Enhancing the Corrosion Protection of AA2024-T3 Alloy by Surface Treatments Based on Piperazine-Modified Hybrid Sol–Gel Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Unmodified Hybrid Sol–Gel Films
2.3. Piperazine-Modified Hybrid Sol–Gel Films
2.4. Coating Thickness
2.5. Viscosity Measurements
2.6. FTIR Spectroscopy
2.7. Liquid-State 29Si-NMR Spectra
2.8. Electrochemical Techniques
3. Results and Discussion
3.1. Sol–Gel Films
3.2. Characterization of Unmodified Sol–Gel Films
3.3. Characterization of Piperazine-Modified Sol–Gel Samples
3.4. EIS Measurements on Unmodified Sol–Gel Films
3.5. EIS Measurements on Modified Sol–Gel Films
3.6. Equivalent Circuit Models for Analyzing the Electrochemical Impedance Spectroscopy Data of the AA2024-T3 Alloy/Sol–Gel Film Systems
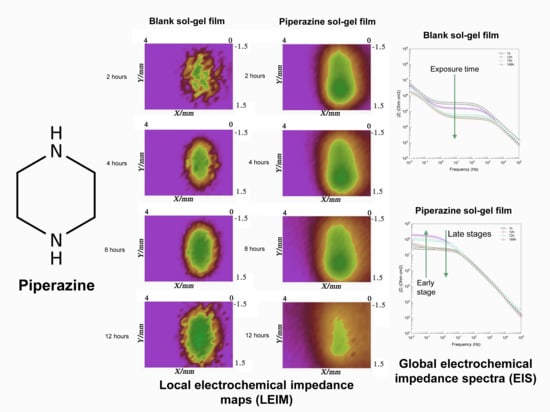
3.7. Local Electrochemical Impedance Mapping (LEIM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminum alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, Y.; Yu, Y.; Liu, B.; Hashimoto, T.; Liu, H.; Dong, Z. Intergranular corrosion in AA2024-T3 aluminum alloy: The influence of stored energy and prediction. Corros. Sci. 2019, 155, 1–12. [Google Scholar] [CrossRef]
- Trdan, U.; Sano, T.; Klobčar, D.; Sano, Y.; Grum, J.; Šturm, R. Improvement of corrosion resistance of AA2024-T3 using femtosecond laser peening without protective and confining medium. Corros. Sci. 2018, 143, 46–55. [Google Scholar] [CrossRef]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Hughes, A.E.; Boag, A.; Glenn, A.M.; McCulloch, D.; Muster, T.H.; Ryan, C.; Luo, C.; Zhou, X.; Thompson, G.E. Corrosion of AA2024-T3 Part II: Co-operative corrosion. Corros. Sci. 2011, 53, 27–39. [Google Scholar] [CrossRef]
- Mitton, D.B.; Carangelo, A.; Acquesta, A.; Monetta, T.; Curioni, M.; Bellucci, F. Selected Cr(VI) replacement options for aluminum alloys: A literature survey. Corros. Rev. 2017, 35, 365–381. [Google Scholar] [CrossRef]
- Becker, M. Chromate-free chemical conversion coatings for aluminum alloys. Corros. Rev. 2019, 37, 321–342. [Google Scholar] [CrossRef]
- Kakde, V.; Mannari, V. Advanced chrome-free organic-inorganic hybrid pretreatments for aerospace aluminum alloy 2024-T3-application of novel bis-ureasil sol-gel precursors. J. Coat. Technol. Res. 2009, 6, 201–211. [Google Scholar] [CrossRef]
- Basu, B.J.; Srinivasan, A.; Manasa, J.; Grips, V.K.W. Improved corrosion protection of aluminum alloy AA 2024 by sol-gel hybrid coatings after surface pretreatment by silanization. Surf. Eng. 2012, 28, 294–299. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Kallip, S.; Starykevich, M.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024. Corros. Sci. 2018, 143, 299–313. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, D.; Hou, L.; Li, X.; Wei, Y. Understanding of the corrosion protection by V(IV) conversion coatings from a sol-gel perspective. Corros. Sci. 2019, 161, 108196. [Google Scholar] [CrossRef]
- Lev, O.; Wu, Z.; Bharathi, S.; Glezer, V.; Modestov, A.; Gun, J.; Rabinovich, L.; Sampath, S. Sol-gel materials in electrochemistry. Chem. Mat. 1997, 9, 2354–2375. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Miranda Salvado, I.M.; Ferreira, M.G.S. Corrosion protective properties of nanostructured sol-gel hybrid coatings to AA2024-T3. Surf. Coat. Technol. 2006, 200, 3084–3094. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol–gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3: Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-filled sol-gel coatings for corrosion protection of AA2024-T3 aluminum alloy. Corr. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Álvarez, D.; Collazo, A.; Hernández, M.; Nóvoa, X.R.; Pérez, C. Characterization of hybrid sol-gel coatings doped with hydrotalcite-like compounds to improve corrosion resistance of AA2024-T3 alloys. Prog. Org. Coat. 2010, 68, 91–299. [Google Scholar] [CrossRef]
