Second Life Application of Automotive Catalysts: Hydrodynamic Cavitation Recovery and Photo Water Splitting
Abstract
:1. Introduction
2. Experimental
2.1. Chemical Composition
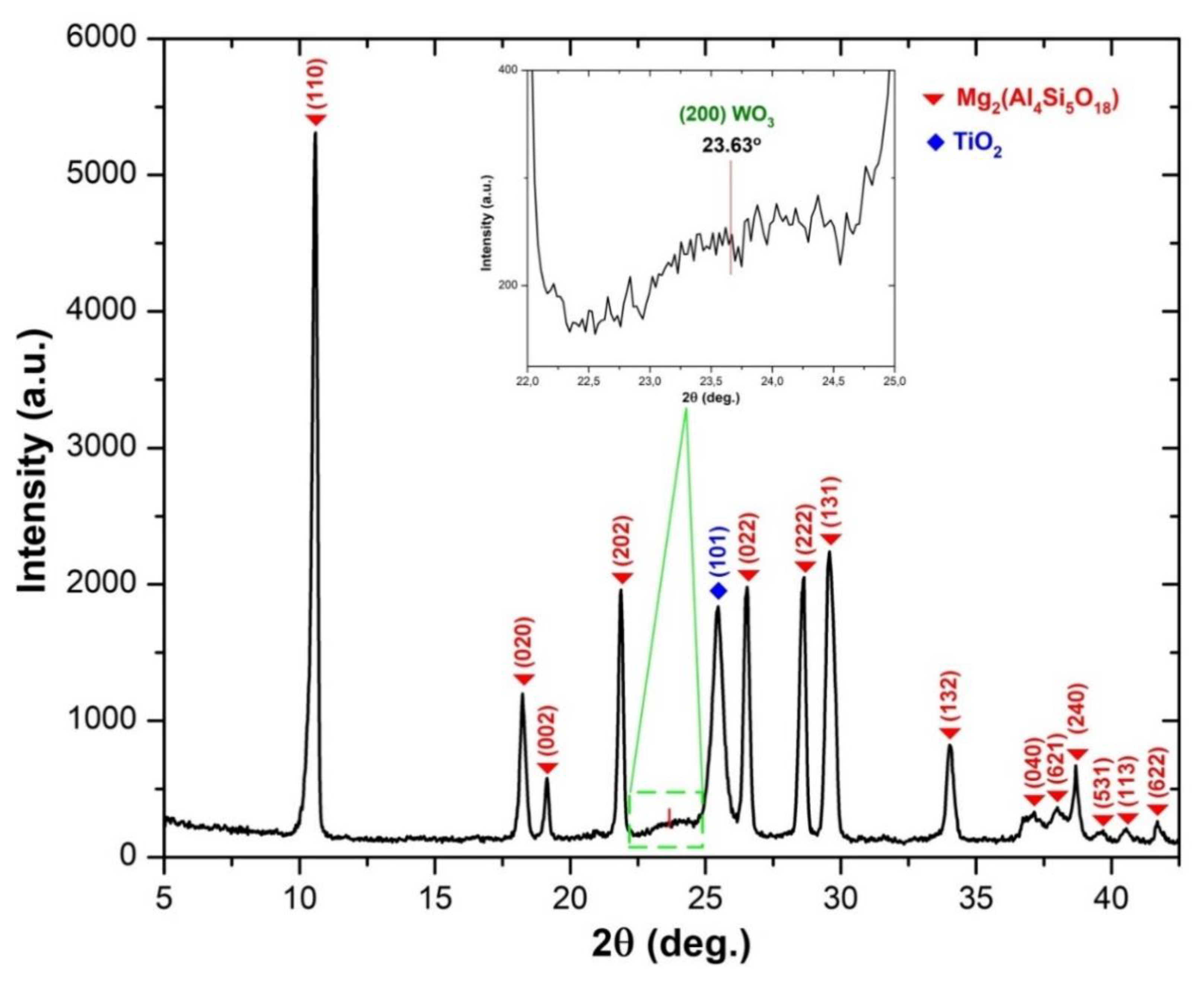
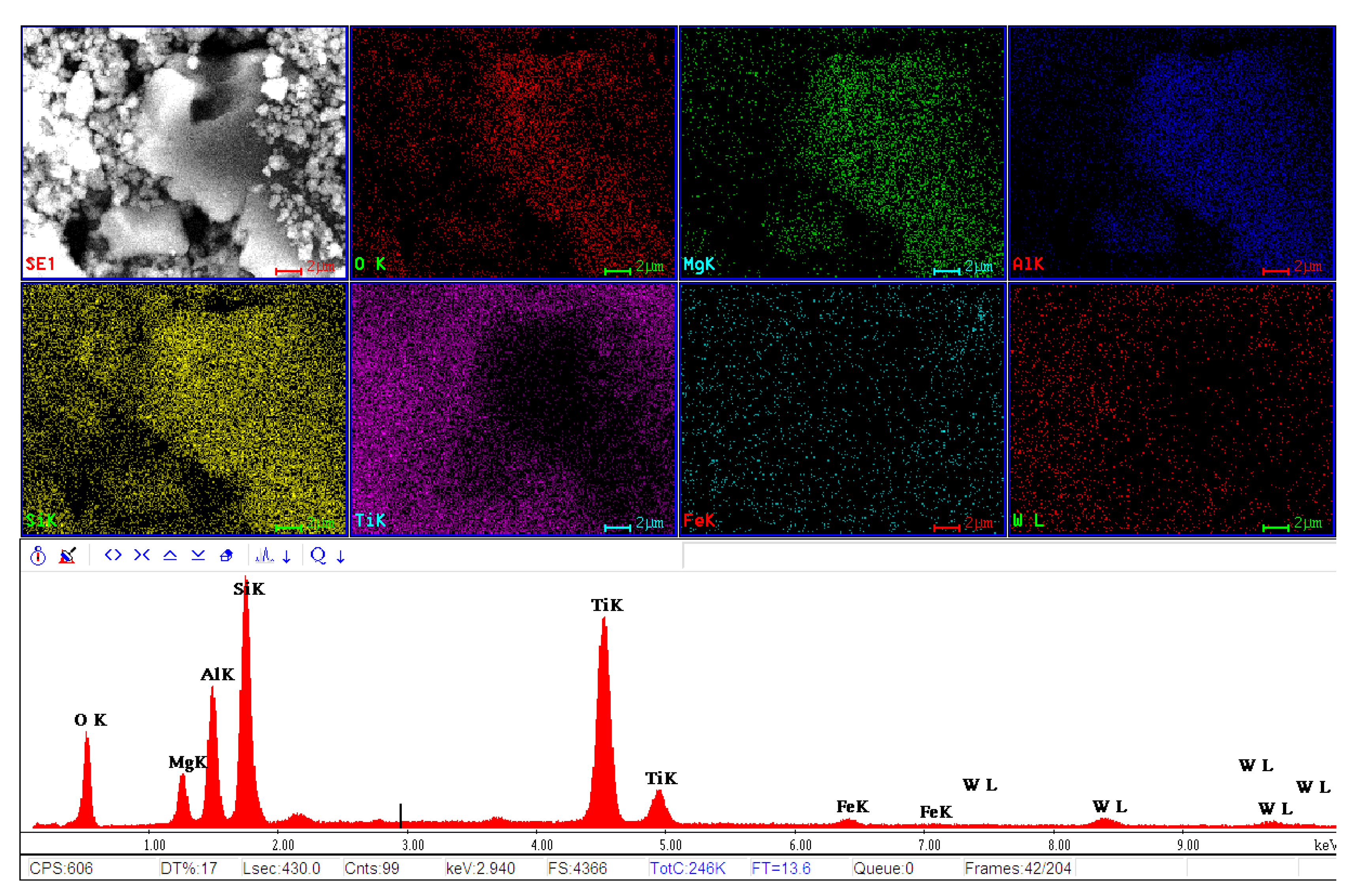
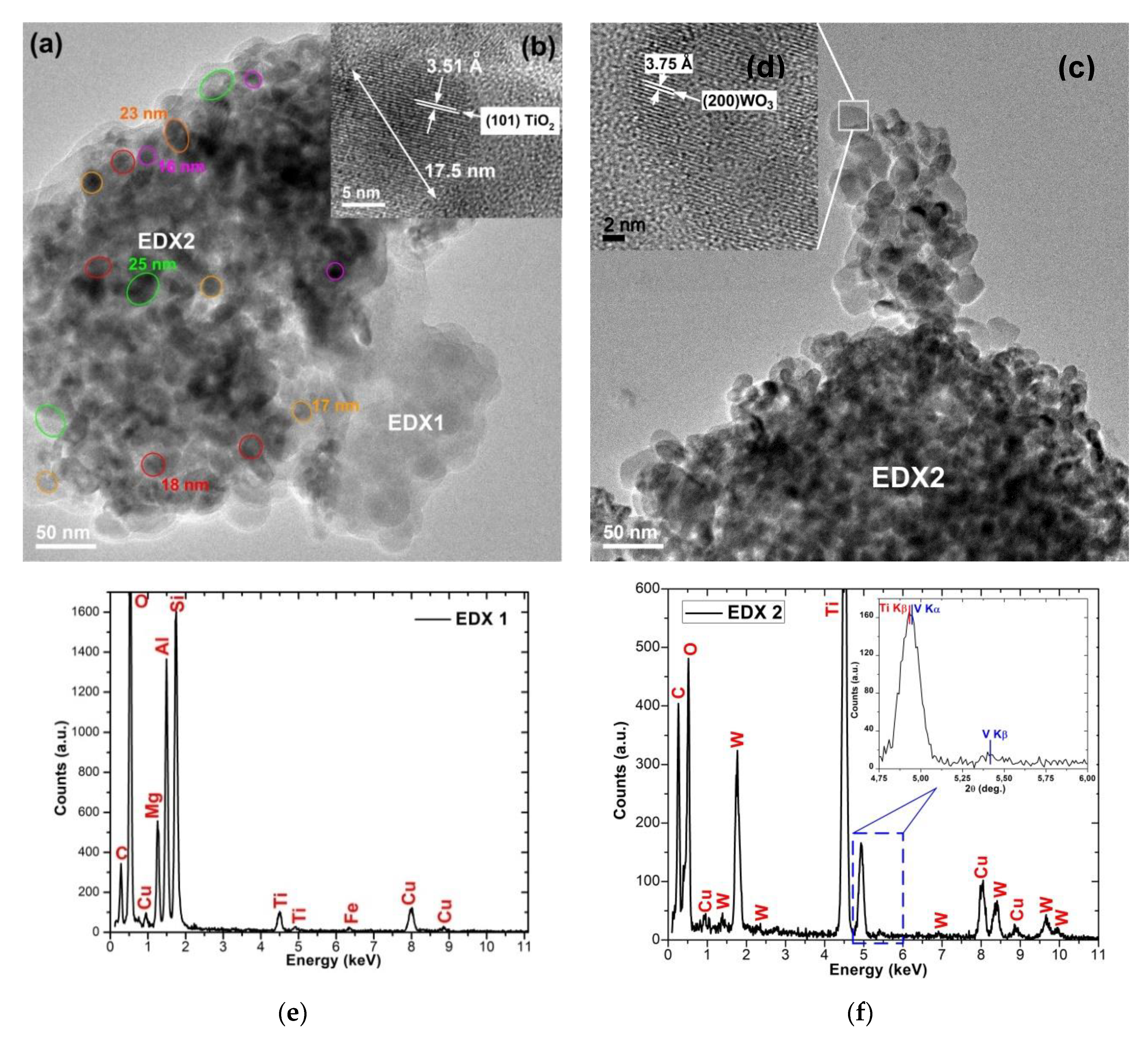
2.2. Structural and Morphological Characterization
2.3. Hydrodynamic Cavitation Method
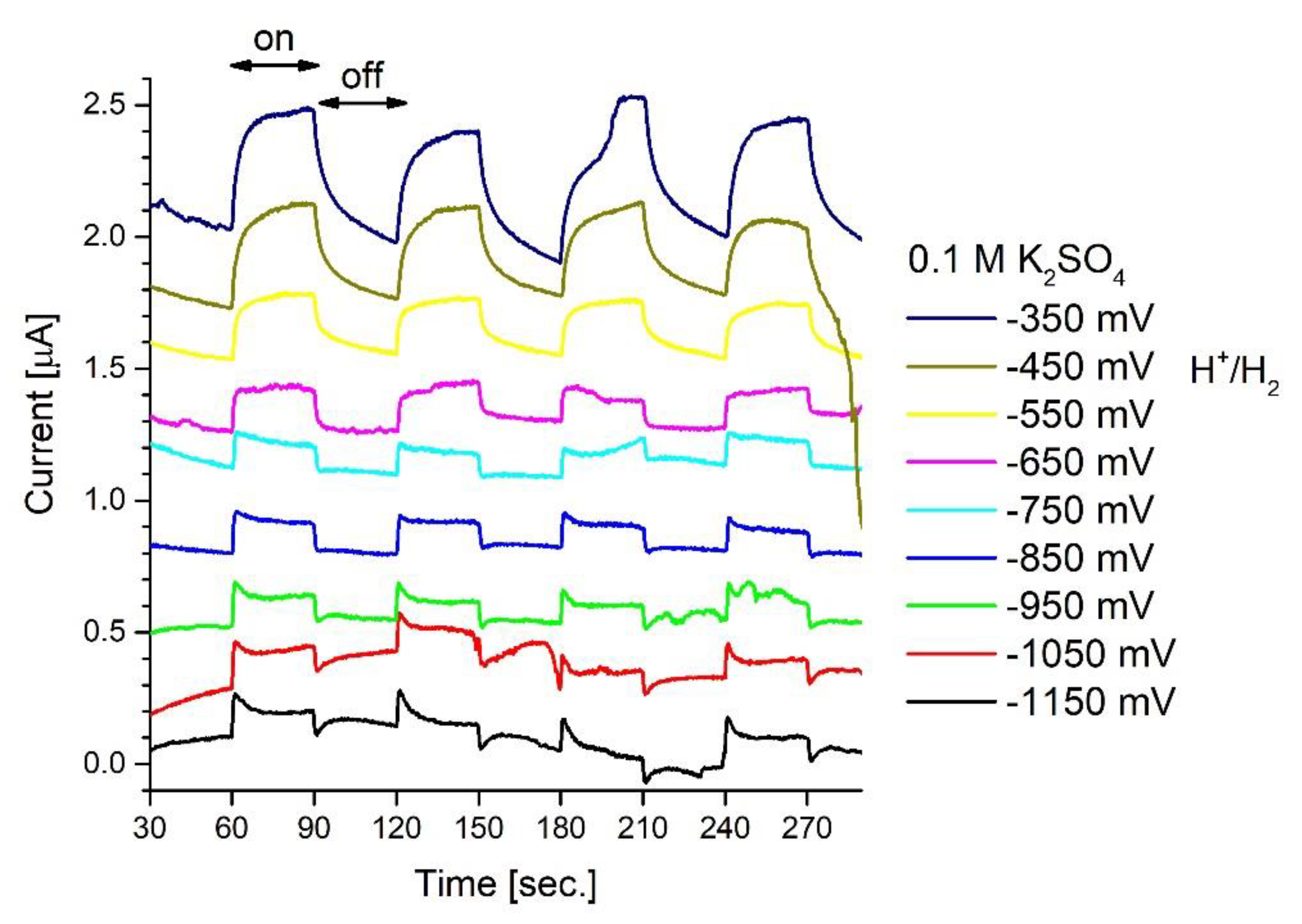
2.4. Electrochemical Measurements
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kröcher, O. Selective Catalytic Reduction of NOx. Catalysts 2018, 8, 459. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Zielińska, K.; Haratym, A. Environmental benefits of the SCR systems: A case study of the Lublin city transport. J. Ecol. Eng. 2017, 18, 202–207. [Google Scholar] [CrossRef]
- Hosoya, M.; Kawada, Y.; Satao, S.; Shimoda, M. The study of NOx and PM Reduction Using Urea Selective Catalytic Reduction System for Heavy Duty Diesel Engine. SAE Tech. Pap. 2007, 1–8. [Google Scholar] [CrossRef]
- Jaworski, P.; Jarosiński, S.; Capetillo, A.C.; Kapusta, Ł.J.; Ziółkowski, A.; Grzywnowicz, R. SCR systems for NOx reduction in heavy and light duty vehicles. Combust. Engines 2016, 164, 32–36. [Google Scholar]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- He, Y.; Ford, M.E.; Zhub, M.; Liu, Q.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B Environ. 2016, 193, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Han, F.; Wang, M.; Han, L.; Zhang, C.; Wang, J.; Bao, W.; Chang, L. Regeneration of the Waste Selective Catalytic Reduction Denitrification Catalyst by Nitric Acid Washing. ACS Omega 2019, 4, 16629–16637. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, W.G.; Hwang, I.S.; Lee, J.Y.; Han, C. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching. J. Ind. Eng. Chem. 2015, 28, 73–77. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Kröcher, O. VOx surface coverage optimization of V2O5-WO3/TiO2 SCR catalysts by variation of the V loading and by aging. Catalyst 2015, 5, 1704–1720. [Google Scholar] [CrossRef]
- Arce-Sarria, A.; Caicedo-Rosero, C.L.; Lara-Ramos, J.A.; Diaz-Angulo, J.; Machuca-Martínez, F. Experimental data on synthesis and characterization of WO3/TiO2 as catalyst. Data Brief 2019, 25, 104151–104152. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Delgado, N.A.; Hinojosa-Reyes, L.; Guzman-Mar, I.L.; Gracia-Pinilla, M.A.; Hernández-Ramírez, A. Synthesis by sol–gel of WO3/TiO2 for solar photocatalytic degradation of malathion pesticide. Catal. Today 2013, 209, 35–40. [Google Scholar] [CrossRef]
- Soares, L.; Alves, A. Photocatalytic properties of TiO2 and TiO2/WO3 films applied as semiconductors in heterogeneous photocatalysis. Mater. Lett. 2018, 211, 339–342. [Google Scholar] [CrossRef]
- Bayati, M.R.; Golestani-Fard, F.; Moshfeghd, A.Z.; Molaei, R. A photocatalytic approach in micro arc oxidation of WO3–TiO2 nanoporous semiconductors under pulse current. Mater. Chem. Phys. 2011, 128, 427–432. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Bao, W.; Wang, L.; Li, H.; Wu, W. Study of the V2O5–WO3/TiO2 Catalyst Synthesized from Waste Catalyst on Selective Catalytic Reduction of NOx by NH3. Catalysts 2017, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency. Emerging Contaminant—Tungsten; 2010 EPA/505/F-10/004; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Lia, M.; Liu, B.; Wang, X.; Yue, X.; Zheng, S.; Du, H.; Dreisinger, D.; Zhang, Y. A promising approach to recover a spent SCR catalyst: Deactivation by arsenic and alkaline metals and catalyst regeneration. Chem. Eng. J. 2018, 342, 1–8. [Google Scholar] [CrossRef]
- Choi, I.-H.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Extraction of tungsten and vanadium from spent selective catalytic reduction catalyst for stationary application by pressure leaching process. J. Clean. Prod. 2018, 197, 163–169. [Google Scholar] [CrossRef]
- Choi, I.-H.; Kim, H.-R.; Moon, G.; Jyothi, R.K.; Lee, J.-Y. Spent V2O5-WO3/TiO2 catalyst processing for valuable metals by soda roasting-water leaching. Hydrometallurgy 2018, 175, 292–299. [Google Scholar] [CrossRef]
- Choi, I.H.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Hydrometallurgical processing of spent selective catalytic reduction (SCR) catalyst for recovery of tungsten. Hydrometallurgy 2018, 178, 137–145. [Google Scholar] [CrossRef]
- Shang, X.; Hu, G.; He, C.; Zhao, J.; Zhang, F.; Xu, Y.; Zhang, Y.; Li, J.; Chen, J. Regeneration of full-scale commercial honeycomb monolith catalyst (V2O5–WO3/TiO2) used in coal-fired power plant. J. Ind. Eng. Chem. 2012, 18, 513–519. