Effect of Roasting Temperature on Phase Transformation in Co-Reduction Roasting of Nickel Slag
Abstract
:1. Introduction
2. Materials
3. Experimental Details
3.1. Experimental Procedure
3.2. Analysis and Characterization
4. Results and Discussion
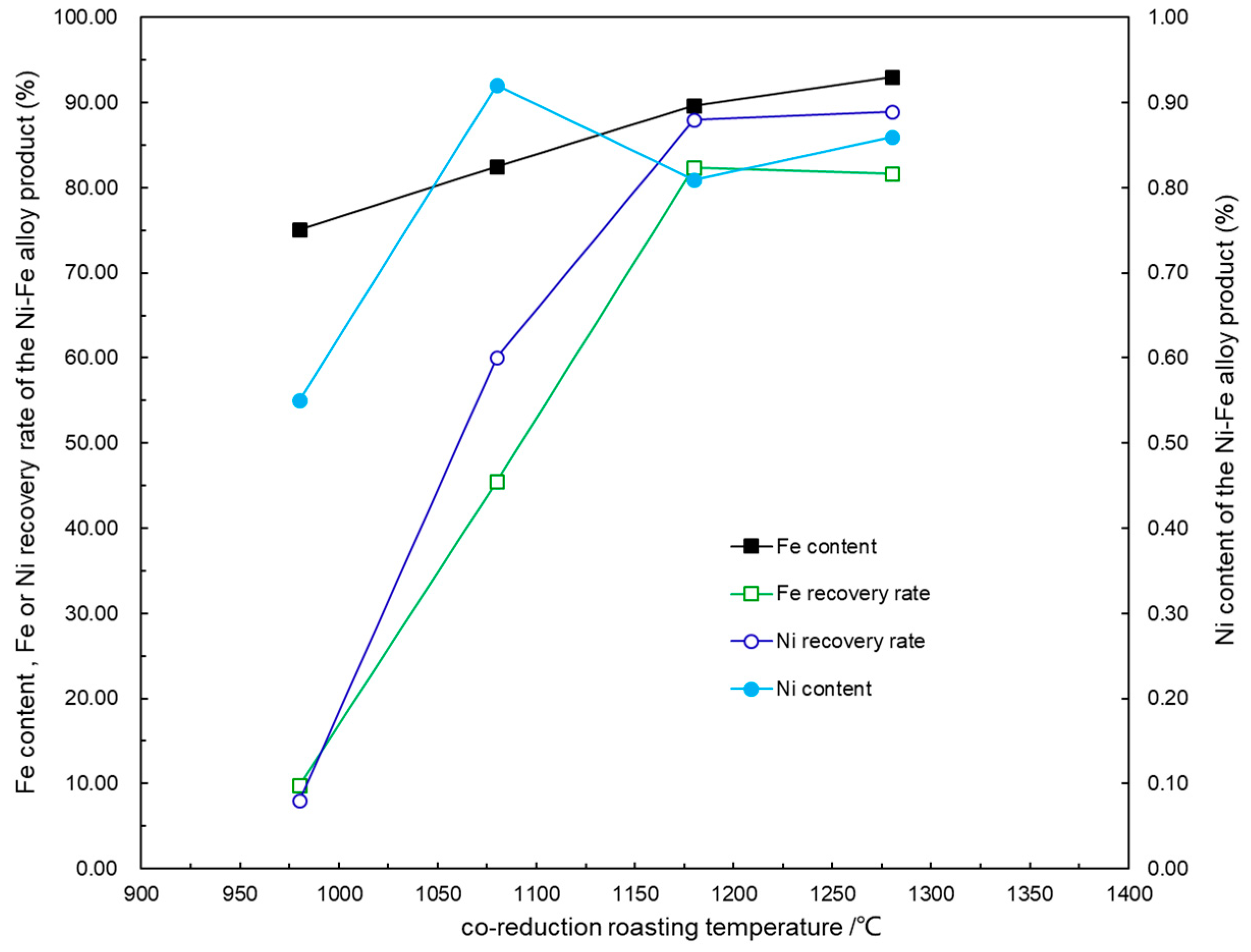
4.1. Effect of Roasting Temperature on the Co-Reduction Process
4.2. Effect of bfd Dosage on the Co-Reduction Process
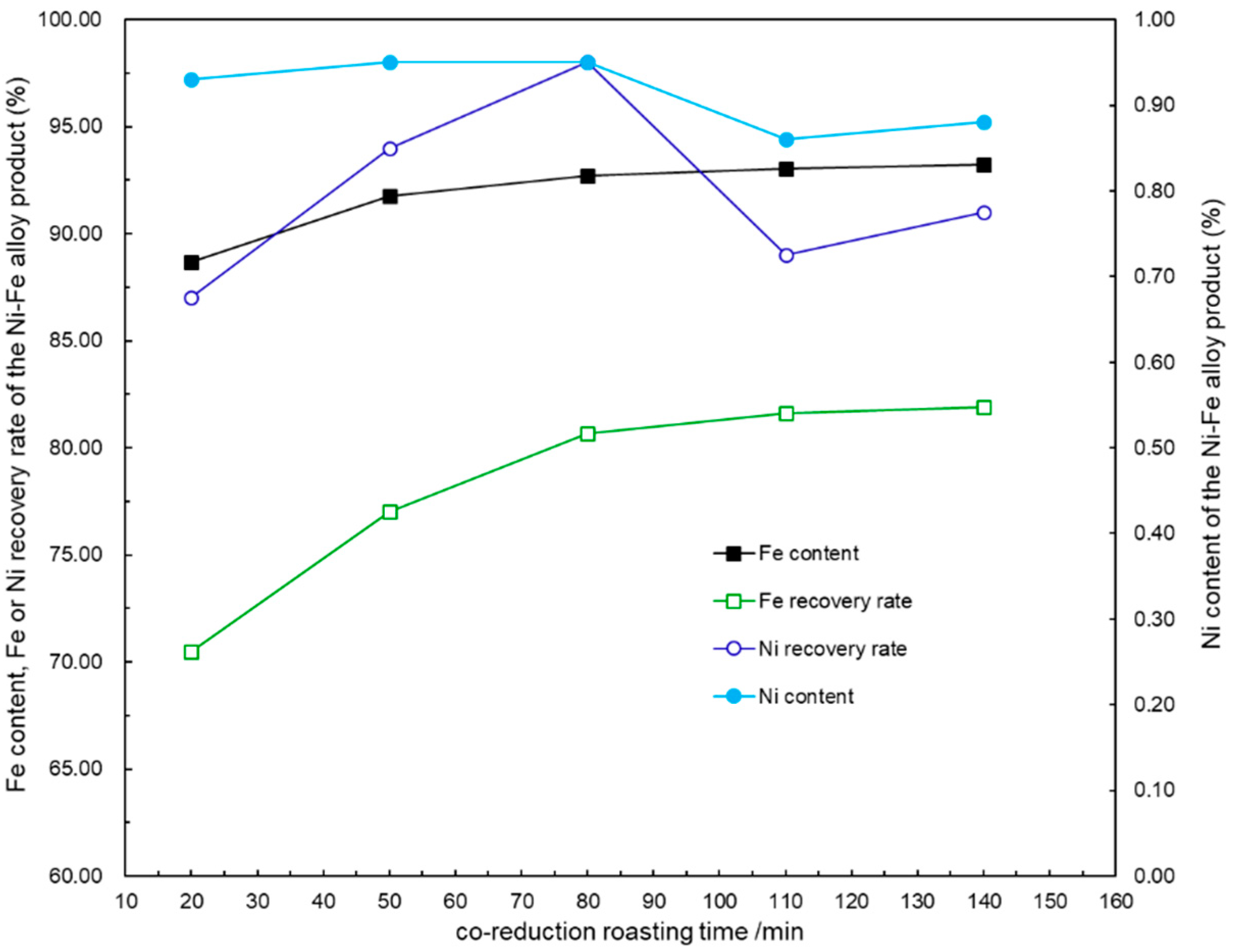
4.3. Effect of Roasting Time on the Co-Reduction Process
5. Effect of Roasting Temperature on the Phase Transformation
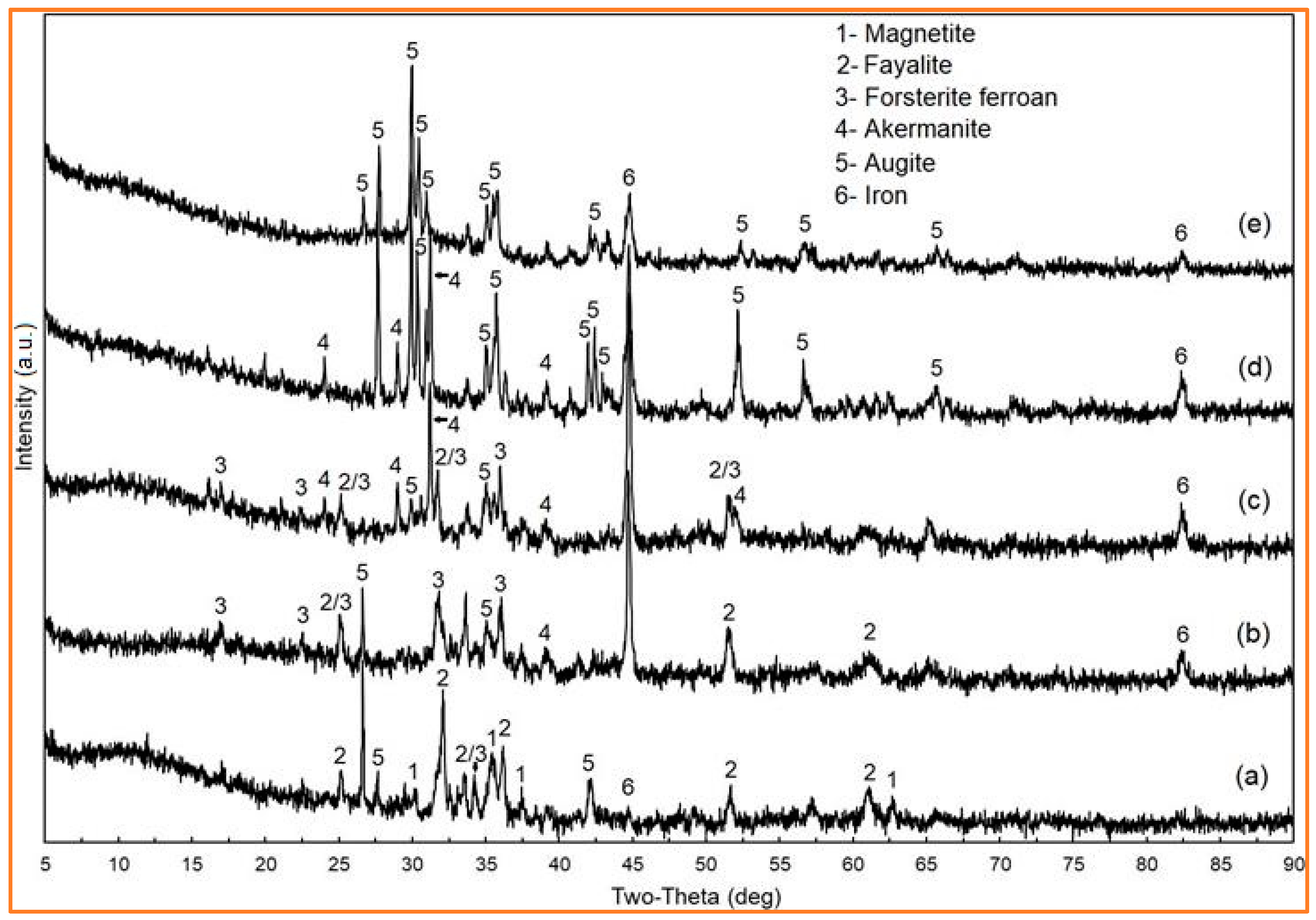
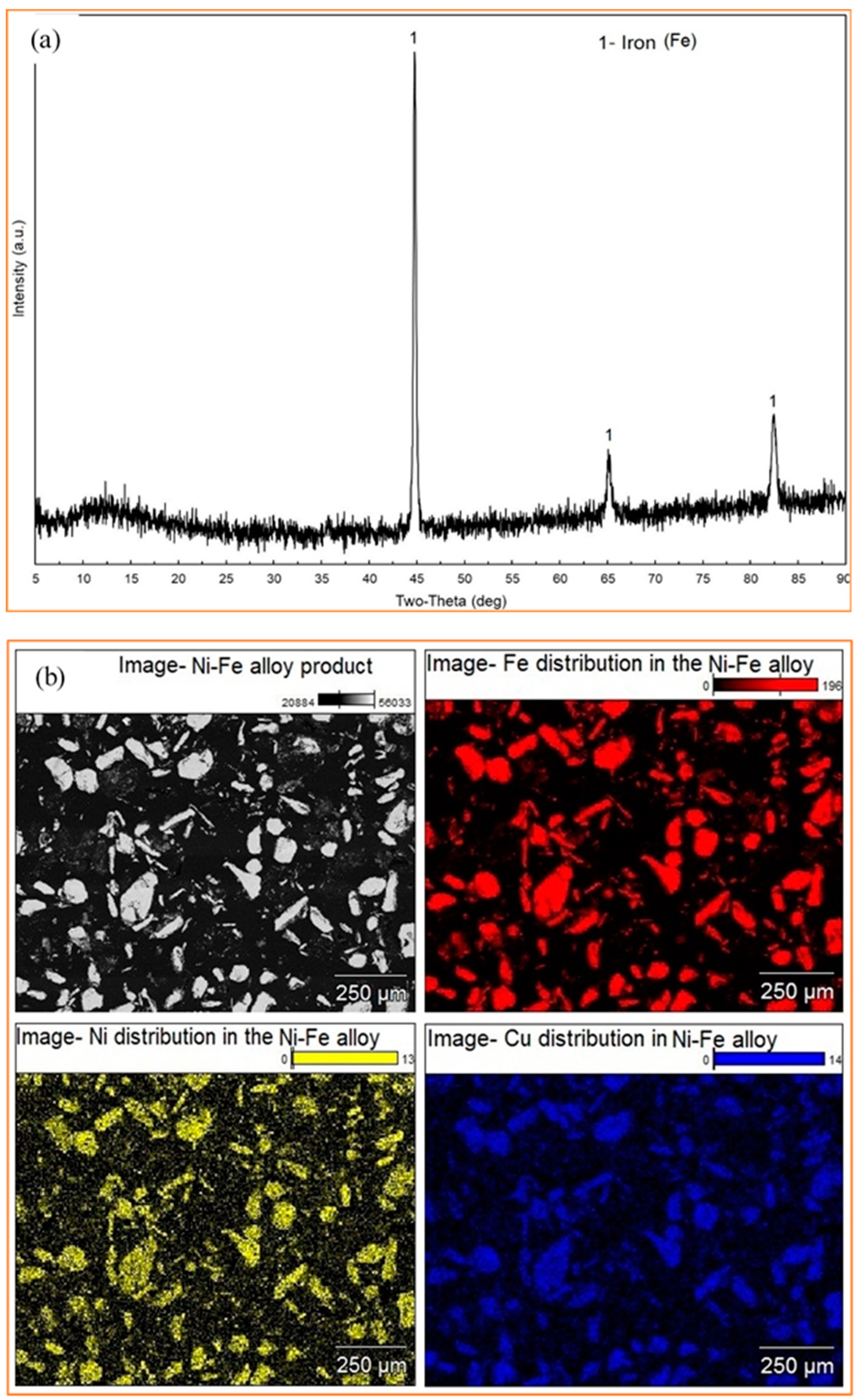
5.1. XRD Analysis
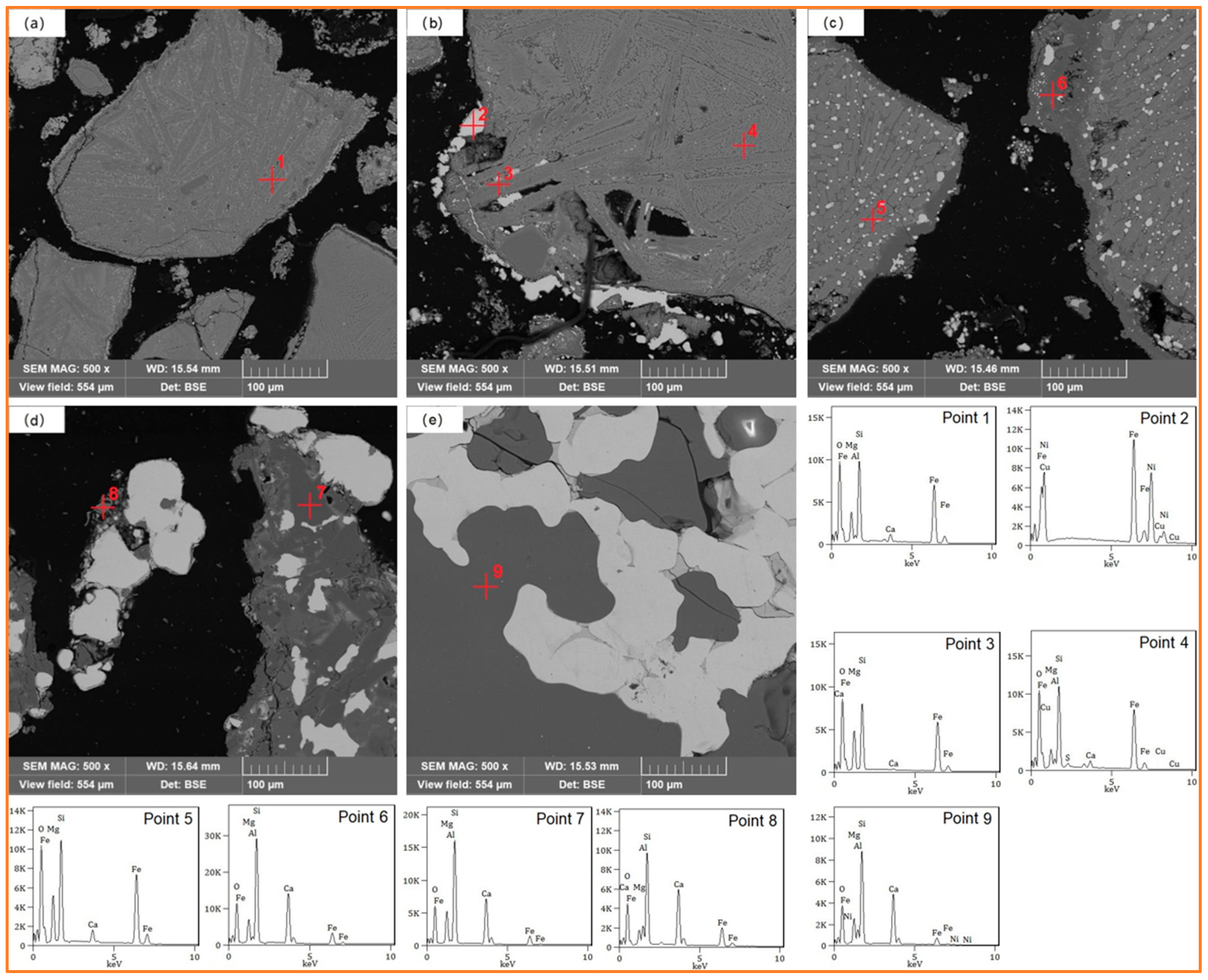
5.2. Analysis of the Microstructural Variation in the Roasted Products
5.3. Analysis of the Ni-Fe Alloy Product
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- China Nonferrous Metals Industry Association. The Yearbook of Nonferrous Metals Industry of China 2018; China Nonferrous Metals Industry Association: Beijing, China, 2019. [Google Scholar]