- Terada, M.; Queiroz, F.M.; Aguiar, D.B.S.; Ayusso, V.H.; Costenaro, H.; Olivier, M.-G.; De Melo, H.G.; Costa, I. Corrosion resistance of tartaric-sulfuric acid anodized AA2024-T3 sealed with Ce and protected with hybrid sol-gel coating. Surf. Coat. Technol. 2019, 372, 422–426. [Google Scholar] [CrossRef]
- Hu, T.-H.; Shi, H.-W.; Wei, T.; Fan, S.-H.; Liu, F.-C.; Han, E.-H. Corrosion protection of AA2024-T3 by cerium malate and cerium malate-doped sol-gel coatings. Acta Metall. Sin. Engl. Lett. 2019, 32, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, R.; Nóvoa, X.R.; Pérez, C. Hydrophobic surface treatments for improving the corrosion resistance of anodized AA2024-T3 alloys. Electroch. Acta 2019, 303, 56–66. [Google Scholar] [CrossRef]
- Hegde, M.; Kavanagh, Y.; Duffy, B.; Tobin, E.F. Preliminary Evaluation of functional coatings for marine based renewable energy applications. Lect. Notes Mech. Eng. 2020, 672–683. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid sol-gel coatings: Smart and green materials for corrosion mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Hitzky, E.; Casal, B.; Aranda, P.; Galván, J.C. Inorganic-organic nanocomposite materials based on macrocyclic compounds. Rev. Inorg. Chem. 2001, 21, 125–159. [Google Scholar] [CrossRef]
- Jiménez-Morales, A.; Galván, J.C.; Aranda, P. A new silver-ion selective sensor based on a polythiacrown-ether entrapped by sol-gel. Electrochim. Acta 2002, 47, 2281–2287. [Google Scholar] [CrossRef]
- Garcia-Heras, M.; Jimenez-Morales, A.; Casal, B.; Galvan, J.C.; Radzki, S.; Villegas, M.A. Preparation and electrochemical study of cerium-silica sol-gel thin films. J. Alloy. Compd. 2004, 380, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Rosero-Navarro, N.C.; Pellice, S.A.; Durán, A.; Aparicio, M. Effects of Ce-containing sol-gel coatings reinforced with SiO2 nanoparticles on the protection of AA2024. Corr. Sci. 2008, 50, 1283–1291. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Paussa, L.; Andreatta, F.; Castro, Y.; Durán, A.; Aparicio, M.; Fedrizzi, L. Optimization of hybrid sol-gel coatings by combination of layers with complementary properties for corrosion protection of AA2024. Prog. Org. Coat. 2010, 69, 167–174. [Google Scholar] [CrossRef]
- Paussa, L.; Rosero-Navarro, N.C.; Andreatta, F.; Castro, Y.; Duran, A.; Aparicio, M.; Fedrizzi, L. Inhibition effect of cerium in hybrid sol-gel films on aluminum alloy AA2024. Surf. Interface Anal. 2010, 42, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Andreatta, F.; Paussa, L.; Lanzutti, A.; Rosero Navarro, N.C.; Aparicio, M.; Castro, Y.; Duran, A.; Ondratschek, D.; Fedrizzi, L. Development and industrial scale-up of ZrO2 coatings and hybrid organic-inorganic coatings used as pre-treatments before painting aluminum alloys. Prog. Org. Coat. 2011, 72, 3–14, Cited 33 times. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Corrosion behavior and chemical stability of transparent hybrid sol-gel coatings deposited on aluminum in acidic and alkaline solutions. Prog. Org. Coat. 2018, 124, 286–295. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Hybrid coatings from novel silane-modified glycidyl carbamate resins and amine crosslinkers. Prog. Org. Coat. 2009, 66, 73–85. [Google Scholar] [CrossRef]
