Electrochemical Recycling of Platinum Group Metals from Spent Catalytic Converters
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Effect of pH and Anion on Platinum Electrochemistry
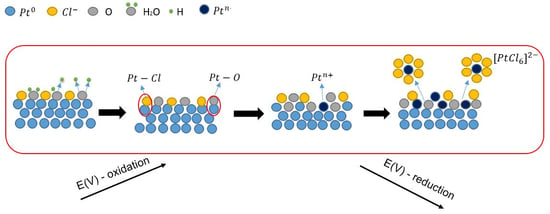
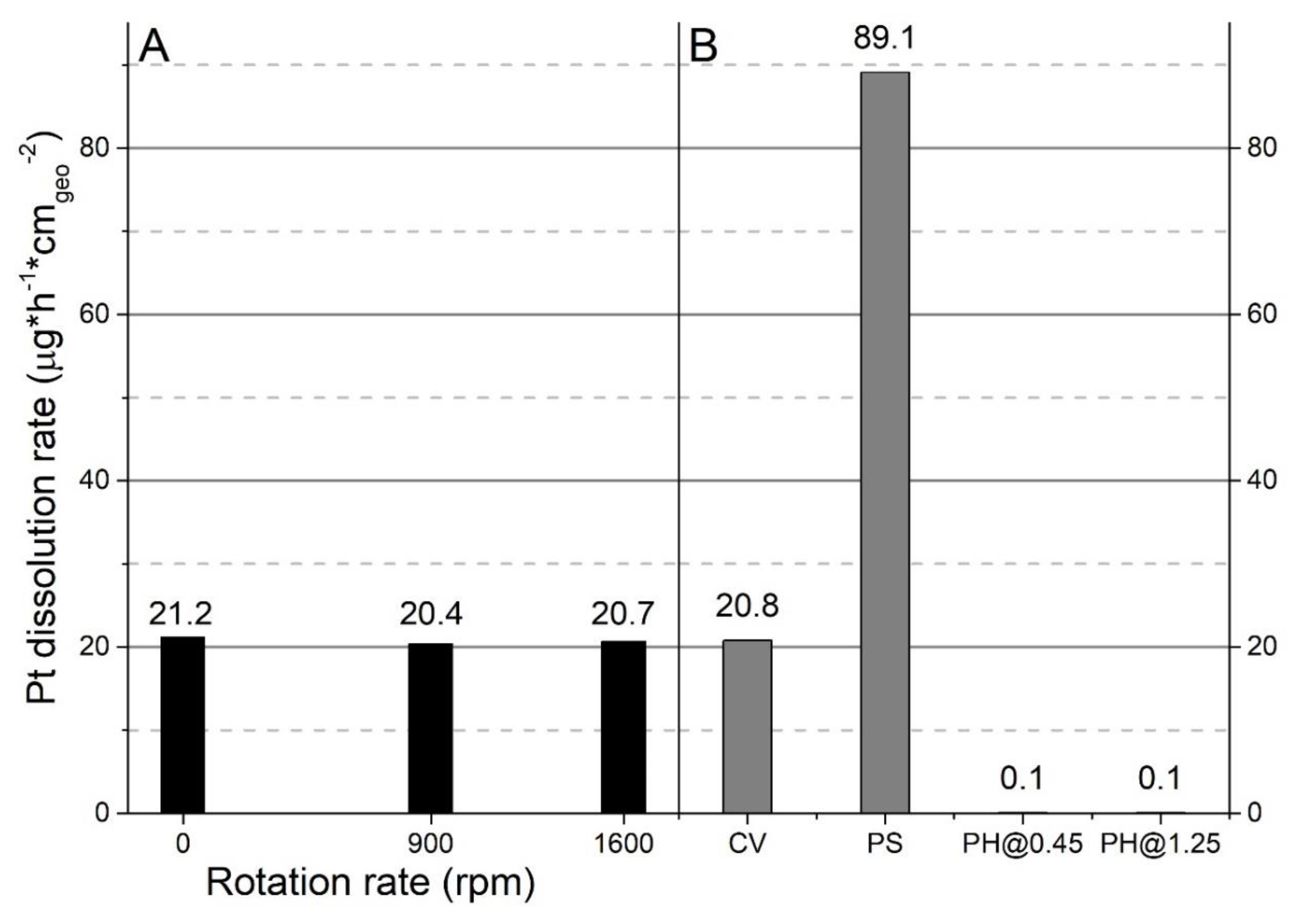
3.2. Electrochemical Dissolution of Pt in Hydrochloric Acid
3.3. Potential Sweep versus Potential Step
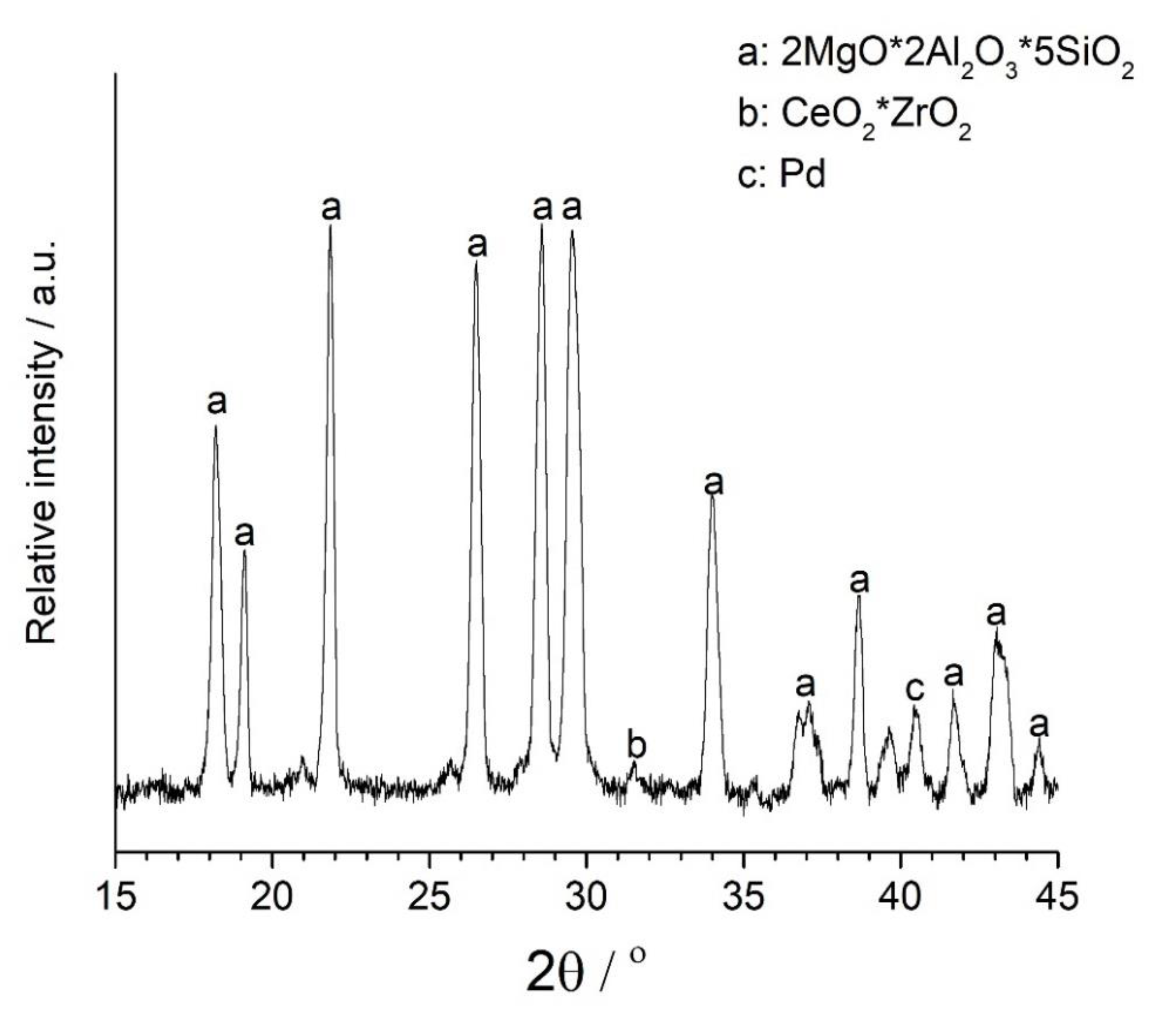
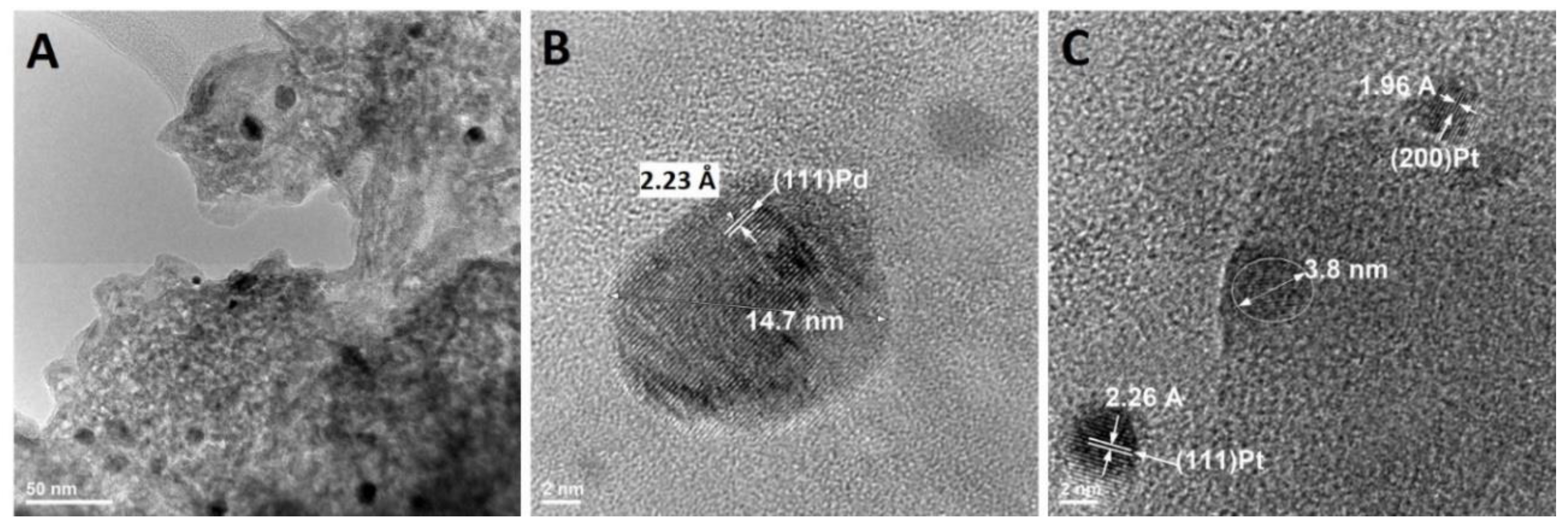
3.4. Morphology and Composition of the Automotive Catalyst
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eu-law-Eur-lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52017DC0490&from=EN (accessed on 10 May 2020).
- Mudd, G.M.; Jowitt, S.M.; Werner, T.T. Global platinum group element resources, reserves and mining—A critical assessment. Sci. Total Environ. 2018, 622, 614–625. [Google Scholar] [CrossRef]