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Mnichowicz, B.; Harinath, A.V.; Li, H.; Bahrami, B. Chemical deactivation of commercial vanadium SCR catalysts in diesel emission control application. Chem. Eng. J. 2016, 287, 680–690. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Peng, Y.; Chang, H.; Zhang, T.; Zhao, S.; Si, W.; Hao, J. Mechanism of arsenic poisoning on SCR catalyst of CeW/Ti and its novel efficient regeneration method with hydrogen. Appl. Catal. B Environ. 2016, 184, 246–257. [Google Scholar] [CrossRef]
- Dular, M.; Griessler-Bulc, T.; Gutierrez-Aguirre, I.; Heath, E.; Kosjek, T.; Klemencic, A.K.; Oder, M.; Petkovšek, M.; Racki, N.; Ravnikar, M.; et al. Use of hydrodynamic cavitation in (waste)water treatment. Ultrason. Sonochem. 2016, 29, 577–588. [Google Scholar] [CrossRef]
- Sivakumar, M.; Pandit, A.B. Wastewater treatment: A novel energy efficient hydrodynamic cavitational technique. Ultrason. Sonochem. 2002, 9, 123–131. [Google Scholar] [CrossRef]
- Jyoti, K.K.; Pandit, A.B. Hybrid cavitation methods for water disinfection: Simultaneous use of chemicals with cavitation. Ultrason. Sonochem. 2003, 10, 255–264. [Google Scholar] [CrossRef]
- Jyoti, K.K.; Pandit, A.B. Effect of cavitation on chemical disinfection efficiency. Water Res. 2004, 38, 2249–2258. [Google Scholar] [CrossRef]
- Prajapat, A.L.; Gogate, P.R. Depolymerization of carboxymethyl cellulose using hydrodynamic cavitation combined with ultraviolet irradiation and potassium persulfate. Ultrason. Sonochem. 2019, 51, 258–263. [Google Scholar] [CrossRef]