- Croes, K.J.; Vreugdenhil, A.J.; Yan, M.; Singleton, T.A.; Boraas, S.; Gelling, V.J. An electrochemical study of corrosion protection by in situ oxidative polymerization in phenylenediamine crosslinked sol–gel hybrid coatings. Electrochim. Acta 2011, 56, 7796–7804. [Google Scholar] [CrossRef]
- Woods, M.E.; Vreugdenhil, A.J. Continuously responsive epoxy-amine cross-linked silicon sol-gel materials. J. Coat. Technol. Res. 2006, 41, 7545–7554. [Google Scholar] [CrossRef]
- Vreugdenhil, A.J.; Gelling, V.J.; Woods, M.E.; Schmelz, J.R.; Enderson, B.P. The role of crosslinkers in epoxy-amine crosslinked silicon sol–gel barrier protection coatings. Thin Solid Films 2008, 517, 538–543. [Google Scholar] [CrossRef]
- Rathi, A.K.; Syed, R.; Shin, H.-S.; Patel, R.V. Piperazine derivatives for therapeutic use: A patent review (2010-present). Expert Opin. Ther. Patents 2016, 26, 777–797. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, X.; Pu, X.-J.; Zheng, X.; Liu, B.; Rao, G.-X.; Wan, C.-P.; Mao, Z.-W. 2-Benzoylbenzofuran derivatives possessing piperazine linker as anticancer agents. Bioorg. Med. Chem. Lett. 2019, 29, 806–810. [Google Scholar] [CrossRef]
- Mazzotta, S.; Marrugal-Lorenzo, J.A.; Vega-Holm, M.; Serna-Gallego, A.; Álvarez-Vidal, J.; Berastegui-Cabrera, J.; Pérez del Palacio, J.; Díaz, C.; Aiello, F.; Pachón, J.; et al. Optimization of piperazine-derived ureas privileged structures for effective antiadenovirus agents. Eur. J. Med. Chem. 2020, 185, 111840. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Li, C.; Peng, W.-X.; Hemmati, A.; Hemmati, A. Assessment of mass transfer correlations used in post-combustion CO2 capture by piperazine activated 2-amino-2-methyl-1-propanol (a-AMP). J. Nat. Gas Sci. Eng. 2020, 73, 103051. [Google Scholar] [CrossRef]
- Yuan, Y.; Rochelle, G.T. CO2 absorption rate and capacity of semi-aqueous piperazine for CO2 capture. Int. J. Greenh. Gas. Control. 2019, 85, 182–186. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Lal, B. Performance of aqueous tetrabutylammonium hydroxide, piperazine and their blends for carbon dioxide capture. J. Mol. Liq. 2018, 266, 522–528. [Google Scholar] [CrossRef]
- Lin, F.-W.; Xu, X.-L.; Wu, J.; Wan, L.-S.; Xu, Z.-K. Cobalt-porphyrin/dansyl piperazine complex coated filter paper for “turn on” fluorescence sensing of ammonia gas. RSC Adv. 2015, 5, 99361–99363. [Google Scholar] [CrossRef]
- Gan, L.H.; Roshan Deen, G.; Gan, Y.Y.; Tam, K.C. Water sorption studies of new pH-responsive N-acryloyl-N′-methyl piperazine and methyl methacrylate hydrogels. Eur. Polym. J. 2001, 37, 1473–1478. [Google Scholar] [CrossRef]
- Mah, C.H.; Wu, Q.Y.; Deen, G.R. Effect of nature of chemical crosslinker on swelling and solubility parameter of a new stimuli-responsive cationic poly (N-acryloyl-N′-propyl piperazine) hydrogel. Polym. Bull. 2018, 75, 221–238. [Google Scholar] [CrossRef]
- Xu, M.-J.; Ma, K.; Liu, C.; Li, B. Synthesis of the poly(phosphoric-boric acid) piperazine and its application as an effective flame retardant for epoxy resins. Polym. Eng. Sci. 2018, 58, 1858–1867. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Yu, L.-X.; Long, J.-W.; Qi, M.; Chen, L.; Wang, Y.-Z. Piperazine-modified ammonium polyphosphate as monocomponent flame-retardant hardener for epoxy resin: Flame retardance, curing behavior and mechanical property. Polym. Chem. 2016, 7, 3003–3012. [Google Scholar] [CrossRef]
- Ousslim, A.; Chetouani, A.; Hammouti, B.; Bekkouch, K.; Al-Deyab, S.S.; Aouniti, A.; Elidrissi, A. Thermodynamics, quantum and electrochemical studies of corrosion of iron by piperazine compounds in sulphuric acid. Int. J. Electrochem. Sci. 2013, 8, 5980–6004. [Google Scholar]
