Microstructure Evolution and Mechanical Properties of Refractory Mo-Nb-V-W-Ti High-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Alloy Design Based on Thermodynamics and Geometrical Effects
3.2. X-ray Diffraction of the RHEAs
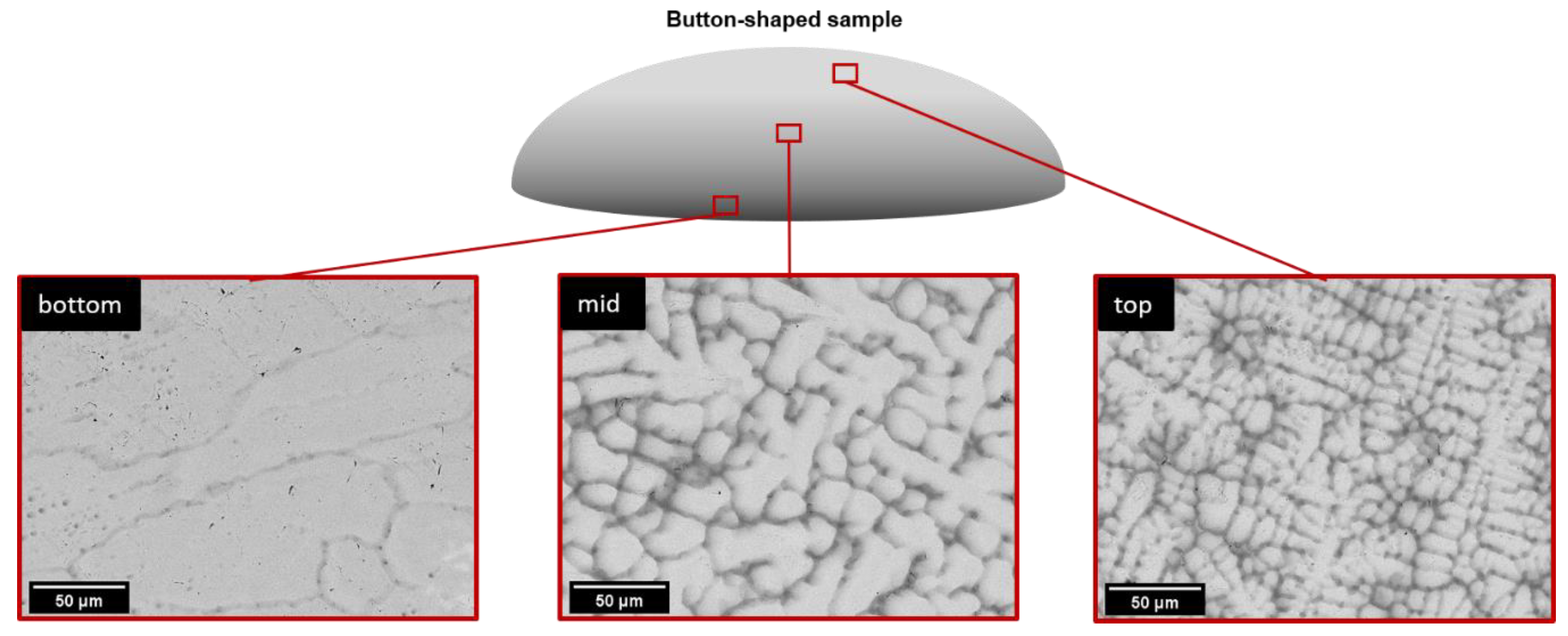
3.3. As-Cast Microstructures of the Alloys Examined
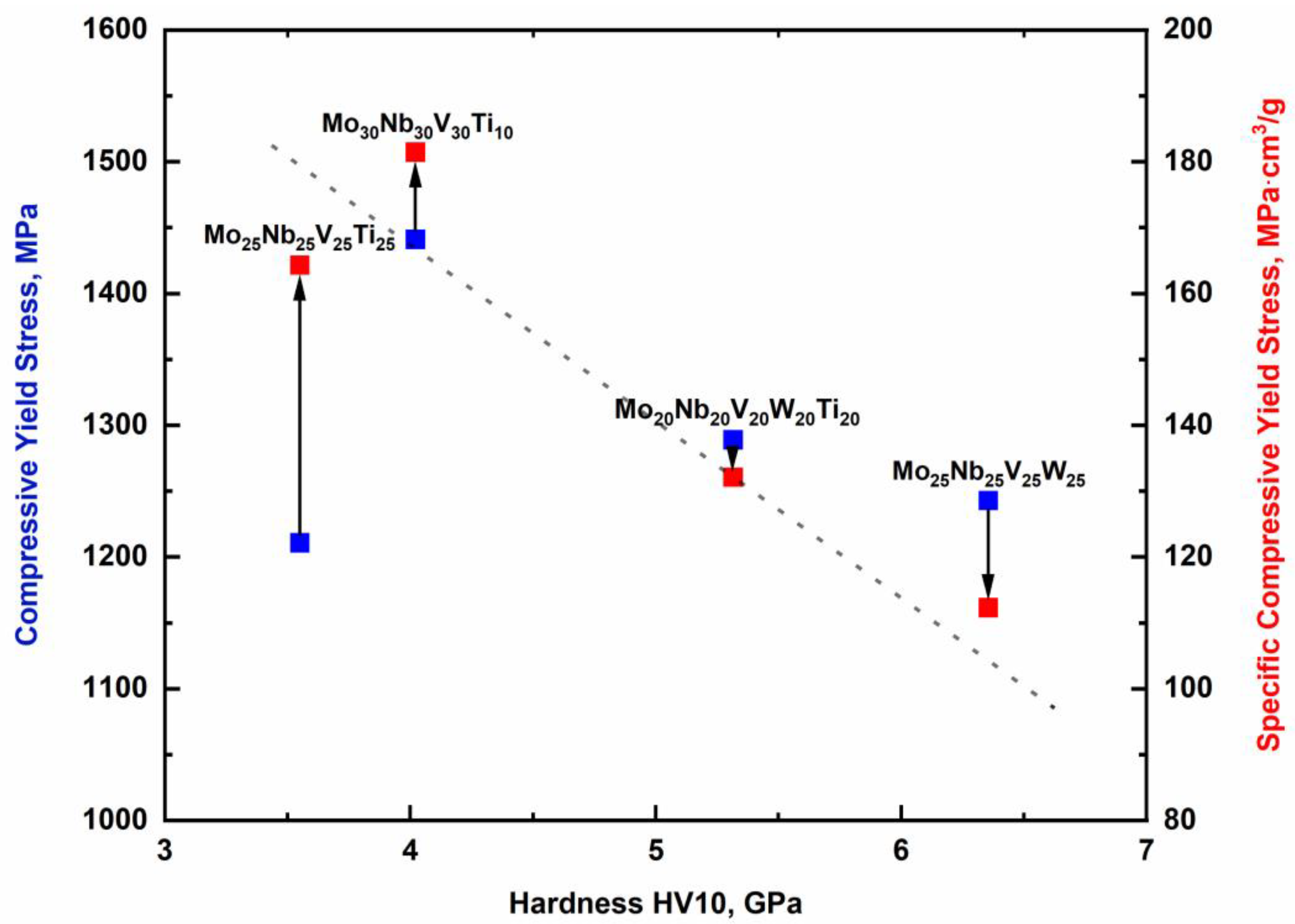
3.4. Mechanical Properties at Room Temperature
3.5. Comparison with Other Works and Superalloys
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speich, G.R.; Schwoeble, A.J.; Leslie, W.C. Elastic constants of binary iron-base alloys. Metall. Trans. 1972, 3, 2031–2037. [Google Scholar] [CrossRef]
- Tawancy, H.M.; Klarstrom, D.L.; Rothman, M.F. Development of a New Nickel-Base Superalloy. JOM 1984, 36, 58–62. [Google Scholar] [CrossRef]
- Hemmersmeier, U.; Feller-Kniepmeier, M. Element distribution in the macro- and microstructure of nickel base superalloy CMSX-4. Mater. Sci. Eng. A 1998, 248, 87–97. [Google Scholar] [CrossRef]
- Dimiduk, D.M.; Perepezko, J.H. Mo-Si-B alloys: Developing a revolutionary turbine-engine material. MRS Bull. 2003, 28, 639–645. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer International Publishing: Basel, Switzerland, 2016; ISBN 9783319270135. [Google Scholar]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Mondal, S.; Van Smaalen, S.; Völkl, R.; Glatzel, U.; Wanderka, N. Investigation of phases in Al23Co15Cr 23Cu8Fe15Ni16 and Al 8Co17Cr17Cu8Fe17Ni 33 high entropy alloys and comparison with equilibrium phases predicted by Thermo-Calc. J. Alloys Compd. 2013, 552, 430–436. [Google Scholar] [CrossRef]
- Manzoni, A.M.; Daoud, H.M.; Voelkl, R.; Glatzel, U.; Wanderka, N. Influence of W, Mo and Ti trace elements on the phase separation in Al8Co17Cr17Cu8Fe17Ni33 based high entropy alloy. Ultramicroscopy 2015, 159, 265–271. [Google Scholar] [CrossRef]