- Pathania, S.; Ho, Q.T.; Hogan, S.A.; McCarthy, N.; Tobin, J.T. Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J. Food Eng. 2018, 225, 18–25. [Google Scholar] [CrossRef]
- Li, K.; Woo, M.W.; Patel, H.; Metzger, L.; Selomulya, C. Improvement of rheological and functional properties of milk protein concentrate by hydrodynamic cavitation. J. Food Eng. 2018, 221, 106–113. [Google Scholar] [CrossRef]
- Find, J.; Emerson, S.C.; Krausz, I.M.; Moser, W.R. Hydrodynamic cavitation as a tool to control macro-, micro-, and nano-properties of inorganic materials. J. Mater. Res. 2001, 16, 3503–3513. [Google Scholar] [CrossRef]
- Moser, W.R.; Marshik, B.J.; Kingsley, J.; Lemberger, M.; Willette, R.; Chan, A.; Sunstrom, J.E.; Boye, A. The synthesis and characterization of solid-state materials produced by high shear-hydrodynamic cavitation. J. Mater. Res. 1995, 10, 2322–2335. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, H.; Ou, L.; Shi, Q. Aggregation of ultra-fine scheelite particles induced by hydrodynamic cavitation. Int. J. Miner. Process. 2016, 157, 236–240. [Google Scholar] [CrossRef]
- Kumar, K.S.; Moholkar, V.S. Conceptual design of a novel hydrodynamic cavitation reactor. Chem. Eng. Sci. 2007, 62, 2698–2711. [Google Scholar] [CrossRef]
- Smoholkar, V.; Pandit, A.B. Modeling of hydrodynamic cavitation reactors: A unified approach. Chem. Eng. Sci. 2001, 56, 6295–6302. [Google Scholar] [CrossRef]