- Mondal, S.K.; Taylor, S.R. The identification and characterization of organic corrosion inhibitors: Correlation of a computational model with experimental results. J. Electrochem. Soc. 2014, 161, C476–C485. [Google Scholar] [CrossRef]
- Nnaji, N.J.N.; Ujam, O.T.; Ibisi, N.E.; Ani, J.U.; Onuegbu, T.O.; Olasunkanmi, L.O.; Ebenso, E.E. Morpholine and piperazine based carboxamide derivatives as corrosion inhibitors of mild steel in HCl medium. J. Mol. Liq. 2017, 230, 652–661. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Aragon, E.; Merlatti, C.; Pébère, N. Delaminated areas beneath organic coating: A local electrochemical impedance approach. Corros. Sci. 2006, 48, 1779–1790. [Google Scholar] [CrossRef]
- Jimenez-Morales, A.; Galván, J.C.; Aranda, P.; Ruiz-Hitzky, E. Hybrid organic-inorganic electrode-membranes based on organo- polysiloxane/macrocycle systems. Mater. Res. Soc. Symp. Proc. 1998, 519, 211–216. [Google Scholar] [CrossRef]
- El Hadad, A.A.; Carbonell, D.; Barranco, V.; Jiménez-Morales, A.; Casal, B.; Galván, J.C. Preparation of sol–gel hybrid materials from γ-methacryloxypropyltrimethoxysilane and tetramethyl orthosilicate: Study of the hydrolysis and condensation reactions. Colloid Polym. Sci. 2011, 289, 1875–1883. [Google Scholar] [CrossRef] [Green Version]
- Aranda, P.; Jiménez-Morales, A.; Galván, J.C.; Casal, B.; Ruiz-Hitzky, E. Composite membranes based on macrocycle/polysiloxanes: Preparation, characterization and electrochemical behavior. J. Mat. Chem. 1995, 5, 817–826. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Sanz, J. Polymerization of hybrid organic-inorganic materials from several silicon compounds followed by TGA/DTA, FTIR and NMR techniques. Prog. Org. Coat. 2014, 77, 880–891. [Google Scholar] [CrossRef]
- Fardjaoui, N.-E.-H.; Wicklein, B.; Aranda, P.; Sobrados, I.; El Berrichi, F.Z.; Ruiz-Hitzky, E. Modulation of inorganic matrices for functional nanoarchitectures fabrication: The simultaneous effect of moisture and temperature in the preparation of metakaolin based geopolymers. Bull. Chem. Soc. Jpn. 2018, 91, 1158–1167. [Google Scholar] [CrossRef]
- Lino, A.M.; Gehlen, M.H. Styryl dye formation promoted by catalytic centers of piperazine bound to a silica surface traced by single molecule fluorescence microscopy. Phys. Chem. Chem. Phys. 2017, 19, 20984–20990. [Google Scholar] [CrossRef] [PubMed]
- Beaunier, L.; Epelboin, I.; Lestrade, J.C.; Takenouti, H. Etude electrochimique, et par microscopie electronique a balayage, du fer recouvert de peinture. Surf. Technol. 1976, 4, 237–254. [Google Scholar] [CrossRef]
- Walter, G.W. A review of impedance plot methods used for corrosion performance analysis of painted metals. Corr. Sci. 1986, 26, 681–703. [Google Scholar] [CrossRef]
- Feliu, S.; Galván, J.C.; Morcillo, M. An interpretation of electrical impedance diagrams for painted galvanized steel. Prog. Org. Coat. 1989, 17, 143–153. [Google Scholar] [CrossRef]
- Chimenti, S.; Vega, J.M.; Aguirre, M.; García-Lecina, E.; Díez, J.A.; Grande, H.-J.; Paulis, M.; Leiza, J.R. Effective incorporation of ZnO nanoparticles by miniemulsion polymerization in waterborne binders for steel corrosion protection. J. Coat. Technol. Res. 2017, 14, 829–1839. [Google Scholar] [CrossRef]
- Koochaki, M.S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ashrafi, A.; Magni, M.; Trasatti, S.P. Facile strategy toward the development of a self-healing coating by electrospray method. Mater. Res. Express 2019, 6, 116444. [Google Scholar] [CrossRef]