- Manzoni, A.M.; Singh, S.; Daoud, H.M.; Popp, R.; Völkl, R.; Glatzel, U.; Wanderka, N. On the path to optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti high entropy alloy family for high temperature applications. Entropy 2016, 18, 104. [Google Scholar] [CrossRef] [Green Version]
- Bolbut, V.; Wessel, E.; Krüger, M. Phase stability and temperature-dependent compressive strength of a low-density Fe32.3Al29.3Cu11.7Ni10.8Ti15.9 alloy. Scr. Mater. 2018, 150, 54–56. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Otto, F.; Dlouhý, A.; Pradeep, K.G.; Kuběnová, M.; Raabe, D.; Eggeler, G.; George, E.P. Decomposition of the single-phase high-entropy alloy CrMnFeCoNi after prolonged anneals at intermediate temperatures. Acta Mater. 2016, 112, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Bei, H. Multi-Component Solid Solution Alloys Having High Mixing Entropy. US Patent US9150945B2, 6 October 2015. [Google Scholar]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta 25W25 and V20Nb20Mo 20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Materialprüfung, DIN-Normenausschuss. Gleitlager, DIN-Normenausschuss Wälz- und DIN 50106 Prüfung Metallischer Werkstoffe—Druckversuch bei Raumtemperatur. 2016. Available online: https://www.din.de/de/mitwirken/normenausschuesse/nawgl (accessed on 16 November 2020).
- Hasegawa, M. Thermodynamic Basis for Phase Diagrams; Elsevier Ltd.: London, UK, 2013; Volume 1, ISBN 9780080969862. [Google Scholar]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Fang, S.; Xiao, X.; Xia, L.; Li, W.; Dong, Y. Relationship between the widths of supercooled liquid regions and bond parameters of Mg-based bulk metallic glasses. J. Non-Cryst. Solids 2003, 321, 120–125. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Wiley John + Sons: Berkeley, CA, USA, 2008; ISBN 047141526X. [Google Scholar]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.P.; Ma, S.G.; Liaw, P.K.; Tang, Z.; Cheng, Y.Q.; Gao, M.C. Guidelines in predicting phase formation of high-entropy alloys. MRS Commun. 2014, 4, 57–62. [Google Scholar] [CrossRef]
