Zn alloy. J. Mater. Chem. B 2017, 5, 7661–7674. [Google Scholar] [CrossRef]
- Mao, L.; Zhou, H.; Chen, L.; Niu, J.; Zhang, L.; Yuan, G.; Song, C. Enhanced biocompatibility and long-term durability in vivo of Mg-Nd-Zn-Zr alloy for vascular stent application. J. Alloys Compd. 2017, 720, 245–253. [Google Scholar] [CrossRef]
- Niu, J.; Xiong, M.; Guan, X.; Zhang, J.; Huang, H.; Pei, J.; Yuan, G. The in vivo degradation and bone-implant interface of Mg-Nd-Zn-Zr alloy screws: 18 months post-operation results. Corros. Sci. 2016, 113, 183–187. [Google Scholar] [CrossRef]
- Nanda, I.P.; Hassim, M.H.; Idris, M.H.; Jahare, M.H.; Abdulmalik, S.S.; Arafat, A. Mechanical and degradation properties of zinc adopted magnesium alloys for biomedical application. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 602, p. 012094. [Google Scholar]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Fereidouni-Lotfabadi, A.; Daroonparvar, M.; Yajid, M.A.M.; Mezbahul-Islam, M.; Kasiri-Asgarani, M.; Medraj, M. Microstructure and bio-corrosion behavior of Mg-Zn and Mg-Zn-Ca alloys for biomedical applications. Mater. Corros. 2014, 65, 1178–1187. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg-Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Song, Y.; Han, E.H.; Shan, D.; Yim, C.D.; You, B.S. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Atrens, A.; StJohn, D. An hydrogen evolution method for the estimation of the corrosion rate of magnesium alloys. In Proceedings of the Magnesium Technology TMS Symposium, New Orleans, LA, USA, 11–15 February 2001; pp. 255–262. [Google Scholar]
- Andrade, C.; Gónzalez, J.A. Quantitative measurements of corrosion rate of reinforcing steels embedded in concrete using polarization resistance measurements. Mater. Corros. 1978, 29, 515–519. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996; pp. 76–77. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical polarization I. A Theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Pérez, P.; Garcés, G.; Maeso, M.; Adeva, P. Effect of Zn content on microstructure and mechanical properties of MgZnYLaMM alloys. Metall. Mater. Trans. A 2012, 43, 4383–4396. [Google Scholar] [CrossRef] [Green Version]
- Buzolin, R.H.; Mohedano, M.; Mendis, C.L.; Mingo, B.; Tolnai, D.; Blawert, C.; Kainer, K.U.; Pinto, H.; Hort, N. As cast microstructures on the mechanical and corrosion behaviour of ZK40 modified with Gd and Nd additions. Mater. Sci. Eng. A 2017, 682, 238–247. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Recent insights into the mechanism of magnesium corrosion and research suggestions. Adv. Eng. Mater. 2007, 9, 177–183. [Google Scholar] [CrossRef]
- Pérez, P.; Cabeza, S.; Garcés, G.; Adeva, P. Influence of long period stacking ordered phase arrangements on the corrosion behaviour of extruded Mg97Y2Zn1 alloy. Corros. Sci. 2016, 107, 107–112. [Google Scholar] [CrossRef]
- Yao, H.; Wen, J.; Xiong, Y.; Liu, Y.; Lu, Y.; Cao, W. Microstructures, mechanical properties, and corrosion behavior of as-cast Mg–2.0Zn–0.5Zr–xGd (wt%) biodegradable alloys. Materials 2018, 11, 1564. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Song, R.; Wang, L.; Li, J. Effect of anodic T phase on surface micro-galvanic corrosion of biodegradable Mg-Zn-Zr-Nd alloys. Appl. Surf. Sci. 2018, 462, 243–254. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, L.; Ni, D.; Chen, J.; Zhao, Y.-C.; Liu, L.; Shuai, C.; Yang, K.; Atrens, A.; Zhao, M.-C. Effect of grain refinement and crystallographic texture produced by friction stir processing on the biodegradation behavior of a Mg-Nd-Zn alloy. J. Mater. Sci. Technol. 2019, 35, 777–783. [Google Scholar] [CrossRef]
- Cai, C.; Song, R.; Wang, L.; Li, J. Surface corrosion behavior and reaction product film deposition mechanism of Mg-Zn-Zr-Nd alloys during degradation process in Hank’s solution. Surf. Coat. Technol. 2018, 342, 57–68. [Google Scholar] [CrossRef]








| Site | O (at. %) | Na (at. %) | Mg (at. %) | P (at. %) | K (at. %) | Nd (at. %) |
|---|---|---|---|---|---|---|
| 1 | 63.6 | 5.3 | 12.8 | 14.5 | 3.8 | - |
| 2 | 58.5 | 6.0 | 17.5 | 13.9 | 3.6 | 0.4 |
| 3 | 62.7 | 6.7 | 13.4 | 13.5 | 3.7 | - |
| Parameter | 0 h | 48 h |
|---|---|---|
| Ecorr (mVAg/AgCl) | −1529.9 | −1486.8 |
| βa (mV/decade) | 186 | 148 |
| βc (mV/decade) | −398 | −345 |
| B (mV) | 55* | 44.9 |
| icorr1 (mA/cm2) | 0.27 | 0.11 |
| Pi1 (mm/year) | 6.2 | 2.5 |
| Rp (Ω*cm2) | 169 | 1094 |
| icorr2 (mA/cm2) | 0.27 | 0.04 |
| Pi2 (mm/year) | 6.2 | 0.9 |
| Equivalent Circuit | Time (h) | RΩ (Ω·cm2) | Rp (Ω·cm2) | C (F/cm2) |
|---|---|---|---|---|
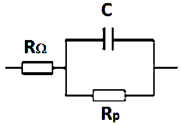 | 0 | 34.30 | 168.58 | 3.15 × 10−5 |
| 3 | 33.96 | 203.15 | 7.25 × 10−5 | |
| 6 | 33.25 | 304.77 | 6.79 × 10−5 | |
| 24 | 47.87 | 1364.63 | 3.63 × 10−5 | |
| 47 | 80.38 | 1093.73 | 4.04 × 10−5 | |
| 72 | 104.29 | 1082.70 | 3.77 × 10−5 | |
| 96 | 112.04 | 890.55 | 5.40 × 10−5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabeza, S.; Pérez Zubiaur, P.; Garcés, G.; Andrade, C.; Adeva, P. Corrosion Behaviour of Mg98.5Nd1Zn0.5 (at. %) Alloy in Phosphate Buffered Saline Solution. Metals 2020, 10, 148. https://doi.org/10.3390/met10010148
Cabeza S, Pérez Zubiaur P, Garcés G, Andrade C, Adeva P. Corrosion Behaviour of Mg98.5Nd1Zn0.5 (at. %) Alloy in Phosphate Buffered Saline Solution. Metals. 2020; 10(1):148. https://doi.org/10.3390/met10010148
Chicago/Turabian StyleCabeza, Sandra, Pablo Pérez Zubiaur, Gerardo Garcés, Carmen Andrade, and Paloma Adeva. 2020. "Corrosion Behaviour of Mg98.5Nd1Zn0.5 (at. %) Alloy in Phosphate Buffered Saline Solution" Metals 10, no. 1: 148. https://doi.org/10.3390/met10010148