- Amand, S.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Constant-phase-element behavior caused by inhomogeneous water uptake in anti-corrosion coatings. Electrochim. Acta 2013, 87, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Determination of resistivity profiles in anti-corrosion coatings from constant-phase-element parameters. Prog. Org. Coat. 2014, 77, 2076–2083. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concernng the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Constant-phase-element behavior caused by coupled resistivity and permittivity distributions in films. J. Electrochem. Soc. 2011, 158, C424–C428. [Google Scholar] [CrossRef]
- Van Westing, E.P.M.; Ferrari, G.M.; De Wit, J.H.W. The determination of coating performance with impedance measurements-II. Water uptake of coatings. Corros. Sci. 1994, 36, 957–977. [Google Scholar] [CrossRef]
- Gharbi, O.; Ngo, K.; Turmine, M.; Vivier, V. Local electrochemical impedance spectroscopy: A window into heterogeneous interfaces. Curr. Opin. Electrochem. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Barranco, V.; Carmona, N.; Galván, J.C.; Grobelny, M.; Kwiatkowski, L.; Villegas, M.A. Electrochemical study of tailored sol–gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy. Prog. Org. Coat. 2010, 68, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Huang, V.M.; Wu, S.-L.; Orazem, M.E.; Pébre, N.; Tribollet, B.; Vivier, V. Local electrochemical impedance spectroscopy: A review and some recent developments. Electrochim. Acta 2011, 56, 8048–8057. [Google Scholar] [CrossRef] [Green Version]
- Montoya, R.; García-Galván, F.R.; Jiménez-Morales, A.; Galván, J.C. Effect of conductivity and frequency on detection of heterogeneities in solid/liquid interfaces using local electrochemical impedance: Theoretical and experimental study. Electrochem. Commun. 2012, 15, 5–9. [Google Scholar] [CrossRef] [Green Version]


















| Sample | 212 | 21PIP |
|---|---|---|
| MAPTMS/TMOS molar ratio | 2:1 | 2:1 |
| Silane/H2O/EtOH molar ratio | 1:3:3 | 1:3:3 |
| H2O/piperazine molar ratio | – | 1:1000 |
| Processing previous to dip-coating | Condensed 2 h at 60 °C | Stirred 2 h at room temperature (ca. 20 °C) |
| Curing temperature of the films | 120 °C | Room temperature |
| Curing time of the films | 2 h | 12 h |
| Sample | 21 | 31 | 41 |
|---|---|---|---|
| Viscosity (mPa·s) | 5.97–6.96 | 5.06–6.33 | 5.48–6.39 |
| Thickness (µm) | 2.78 ± 0.60 | 2.43 ± 0.67 | 2.23 ± 0.95 |
| Sample | 212 | 21PIP |
|---|---|---|
| Viscosity (mPa·s) | 8.10–8.75 | 9.83–10.80 |
| Thickness (µm) | 10.37 ± 1.83 | 9.11 ± 1.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonell, D.J.; Montoya, R.; Gelling, V.J.; Galván, J.C.; Jiménez-Morales, A. Enhancing the Corrosion Protection of AA2024-T3 Alloy by Surface Treatments Based on Piperazine-Modified Hybrid Sol–Gel Films. Metals 2020, 10, 539. https://doi.org/10.3390/met10040539
Carbonell DJ, Montoya R, Gelling VJ, Galván JC, Jiménez-Morales A. Enhancing the Corrosion Protection of AA2024-T3 Alloy by Surface Treatments Based on Piperazine-Modified Hybrid Sol–Gel Films. Metals. 2020; 10(4):539. https://doi.org/10.3390/met10040539
Chicago/Turabian StyleCarbonell, Diógenes J., Rodrigo Montoya, Victoria J. Gelling, Juan Carlos Galván, and Antonia Jiménez-Morales. 2020. "Enhancing the Corrosion Protection of AA2024-T3 Alloy by Surface Treatments Based on Piperazine-Modified Hybrid Sol–Gel Films" Metals 10, no. 4: 539. https://doi.org/10.3390/met10040539