- Wu, C.S.; Tsai, P.H.; Kuo, C.M.; Tsai, C.W. Effect of atomic size difference on the microstructure and mechanical properties of high-entropy alloys. Entropy 2018, 20, 967. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Becker, J.; Betke, U.; Wessel, E.; Krüger, M. Alloying effects in Mo-5X (X = Zr, Ti,V)—Microstructural modifications and mechanical properties. Mater. Today Commun. 2018, 15, 314–321. [Google Scholar] [CrossRef]
- Sahm, P.R.; Egry, I.; Volkmann, T. Schmelze, Erstarrung, Grenzflächen; Sahm, P.R., Egry, I., Volkmann, T., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1999; ISBN 978–3–540–41566–4. [Google Scholar]
- Sheikh, S.; Shafeie, S.; Hu, Q.; Ahlström, J.; Persson, C.; Veselý, J.; Zýka, J.; Klement, U.; Guo, S. Alloy design for intrinsically ductile refractory high-entropy alloys. J. Appl. Phys. 2016, 120, 164902. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Qiao, J.W.; Gao, M.C.; Hawk, J.A.; Ma, S.G.; Zhou, H. MoNbTaV medium-entropy alloy. Entropy 2016, 18, 189. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.W.; Qiao, J.W.; Gao, M.C.; Hawk, J.A.; Ma, S.G.; Zhou, H.F.; Zhang, Y. NbTaV-(Ti,W) refractory high-entropy alloys: Experiments and modeling. Mater. Sci. Eng. A 2016, 674, 203–211. [Google Scholar] [CrossRef]
- Special Metals IN718 Datasheet; Special Metals Corporation: New Hartford, NY, USA, 2007; Volume 75.
- Sengupta, A.; Putatunda, S.K.; Bartosiewicz, L.; Hangas, J.; Nailos, P.J.; Peputapeck, M.; Alberts, F.E. Tensile behavior of a new single crystal nickel-based superalloy (CMSX-4) at room and elevated temperatures. J. Mater. Eng. Perform. 1994, 3, 664–672. [Google Scholar] [CrossRef]
- Basak, A.; Holenarasipura Raghu, S.; Das, S. Microstructures and Microhardness Properties of CMSX-4® Additively Fabricated Through Scanning Laser Epitaxy (SLE). J. Mater. Eng. Perform. 2017, 26, 5877–5884. [Google Scholar] [CrossRef]
- HAYNES® 282® Alloy—Principal Features; Haynes International: Kokomo, IN, USA, 2020.





