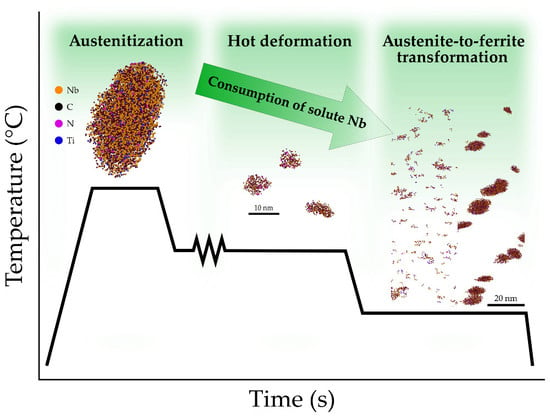
Tracing Microalloy Precipitation in Nb-Ti HSLA Steel during Austenite Conditioning
Abstract
1. Introduction
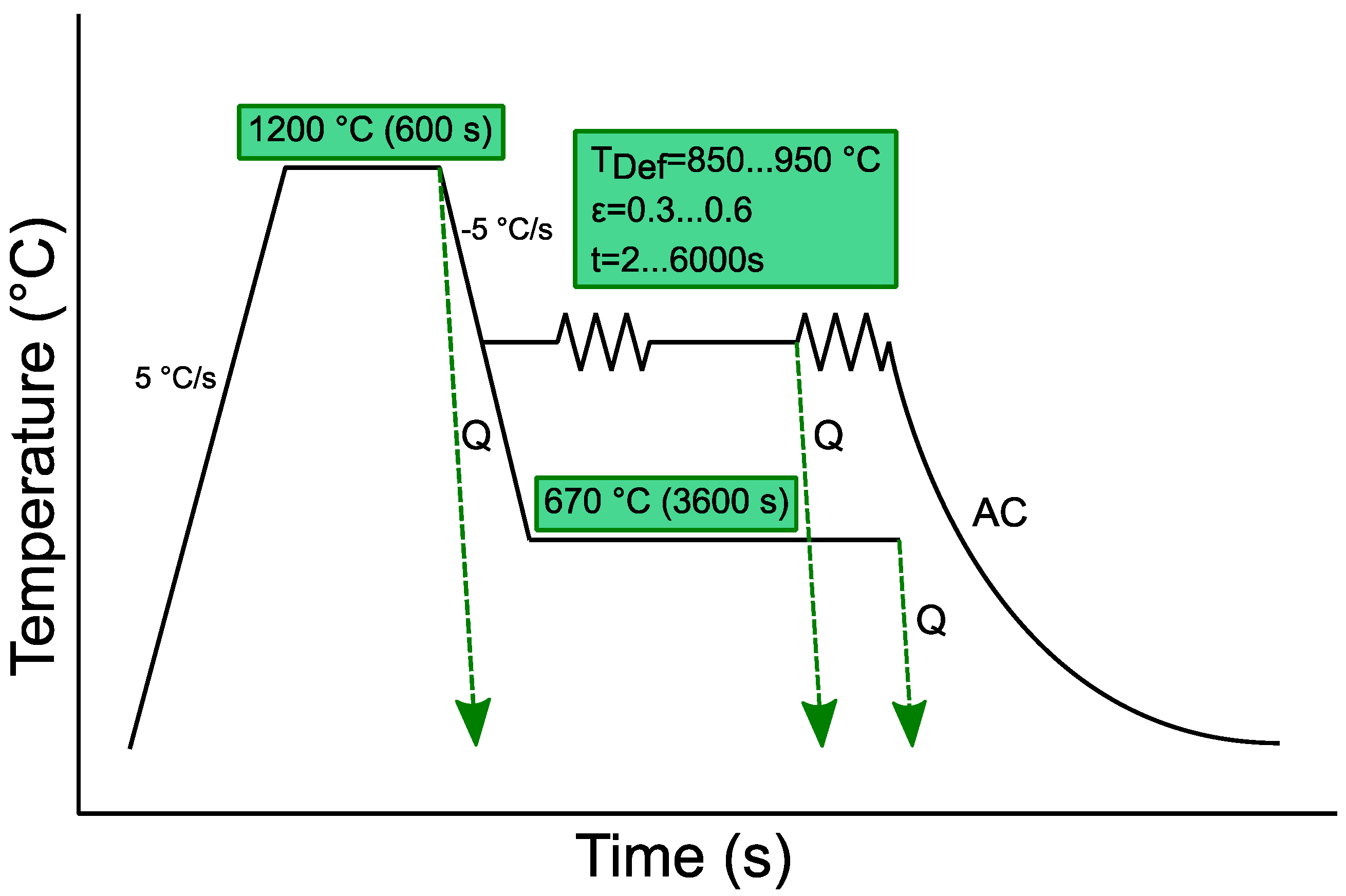
2. Materials and Methods
3. Results
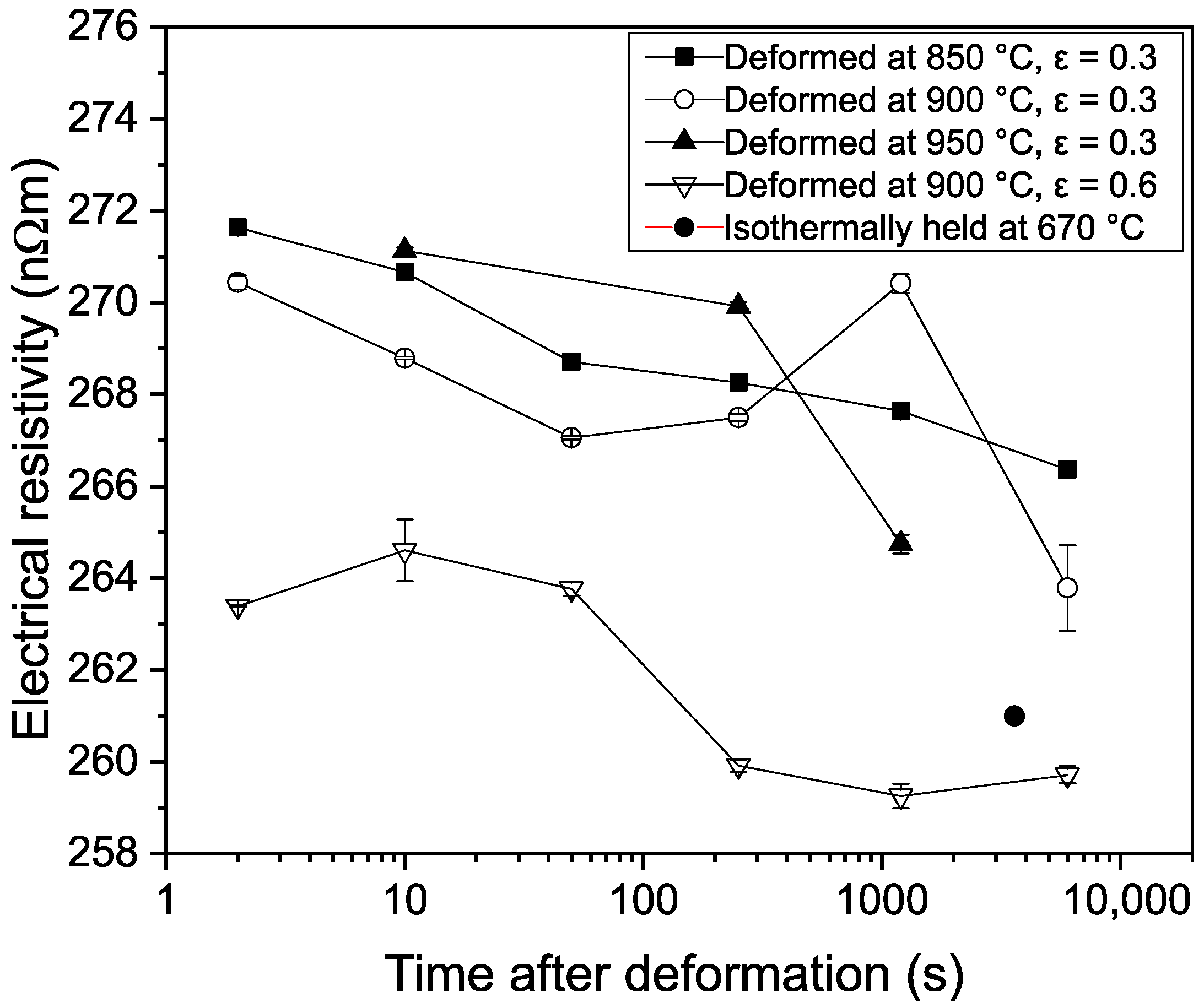
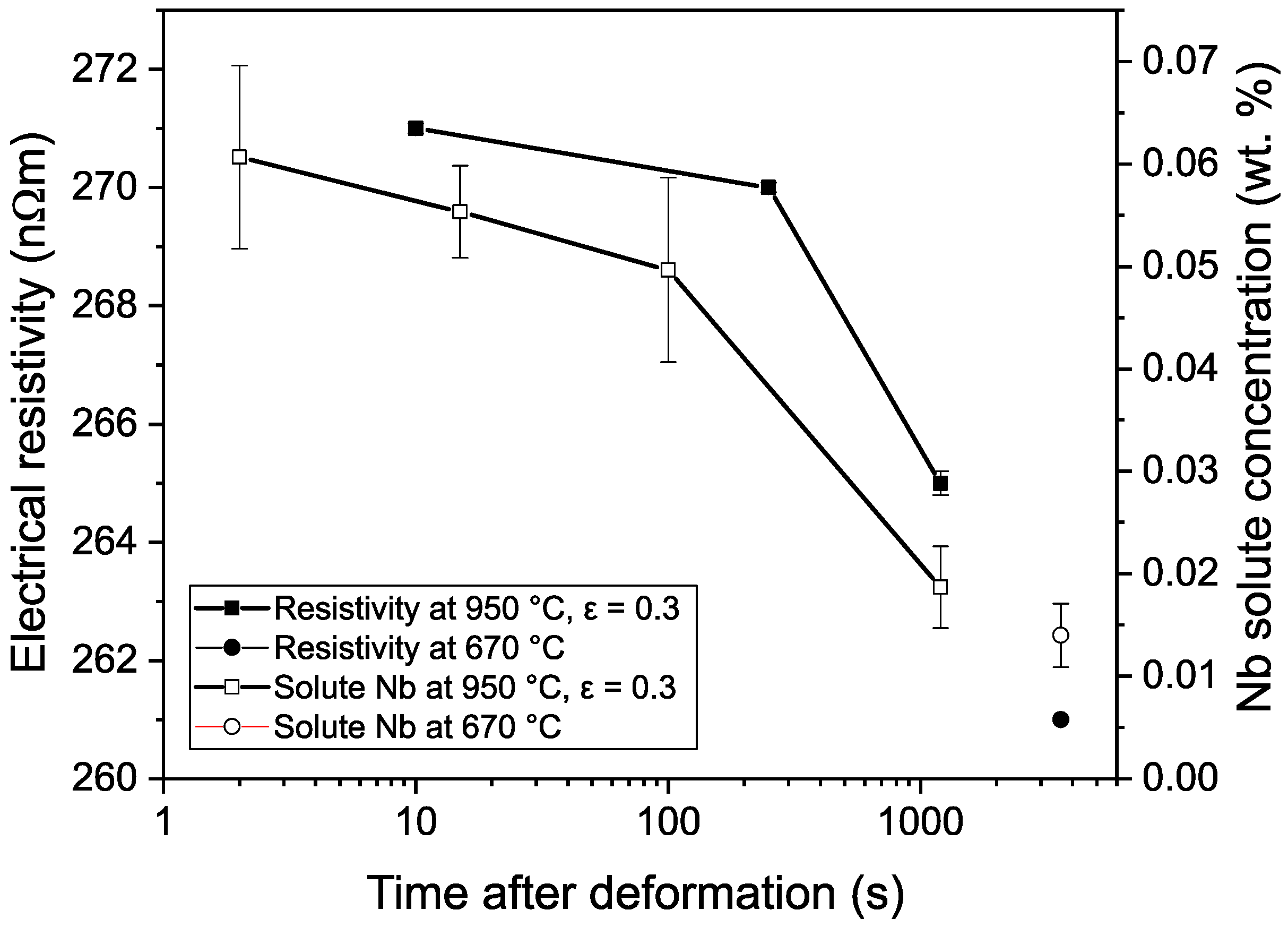
3.1. Electrical Resistivity Measurements
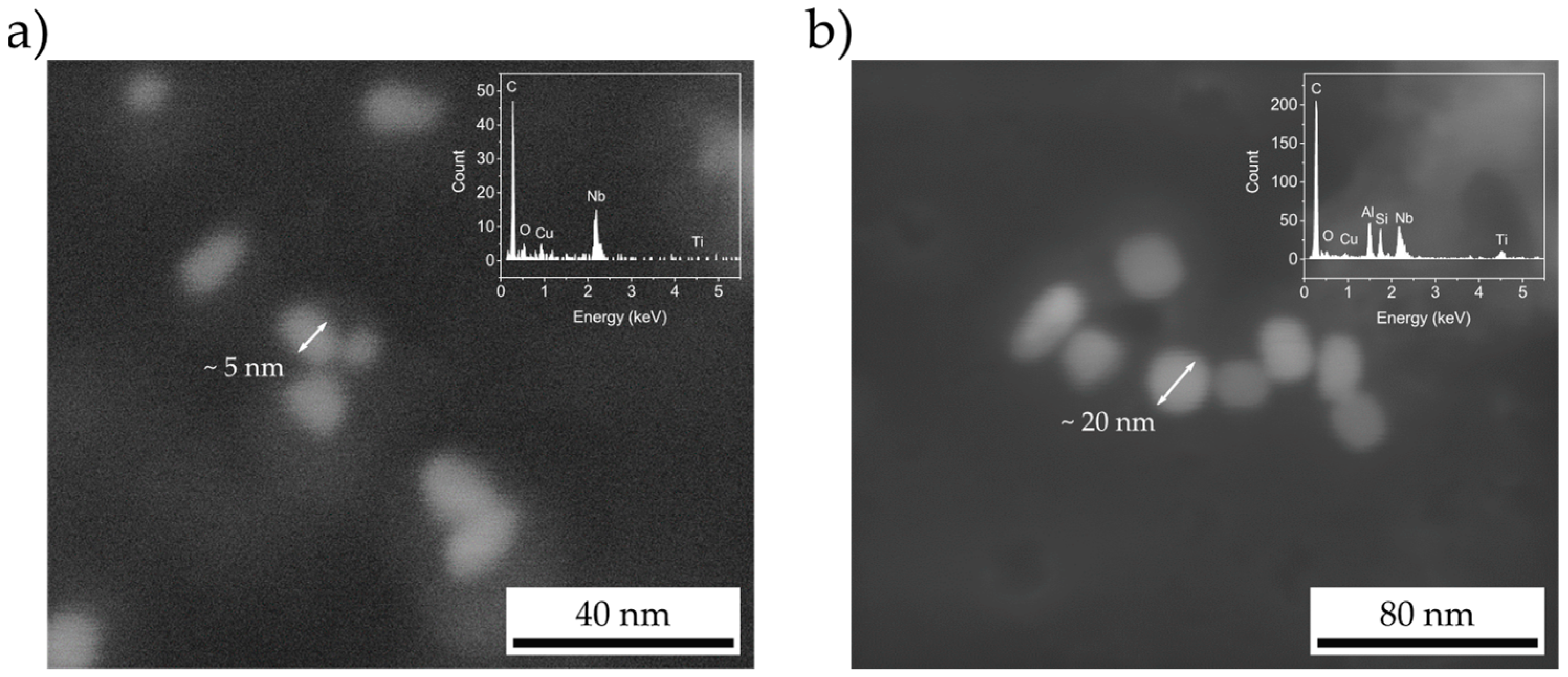
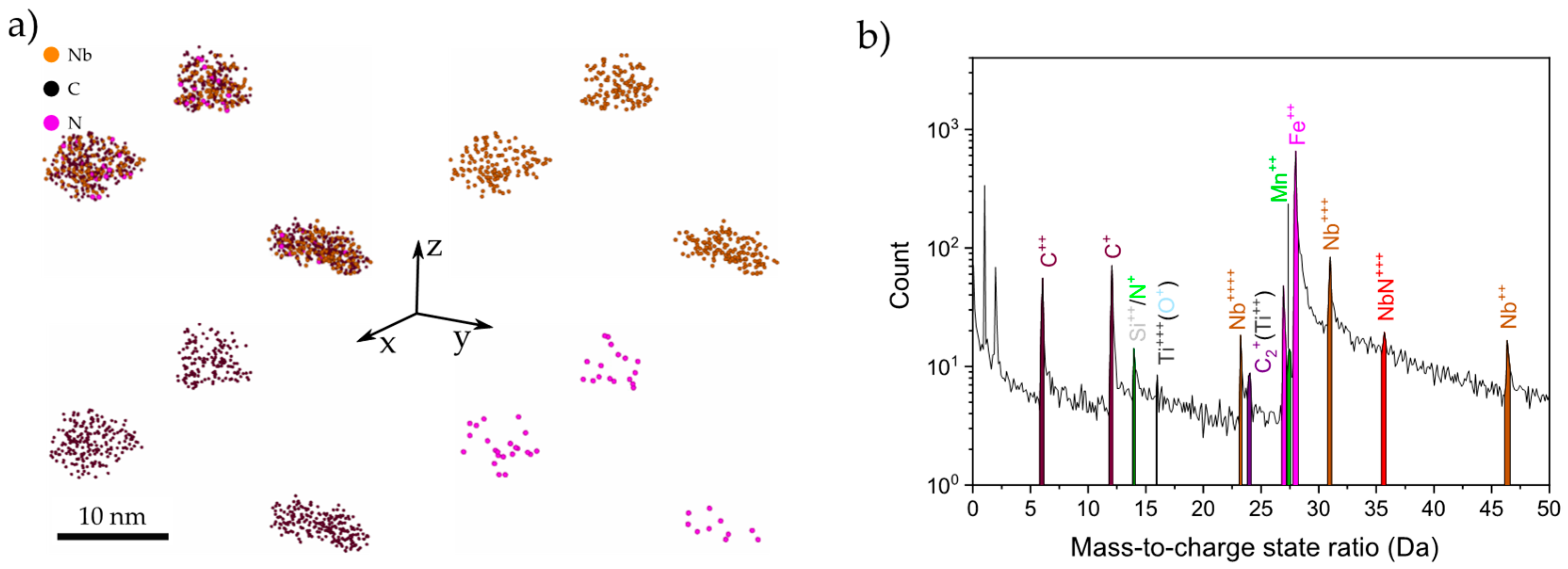
3.2. Precipitate Characterization by STEM
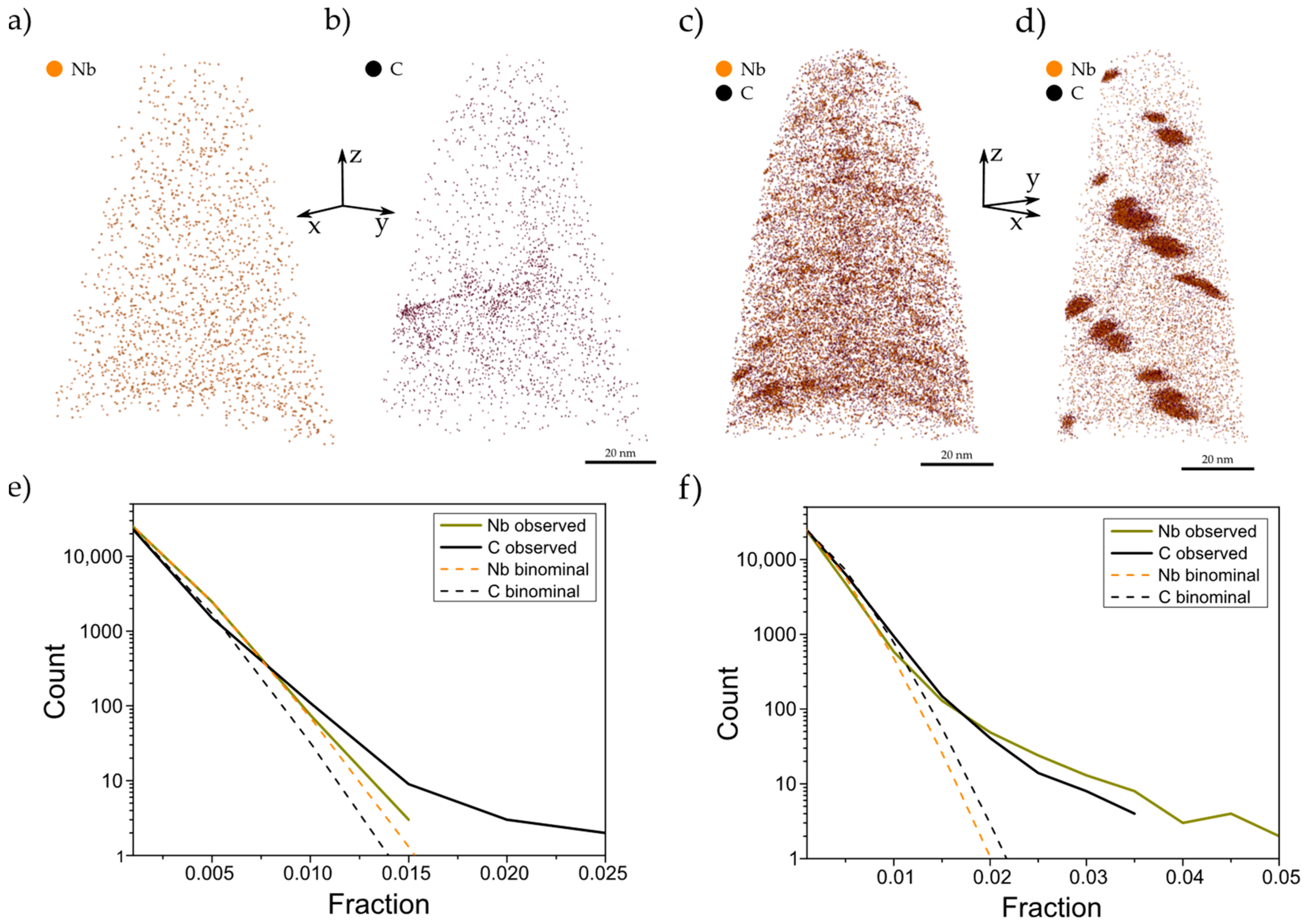
3.3. APT Analysis of Precipitates and Solute Niobium Dtatus
4. Discussion
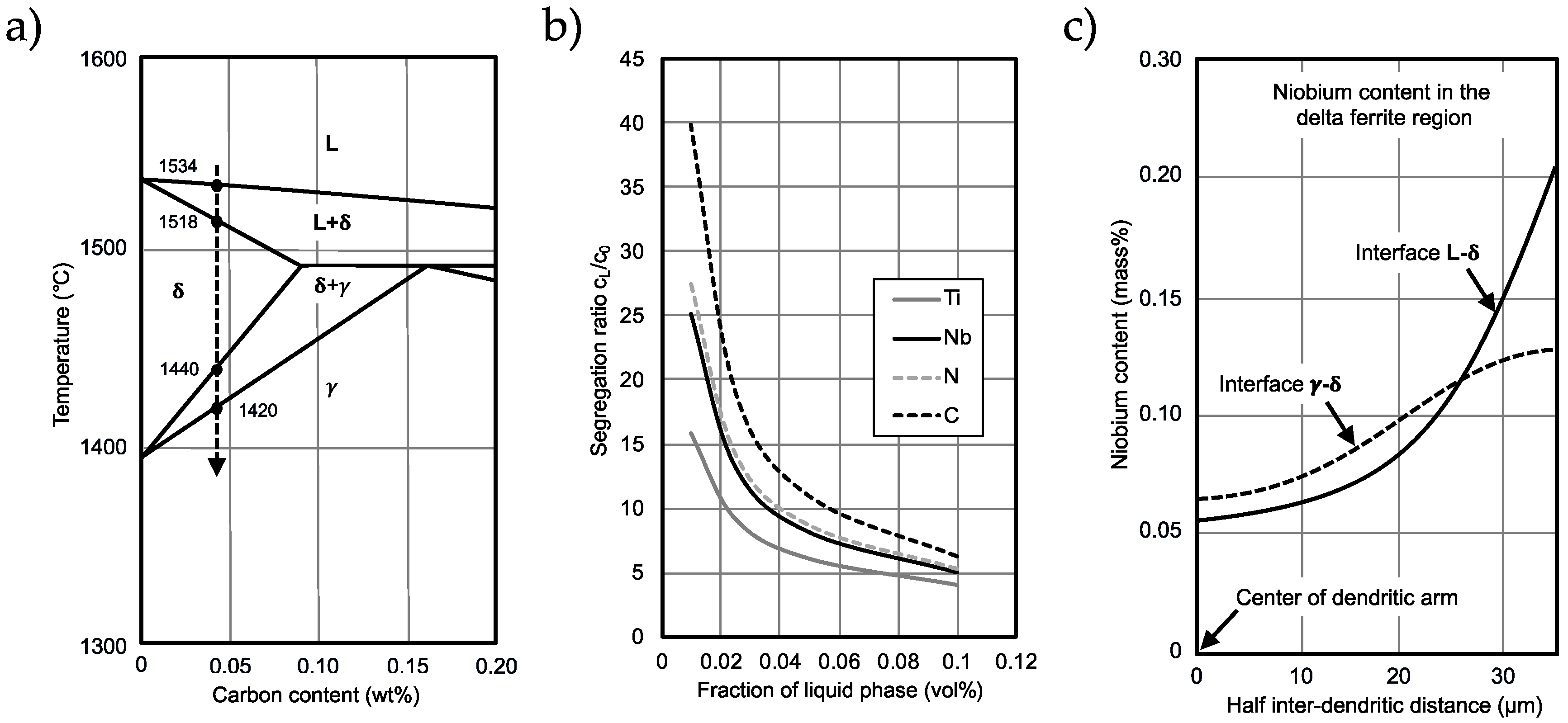
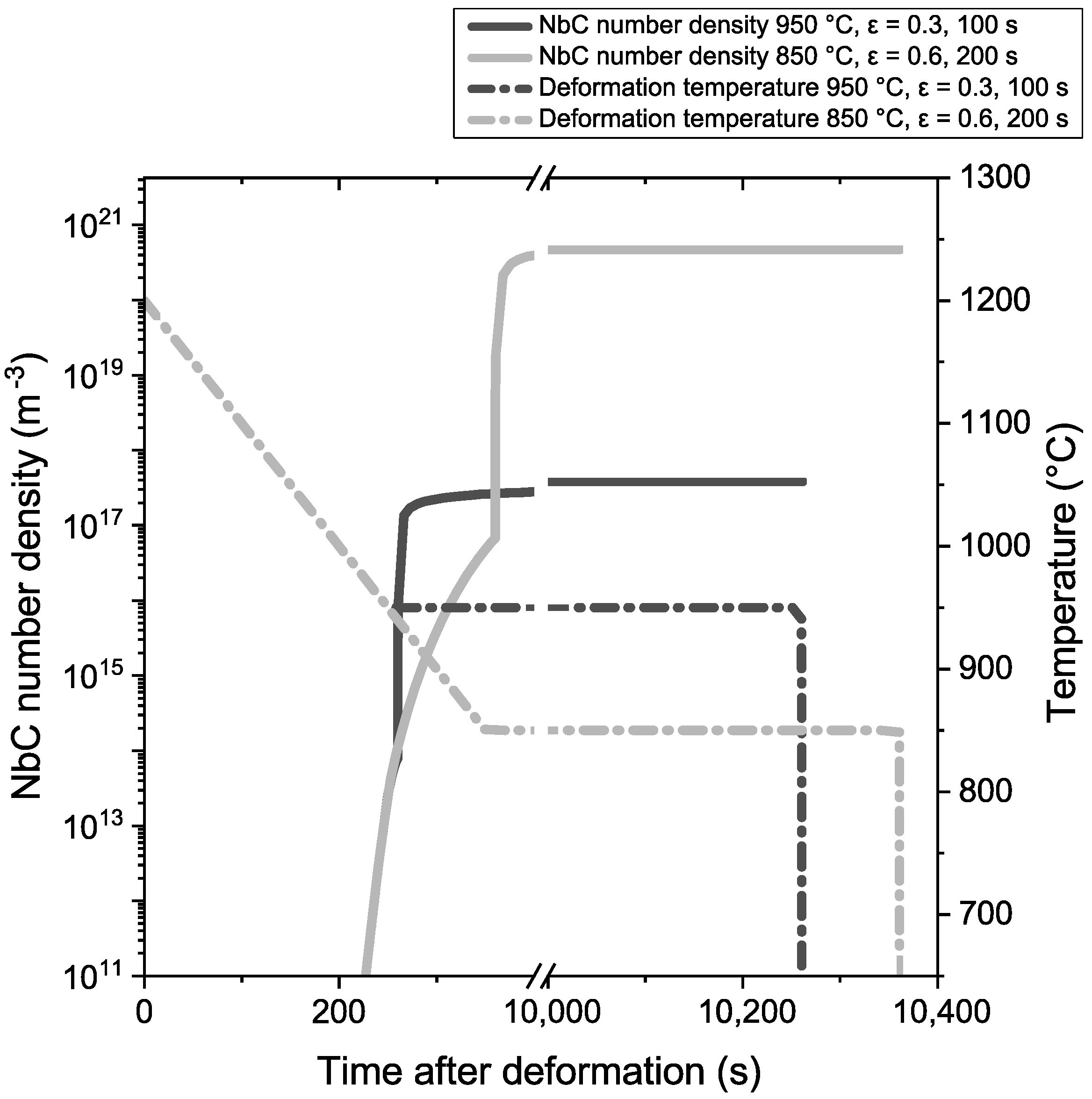
4.1. Precipitation Behavior before TMCP Treatment
4.2. Precipitation Behavior during TMCP Treatment
4.3. Precipitation during Austenite-to-Ferrite Transformation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dutta, B.; Palmiere, E.J. Effect of prestrain and deformation temperature on the recrystallization behavior of steels microalloyed with niobium. Metall. Mater. Trans. A 2003, 34, 1237–1247. [Google Scholar] [CrossRef]
- Dutta, B.; Sellars, C.M. Effect of composition and process variables on Nb(C,N) precipitation in niobium microalloyed austenite. Mater. Sci. Technol. 1987, 3, 197–206. [Google Scholar] [CrossRef]
- Deardo, A.J. Niobium in modern steels. Int. Mater. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Morrison, W.B. Microalloy steels-the beginning. Mater. Sci. Technol. 2009, 25, 1066–1073. [Google Scholar] [CrossRef]
- Hong, S.G.; Kang, K.B.; Park, C.G. Strain-induced precipitation of NbC in Nb and Nb-Ti microalloyed HSLA steels. Scr. Mater. 2002, 46, 163–168. [Google Scholar] [CrossRef]