- Ambulgekar, G.V.; Samant, S.D.; Pandit, A.B. Oxidation of alkylarenes to the corresponding acids using aqueous potassium permanganate by hydrodynamic cavitation. Ultrason. Sonochem. 2004, 11, 191–196. [Google Scholar] [CrossRef]
- Wood, R.J.; Lee, J.; Bussemaker, M.J. A parametric review of sonochemistry: Control and augmentation of sonochemical activity in aqueous solutions. Ultrason. Sonochem. 2017, 38, 351–370. [Google Scholar] [CrossRef]
- Hutli, E.; Nedeljkovic, S.M.; Radovic, N.A.; Bonyár, A. The relation between the high speed submerged cavitating jet behaviour and the cavitation erosion process. Int. J. Multiph. Flow 2016, 83, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-K.; Jayaprakash, A.; Chahine, G.L. Scaling of cavitation erosion progression with cavitation intensity and cavitation source. Wear 2012, 278, 53–61. [Google Scholar] [CrossRef]
- Kim, W.T.; Kim, H.S.; Cho, S.B.; Lee, J.C.; Kim, S.B. Selective recovery of catalyst layer from supporting matrix of ceramic-honeycomb-type automobile catalyst. J. Hazard. Mater. 2010, 183, 29–34. [Google Scholar] [CrossRef]
- Tao, P.; Sun, M.H.; Qu, S.C.; Song, C.W.; Li, C.; Yin, Y.Y.; Cheng, M.R. Effects of V2O5 and WO3 loadings on the catalytic performance of V2O5-WO3/TiO2 catalyst for SCR of NO with NH3. Glob. NEST J. 2017, 19, 160–166. [Google Scholar]
- Wu, W.; Wang, C.; Bao, W.; Li, H. Selective reduction leaching of vanadium and iron by oxalic acid from spent V2O5-WO3/TiO2. Hydrometallurgy 2018, 179, 52–59. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.H.; Si, W.Z.; Luo, J.M.; Wang, Y.; Fu, J.; Li, X.; Crittenden, J.; Hao, J.M. Deactivation and regeneration of a commercial SCR catalyst: Comparison with alkali metals and arsenic. Appl. Catal. B Environ. 2015, 168, 195–202. [Google Scholar] [CrossRef]