| Alloy | ΔSmix, J·K−1·mol−1 | ΔHmix, kJ·mol−1 | Ω | δr, % |
|---|---|---|---|---|
| Mo25Nb25V25W25 | 11.52 | −4.00 | 8.27 | 2.98 |
| Mo25Nb25V25Ti25 | 11.52 | −2.75 | 10.20 | 4.46 |
| Mo30Nb30V30Ti10 | 10.92 | −3.00 | 9.23 | 4.01 |
| Mo20Nb20V20W20Ti20 | 13.38 | −4.16 | 8.63 | 4.05 |
| Mo22.5Nb22.5V22.5W22.5Ti10 | 13.08 | −4.14 | 8.77 | 3.65 |
| Alloy | Theor. Lattice Parameter, Å | Exp. Lattice Parameter, Å | Deviation |
|---|---|---|---|
| Mo25Nb25V25W25 | 3.160 | 3.157 | ±0.11 |
| Mo25Nb25V25Ti25 | 3.196 | 3.174 | ±0.009 |
| Mo30Nb30V30Ti10 | 3.173 | 3.156 | ±0.002 |
| Mo20Nb20V20W20Ti20 | 3.189 | 3.164 | ±0.013 |
| Mo22.5Nb22.5V22.5W22.5Ti10 | 3.175 | 3.159 | ±0.008 |
| Alloy | Mo | V | Nb | W | Ti |
|---|---|---|---|---|---|
| Mo25Nb25V25W25 | 28 ± 1 | 21 ± 1 | 26 ± 1 | 25 ± 2 | - |
| Mo25Nb25V25Ti25 | 26 ± 1 | 24 ± 0.1 | 26 ± 0.1 | - | 24 ± 1 |
| Mo30Nb30V30Ti10 | 31 ± 0.1 | 28 ± 0.1 | 31 ± 0.1 | - | 10 ± 0.2 |
| Mo20Nb20V20W20Ti20 | 21 ± 1 | 19 ± 1 | 21 ± 0.4 | 20 ± 2 | 19 ± 1 |
| Mo22.5Nb22.5V22.5W22.5Ti10 | 26 ± 1 | 23 ± 0.2 | 22 ± 0.2 | 20 ± 0.2 | 10 ± 0.1 |
| Alloy | Area | Mo, at.% | Nb, at.% | V, at.% | W, at.% | Ti, at.% |
|---|---|---|---|---|---|---|
| Mo25Nb25V25Ti25 | Cd | 28 | 28 | 22 | - | 22 |
| Cid | 16 | 22 | 31 | - | 30 | |
| Mo20Nb20V20W20Ti20 | Cd | 23 | 19 | 14 | 32 | 12 |
| Cid | 19 | 25 | 26 | 11 | 19 | |
| Cpr | 2 | 7 | 5 | 2 | 85 |
| Alloy | ρtheor., g·cm−3 | σ0.2, MPa | σ0.2 spec., MPa∙cm3/g | Hardness HV10, GPa |
|---|---|---|---|---|
| Mo25Nb25V25Ti25 | 7.4 | 1210 ± 36 | 164 ± 5 | 4 ± 0.1 |
| Mo30Nb30V30Ti10 | 7.9 | 1441 ± 39 | 182 ± 5 | 4 ± 0.2 |
| Mo20Nb20V20W20Ti20 | 9.8 | 1289 ± 42 | 132 ± 4 | 5 ± 0.1 |
| Mo25Nb25V25W25 | 11.1 | 1243 ± 49 | 112 ± 4 | 6 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regenberg, M.; Hasemann, G.; Wilke, M.; Halle, T.; Krüger, M. Microstructure Evolution and Mechanical Properties of Refractory Mo-Nb-V-W-Ti High-Entropy Alloys. Metals 2020, 10, 1530. https://doi.org/10.3390/met10111530
Regenberg M, Hasemann G, Wilke M, Halle T, Krüger M. Microstructure Evolution and Mechanical Properties of Refractory Mo-Nb-V-W-Ti High-Entropy Alloys. Metals. 2020; 10(11):1530. https://doi.org/10.3390/met10111530
Chicago/Turabian StyleRegenberg, Maximilian, Georg Hasemann, Markus Wilke, Thorsten Halle, and Manja Krüger. 2020. "Microstructure Evolution and Mechanical Properties of Refractory Mo-Nb-V-W-Ti High-Entropy Alloys" Metals 10, no. 11: 1530. https://doi.org/10.3390/met10111530