- Cuddy, L.J.; Raley, J.C. Austenite grain coarsening in microalloyed steels. Metall. Trans. A 1983, 14, 1989–1995. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Jia, T.; Militzer, M. The Effect of Solute Nb on the Austenite-to-Ferrite Transformation. Metall. Mater. Trans. A 2014, 46, 614–621. [Google Scholar] [CrossRef]
- Altuna, M.A.; Iza-Mendia, A.; Gutiérrez, I. Precipitation of Nb in ferrite after austenite conditioning. Part II: Strengthening contribution in high-strength low-alloy (HSLA) steels. Metall. Mater. Trans. A 2012, 43, 4571–4586. [Google Scholar] [CrossRef]
- Kunze, J.; Mickel, C.; Backmann, G.; Beyer, B.; Reibold, M.; Klinkenberg, C. Precipitation of titanium nitride in low-alloyed steel during cooling and deformation. Steel Res. 1997, 68, 441–449. [Google Scholar] [CrossRef]
- Klinkenberg, C.; Hulka, K.; Bleck, W. Niobium Carbide Precipitation in Microalloyed Steel. Steel Res. Int. 2004, 75, 744–752. [Google Scholar] [CrossRef]
- Zhou, C.; Priestner, R. The evolution of precipitates in Nb-Ti microalloyed steels during solidification and post-solidification cooling. ISIJ Int. 1996, 36, 1397–1405. [Google Scholar] [CrossRef]
- Craven, A.J.; He, K.; Garvie, L.A.J.; Baker, T.N. Complex heterogeneous precipitation in titanium–niobium microalloyed Al-killed HSLA steels—I.(Ti,Nb)(C,N) particles. Acta Mater. 2000, 48, 3857–3868. [Google Scholar] [CrossRef]
- Hegetschweiler, A.; Borovinskaya, O.; Staudt, T.; Kraus, T. Single-particle mass spectrometry of titanium and niobium carbonitride precipitates in steels. Anal. Chem. 2019, 91, 943–950. [Google Scholar] [CrossRef]