- Chavez, C.A.; Toledo, J.; Cortes-Jacome, M.A. Chemical Quantification of Mo-S, W-Si and Ti-V by Energy Dispersive X-ray Spectroscopy; Intech Open: London, UK, 2012; p. 133. Available online: https://www.intechopen.com/books/x-ray-spectroscopy/chemical-quantification-of-mo-s-w-si-and-ti-v-by-energy-dispersive-x-ray-spectroscopy (accessed on 15 May 2020).
- Lee, B.W.; Cho, H.; Shin, D.W. Characterization and De-NOX activity of binary V2O5/TiO2 and WO3/TiO2, and ternary V2O5-WO3/TiO2 SCR catalysts. J. Ceram. Process. Res. 2007, 8, 203–207. [Google Scholar]
- Kompio, P.G.W.A.; Bruckner, A.; Hipler, F.; Auer, G.; Loffler, E.; Grünert, W. A new view on the relations between tungsten and vanadium in V2O5WO3/TiO2 catalysts for the selective reduction of NO with NH3. J. Catal. 2012, 286, 237–247. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of tungsten oxide on SCR performance of V2O5WO3/TiO2: Acidity, surface species and catalytic activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Pini, M.; Salieri, B.; Ferrari, A.M.; Nowack, B.; Hischier, R. Human health characterization factors of nano-TiO2 for indoor and outdoor environments. Int. J. LCA 2016, 21, 1452–1462. [Google Scholar] [CrossRef]