- Zhang, Q.L.; Ji, T.; Yang, Z.X.; Wang, C.Q.; Wu, H.C. Influence of different activators on microstructure and strength of alkali-activated nickel slag cementitious materials. Constr. Build. Mater. 2020, 235, 117449. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S.P. Geopolymerization of solid waste of non-ferrous metallurgy—A review. J. Environ. Manag. 2019, 251, 109571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, C.J.; Yang, J.M.; Chong, L.L.; Xu, X.C.; Xu, X.C.; Wu, Q.S. Influence of nickel slag powders on properties of magnesium potassium phosphate cement paste. Constr. Build. Mater. 2019, 205, 668–678. [Google Scholar] [CrossRef]
- Liu, X.M.; Gao, S.L.; Li, J.; Kou, J.; Sun, C.B. Process mineralogy of Jinchuan nickel slag in a settlement furnace. Chin. J. Eng. 2017, 39, 349–353. [Google Scholar]
- Huang, F.R.; Liao, Y.L.; Zhou, J.; Wang, Y.Y.; Li, H. Selective recovery of valuable metals from nickel converter slag at elevated temperature with sulfuric acid solution. Sep. Purif. Technol. 2015, 156, 572–581. [Google Scholar] [CrossRef]
- Wu, Q.S.; Wu, Y.; Tong, W.H.; Ma, H.G. Utilization of nickel slag as raw material in the production of Portland cement for road construction. Constr. Build. Mater. 2018, 193, 426–434. [Google Scholar] [CrossRef]
- Li, Y.J.; Papangelakis, V.G.; Perederiy, I. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 97, 185–193. [Google Scholar] [CrossRef]
- Xu, J.; Wang, N.; Zhou, Z.Y.; Chen, M.; Wang, P.F. Experimental and numerical studies of the gas-molten reduction behavior of blast furnace dust particles during in-flight process. Powder Technol. 2020, 361, 226–237. [Google Scholar] [CrossRef]
- Hu, W.T.; Xia, H.W.; Pan, D.L.; Wei, X.L.; Li, J.; Dai, X.J.; Yang, F.H.; Lu, X.; Wang, H.J. Difference of zinc volatility in diverse carrier minerals: The critical limit of blast furnace dust recycle. Miner. Eng. 2018, 116, 24–31. [Google Scholar] [CrossRef]