- Miao, C.L.; Shang, C.J.; Zhang, G.D.; Subramanian, S.V. Recrystallization and strain accumulation behaviors of high Nb-bearing line pipe steel in plate and strip rolling. Mater. Sci. Eng. A 2010, 527, 4985–4992. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, F.; Qiao, G.; Huang, C.; Zhang, X.; Wu, Z.; Liao, B. Strain-induced precipitation and softening behaviors of high Nb microalloyed steels. Mater. Sci. Eng. A 2012, 552, 502–513. [Google Scholar] [CrossRef]
- Akben, M.G.; Bacroix, B.; Jonas, J.J. Effect of vanadium and molybdenum addition on high temperature recovery, recrystallization and precipitation behavior of niobium-based microalloyed steels. Acta Metall. 1983, 31, 161–174. [Google Scholar] [CrossRef]
- Watanabe, H.; Smith, Y.E.; Pehlke, R.D. Precipitation kinetics of niobium carbonitride in austenite of high-strength low-alloy steels. In The Hot Deformation of Austenite; TMS-AIME: New York, NY, USA, 1977; pp. 140–168. [Google Scholar]
- Herman, J.C.; Donnay, B.; Leroy, V. Precipitation kinetics of microalloying additions during hot-rolling of HSLA steels. ISIJ Int. 1992, 32, 779–785. [Google Scholar] [CrossRef]
- Okamoto, R.; Borgenstam, A.; Ågren, J. Interphase precipitation in niobium-microalloyed steels. Acta Mater. 2010, 58, 4783–4790. [Google Scholar] [CrossRef]
- Hin, C.; Bréchet, Y.; Maugis, P.; Soisson, F. Kinetics of heterogeneous dislocation precipitation of NbC in alpha-iron. Acta Mater. 2008, 56, 5535–5543. [Google Scholar] [CrossRef]