- Salieri, B.; Righi, S.; Pasteris, A.; Olsen, S.I. Freshwater ecotoxicity characterisation factor for metal oxide nanoparticles: A case study on titanium dioxide nanoparticle. Sci. Total Environ. 2015, 505, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhua, S.; Cindrellaa, L.; Kwonb, O.J.; Mohanrajub, K. Photoelectrochemical and photocatalytic activity of TiO2-WO3 heterostructures boosted by mutual interaction. Mater. Sci. Semicond. Process. 2018, 88, 10–19. [Google Scholar] [CrossRef]




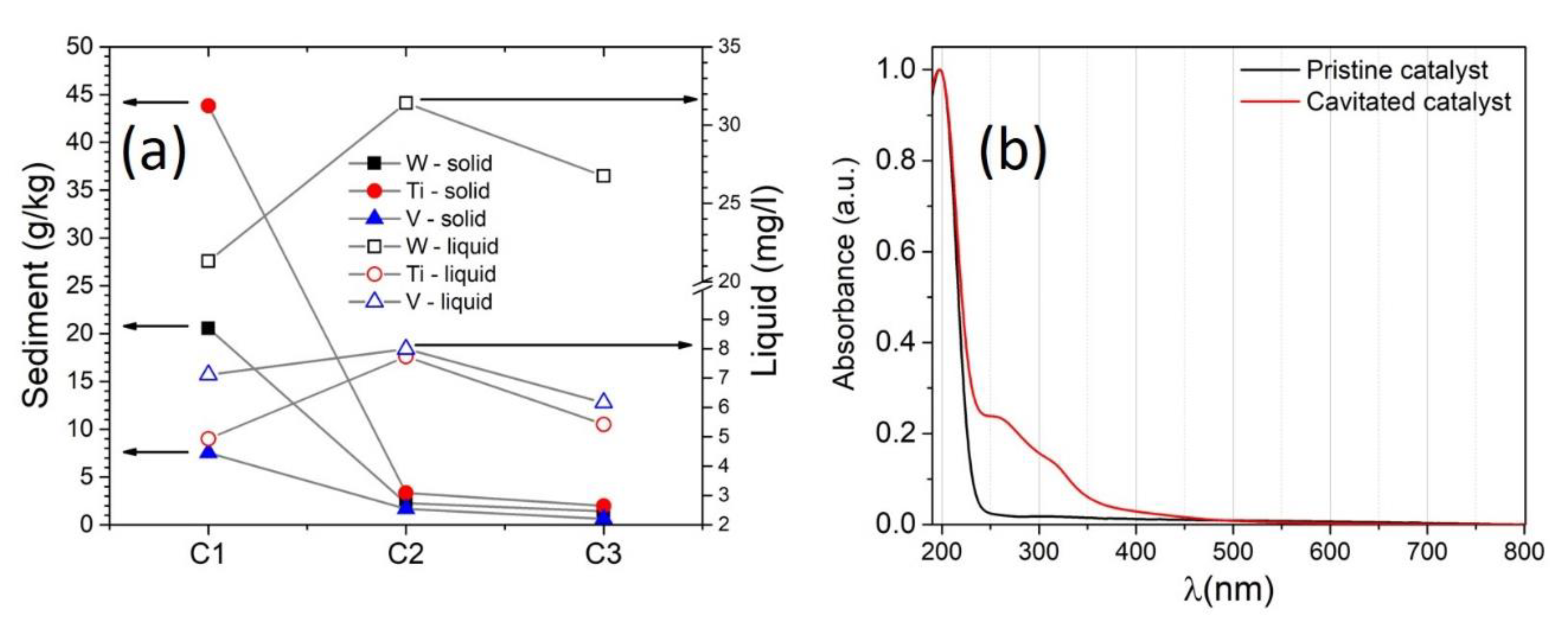
| Sample Identification | Ti [g/kg] | W [g/kg] | V [g/kg] |
|---|---|---|---|
| initial catalyst | 2.1 | 1.4 | 3.2 |
| first cavitation—C1 | 43.8 | 20.6 | 7.5 |
| second cavitation—C2 | 3.4 | 2.3 | 1.7 |
| third cavitation—C3 | 2.0 | 1.41 | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciocanea, A.; Vasile, E.; Ionescu, V.; Maxim, F.I.; Diac, C.; Miron, C.; Stamatin, S.N. Second Life Application of Automotive Catalysts: Hydrodynamic Cavitation Recovery and Photo Water Splitting. Metals 2020, 10, 1307. https://doi.org/10.3390/met10101307
Ciocanea A, Vasile E, Ionescu V, Maxim FI, Diac C, Miron C, Stamatin SN. Second Life Application of Automotive Catalysts: Hydrodynamic Cavitation Recovery and Photo Water Splitting. Metals. 2020; 10(10):1307. https://doi.org/10.3390/met10101307
Chicago/Turabian StyleCiocanea, Adrian, Eugeniu Vasile, Viorel Ionescu, Florentina Iuliana Maxim, Cornelia Diac, Cristina Miron, and Serban N. Stamatin. 2020. "Second Life Application of Automotive Catalysts: Hydrodynamic Cavitation Recovery and Photo Water Splitting" Metals 10, no. 10: 1307. https://doi.org/10.3390/met10101307