- Xu, J.; Wang, N.; Chen, M.; Zhou, Z.Y.; Wang, P.F. Evaluation of reduction behavior of blast furnace dust particles during in-flight process with experiment aided mathematical modeling. Appl. Math. Model. 2019, 75, 535–552. [Google Scholar] [CrossRef]
- Wu, Z.J.; Wang, L.C.; Gao, Z.F.; Liu, W.M.; Wu, X.R. Recycling blast furnace dust into metals (Al, Zn and Ti)-doped hematite with enhanced photocatalytic activity. J. Environ. Chem. Eng. 2016, 4, 341–345. [Google Scholar] [CrossRef]
- Zeng, G.W. Review on utilization technologies of blast-furnace gas sludge. Environ. Prot. Chem. Ind. 2015, 35, 279–283. [Google Scholar]
- Makkonen, H.T.; Heino, J.; Laitila, L.; Hiltunen, A.; Pöyliö, E.; Härkki, J. Optimisation of steel plant recycling in Finland: Dusts, scales and sludge. Resour. Conserv. Recy. 2002, 35, 77–84. [Google Scholar] [CrossRef]
- Hu, T.Y.; Sun, T.C.; Kou, J.; Geng, C.; Wang, X.P.; Chen, C. Recovering titanium and iron by co-reduction roasting of seaside titanomagnetite and blast furnace dust. Int. J. Miner. Process. 2017, 165, 28–33. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhou, W.T.; Han, Y.X.; Li, Y.J. Effect of different additives on reaction characteristics of fluorapatite during coal-based reduction of iron ore. Metals 2019, 9, 923. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Li, G.F.; Han, Y.X.; Sun, Y.S. Reaction behavior of phosphorus in coal-based reduction of an oolitic hematite ore and pre-dephosphorization of reduced iron. Metals 2016, 6, 82. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, T.C.; Kou, J.; Guo, Q.; Xu, C.Y. Study on phosphorus removal of high-phosphorus oolitic hematite by coal-based direct reduction and magnetic separation. Miner. Process. Extr. Metall. Rev. 2014, 35, 66–73. [Google Scholar] [CrossRef]