- Perrard, F.; Deschamps, A.; Bley, F.; Donnadieu, P.; Maugis, P. A small-angle neutron scattering study of fine-scale NbC precipitation kinetics in the α-Fe–Nb–C system. J. Appl. Crystallogr. 2006, 39, 473–482. [Google Scholar] [CrossRef]
- Huang, B.M.; Yang, J.R.; Yen, H.W.; Hsu, C.H.; Huang, C.Y.; Mohrbacher, H. Secondary hardened bainite. Mater. Sci. Technol. 2014, 30, 1014–1023. [Google Scholar] [CrossRef]
- Costa e Silva, A. Challenges and opportunities in thermodynamic and kinetic modeling microalloyed HSLA steels using computational thermodynamics. Calphad Comput. Coupling Phase Diagrams Thermochem. 2020, 68, 101720. [Google Scholar] [CrossRef]
- LeBon, A.; Rofes-Vernis, J.; Rossard, C. Recristallisation et précipitation provoquées par la déformation à chaud: Cas d’un acier de construction sondable au niobium. Mem. Soc. Rev. Met. 1973, 70, 577–588. [Google Scholar]
- Simoneau, R.; Bégin, G.; Marquis, A.H. Progress of NbCN precipitation in HSLA steels as determined by electrical resistivity measurements. Met. Sci. 1978, 12, 381–386. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, Y.K. Determination of Nb(C,N) dissolution temperature by electrical resistivity measurement in a low-carbon microalloyed steel. Scr. Mater. 2007, 56, 225–228. [Google Scholar] [CrossRef]
- Jung, J.-G.; Bae, J.-H.; Lee, Y.-K. Quantitative evaluation of dynamic precipitation kinetics in a complex Nb-Ti-V microalloyed steel using electrical resistivity measurements. Met. Mater. Int. 2013, 19, 1159–1162. [Google Scholar] [CrossRef]
- Jung, J.-G.; Park, J.-S.; Kim, J.; Lee, Y.-K. Carbide precipitation kinetics in austenite of a Nb–Ti–V microalloyed steel. Mater. Sci. Eng. A 2011, 528, 5529–5535. [Google Scholar] [CrossRef]
- Rivas, A.L.; Matlock, D.K.; Speer, J.G. Quantitative analysis of Nb in solution in a microalloyed carburizing steel by electrochemical etching. Mater. Charact. 2008, 59, 571–577. [Google Scholar] [CrossRef]
- Gault, B.; Moody, M.P.; Cairney, J.M.; Ringer, S.P. Atom Probe Microscopy; Springer Series in Materials Science; Springer: New York, NY, USA, 2012; Volume 160, ISBN 978-1-4614-3435-1. [Google Scholar]
- Palmiere, E.J.; Garcia, C.I.; De Ardo, A.J. Compositional and microstructural changes which attend reheating and grain coarsening in steels containing niobium. Metall. Mater. Trans. A 1994, 25, 277–286. [Google Scholar] [CrossRef]
- Nöhrer, M.; Mayer, W.; Primig, S.; Zamberger, S.; Kozeschnik, E.; Leitner, H. Influence of Deformation on the Precipitation Behavior of Nb(CN) in Austenite and Ferrite. Metall. Mater. Trans. A 2014, 45, 4210–4219. [Google Scholar] [CrossRef]
- Pereloma, E.V.; Kostryzhev, A.G.; AlShahrani, A.; Zhu, C.; Cairney, J.M.; Killmore, C.R.; Ringer, S.P. Effect of austenite deformation temperature on Nb clustering and precipitation in microalloyed steel. Scr. Mater. 2014, 75, 74–77. [Google Scholar] [CrossRef]
- Kostryzhev, A.G.; Al Shahrani, A.; Zhu, C.; Ringer, S.P.; Pereloma, E.V. Effect of deformation temperature on niobium clustering, precipitation and austenite recrystallisation in a Nb-Ti microalloyed steel. Mater. Sci. Eng. A 2013, 581, 16–25. [Google Scholar] [CrossRef]
- Kapoor, M.; O’Malley, R.; Thompson, G.B. Atom probe tomography study of multi-microalloyed carbide and carbo-nitride precipitates and the precipitation sequence in Nb-Ti HSLA steels. Metall. Mater. Trans. A 2016, 47, 1984–1995. [Google Scholar] [CrossRef]