- Wang, X.P.; Sun, T.C.; Chen, C.; Kou, J. Effects of Na2SO4 on iron and nickel reduction in a high-iron and low-nickel laterite ore. Int. J. Min. Met. Mater. 2018, 25, 383–390. [Google Scholar] [CrossRef]
- Geng, C.; Sun, T.C.; Ma, Y.W.; Xu, C.Y.; Yang, H.F. Effects of embedding direct reduction followed by magnetic separation on recovering titanium and iron of beach titanomagnetite concentrate. J. Iron Steel Res. Int. 2017, 24, 156–164. [Google Scholar] [CrossRef]
- Sun, Y.S.; Han, Y.X.; Li, Y.F.; Li, Y.J. Formation and characterization of metallic iron grains in coal-based reduction of oolitic iron ore. Int. J. Miner. Metall. Mater. 2017, 24, 123–129. [Google Scholar] [CrossRef]
- Li, X.M.; Wen, Z.Y.; Li, Y.; Yang, H.B.; Xing, X.D. Improvement of carbothermic reduction of nickel slag by addition of CaCO3. Trans. Nonferr. Met. Soc. China 2019, 29, 2658–2666. [Google Scholar] [CrossRef]
- Cao, Z.C.; Sun, T.C.; Xue, X.; Liu, Z.H. Iron recovery from discarded copper slag in a RHF direct reduction and subsequent grinding/magnetic separation process. Minerals 2016, 6, 119. [Google Scholar] [CrossRef] [Green Version]



| Reductant | Code | Mad (wt%) | Aad (wt%) | Vad (wt%) | FCad (wt%) |
|---|---|---|---|---|---|
| blast furnace dust | bfd | 2.62 | 68.04 | 8.79 | 20.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Xu, C.; Tian, Y.; Hou, Y. Effect of Roasting Temperature on Phase Transformation in Co-Reduction Roasting of Nickel Slag. Metals 2020, 10, 550. https://doi.org/10.3390/met10040550
Cao Y, Xu C, Tian Y, Hou Y. Effect of Roasting Temperature on Phase Transformation in Co-Reduction Roasting of Nickel Slag. Metals. 2020; 10(4):550. https://doi.org/10.3390/met10040550
Chicago/Turabian StyleCao, Yunye, Chengyan Xu, Yuechao Tian, and Yanqing Hou. 2020. "Effect of Roasting Temperature on Phase Transformation in Co-Reduction Roasting of Nickel Slag" Metals 10, no. 4: 550. https://doi.org/10.3390/met10040550