- Maruyama, N.; Uemori, R.; Sugiyama, M. The role of niobium in the retardation of the early stage of austenite recovery in hot-deformed steels. Mater. Sci. Eng. A 1998, 250, 2–7. [Google Scholar] [CrossRef]
- Thompson, K.; Lawrence, D.; Larson, D.J.; Olson, J.D.; Kelly, T.F.; Gorman, B. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 2007, 107, 131–139. [Google Scholar] [CrossRef]
- Vaumousse, D.; Cerezo, A.; Warren, P.J. A procedure for quantification of precipitate microstructures from three-dimensional atom probe data. Ultramicroscopy 2003, 95, 215–221. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Hodgson, P.D.; Ringer, S.P.; Zheng, R.K.; Pereloma, E.V. Precipitate characterisation of an advanced high-strength low-alloy (HSLA) steel using atom probe tomography. Scr. Mater. 2007, 56, 601–604. [Google Scholar] [CrossRef]
- Breen, A.J.; Xie, K.Y.; Moody, M.P.; Gault, B.; Yen, H.-W.; Wong, C.C.; Cairney, J.M.; Ringer, S.P. Resolving the morphology of niobium carbonitride nano-precipitates in steel using atom probe tomography. Microsc. Microanal. 2014, 20, 1100–1110. [Google Scholar] [CrossRef]
- Scheil, E. Bemerkungen zur Schichtkristallbildung. Z. Met. 1942, 34, 70–72. [Google Scholar]
- Morita, Z.; Tanaka, T. Thermodynamics of solute distributions between solid and liquid phases in iron-base ternary alloys. Trans. Iron Steel Inst. Jpn. 1983, 23, 824–833. [Google Scholar] [CrossRef]
- Chen, Z.; Loretto, M.H.; Cochrane, R.C. Nature of large precipitates in titanium-containing HSLA steels. Mater. Sci. Technol. 1987, 3, 836–844. [Google Scholar] [CrossRef]
- Narita, K. Physical Chemistry of the groups IVa(Ti,Zr), Va(V,Nb,Ta) and the rare earth elements in steel. Trans. Iron Steel Inst. Jpn. 1975, 15, 145–152. [Google Scholar] [CrossRef]
- Vurpillot, F.; Bostel, A.; Blavette, D. Trajectory overlaps and local magnification in three-dimensional atom probe. Appl. Phys. Lett. 2000, 76, 3127–3129. [Google Scholar] [CrossRef]
- Thuvander, M.; Weidow, J.; Angseryd, J.; Falk, L.K.L.; Liu, F.; Sonestedt, M.; Stiller, K.; Andrén, H.-O. Quantitative atom probe analysis of carbides. Ultramicroscopy 2011, 111, 604–608. [Google Scholar] [CrossRef]
- Danoix, F.; Bémont, E.; Maugis, P.; Blavette, D. Atom probe tomography i. early stages of precipitation of nbc and nbn in ferritic steels. Adv. Eng. Mater. 2006, 8, 1202–1205. [Google Scholar] [CrossRef]
- Li, Y.J.; Ponge, D.; Choi, P.; Raabe, D. Segregation of boron at prior austenite grain boundaries in a quenched martensitic steel studied by atom probe tomography. Scr. Mater. 2015, 96, 13–16. [Google Scholar] [CrossRef]
- Li, Y.J.; Ponge, D.; Choi, P.; Raabe, D. Atomic scale investigation of non-equilibrium segregation of boron in a quenched Mo-free martensitic steel. Ultramicroscopy 2015, 159, 240–247. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1992; ISBN 0748757414. [Google Scholar]





| Element | C | Si | Mn | P | Cr | Mo | Ni | Cu | Al | N | Nb | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 0.042 | 0.32 | 1.69 | 0.012 | 0.036 | 0.021 | 0.195 | 0.215 | 0.033 | 0.005 | 0.085 | 0.017 |
| Inter-Pass Time | Mean PAG Equivalent Grain Diameter (µm) | Number of Grains |
|---|---|---|
| 2 s | 27.35 | 4144 |
| 15 s | 24.33 | 5121 |
| 100 s | 22.59 | 5634 |
| Element | Nb | C | Ti | N |
|---|---|---|---|---|
| Atom count | 4582 | 4400 | 480 | 177 |
| at. % | 47.5 | 45.6 | 5.0 | 1.7 |
| Element | Nb | C | Ti | N |
|---|---|---|---|---|
| Atom count | 410 | 688 | - | 74 |
| at. % | 35.0 | 58.7 | - | 6.3 |
| Element | Nb | C | Ti | N |
|---|---|---|---|---|
| IP (Figure 11d) | - | - | - | - |
| Atom count | 752 | 846 | 54 | 7 |
| at. % | 45.3 | 51.0 | 3.3 | 0.4 |
| Clusters (Figure 11c) | - | - | - | - |
| Atom count | 7708 | 8566 | 405 | 190 |
| at. % | 45.7 | 50.8 | 2.4 | 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webel, J.; Herges, A.; Britz, D.; Detemple, E.; Flaxa, V.; Mohrbacher, H.; Mücklich, F. Tracing Microalloy Precipitation in Nb-Ti HSLA Steel during Austenite Conditioning. Metals 2020, 10, 243. https://doi.org/10.3390/met10020243
Webel J, Herges A, Britz D, Detemple E, Flaxa V, Mohrbacher H, Mücklich F. Tracing Microalloy Precipitation in Nb-Ti HSLA Steel during Austenite Conditioning. Metals. 2020; 10(2):243. https://doi.org/10.3390/met10020243
Chicago/Turabian StyleWebel, Johannes, Adrian Herges, Dominik Britz, Eric Detemple, Volker Flaxa, Hardy Mohrbacher, and Frank Mücklich. 2020. "Tracing Microalloy Precipitation in Nb-Ti HSLA Steel during Austenite Conditioning" Metals 10, no. 2: 243. https://doi.org/10.3390/met10020243
APA StyleWebel, J., Herges, A., Britz, D., Detemple, E., Flaxa, V., Mohrbacher, H., & Mücklich, F. (2020). Tracing Microalloy Precipitation in Nb-Ti HSLA Steel during Austenite Conditioning. Metals, 10(2), 243. https://doi.org/10.3390/met10020243