- Shelef, M.; Mccabe, R.W. Twenty-five years after introduction of automotive catalysts: What next? Catal. Today 2000, 62, 35–50. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling of (critical) metals. In Critical Metals Handbook; Gunn, G., Ed.; John Wiley & Sons: Chichester, UK, 2013; pp. 41–69. [Google Scholar]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Saguru, C.; Ndlovu, S.; Moropeng, D. A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy 2018, 182, 44–56. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.C.; Park, S.W.; Jeong, J.; Kumar, V. Dissolution behaviour of platinum by electro-generated chlorine in hydrochloric acid solution. J. Chem. Technol. Biotechnol. 2013, 88, 1212–1219. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Cao, H.; Ning, P.; Jin, W.; Yang, Z.; Wang, H.; Sun, Z. Understanding the features of PGMs in spent ternary automobile catalysts for development of cleaner recovery technology. J. Clean. Prod. 2019, 239, 118031. [Google Scholar] [CrossRef]
- Fornalczyk, A.; Saternus, M. Platinum Recovery From Used Auto Catalytic. Metalurgia 2013, 52, 219–222. [Google Scholar]
- Carmo, M.; Stolten, D. Energy Storage Using Hydrogen Produced From Excess Renewable Electricity: Power to Hydrogen. In Science and Engineering of Hydrogen-Based Energy Technologies; Elsevier Inc.: London, UK, 2019; pp. 165–199. [Google Scholar] [CrossRef]
- Wang, S.; Tarroja, B.; Smith, L.; Sha, B.; Samuelsen, S. Prioritizing among the end uses of excess renewable energy for cost-effective greenhouse gas emission reductions. Appl. Energy 2019, 235, 284–298. [Google Scholar] [CrossRef]
- Geiger, S.; Cherevko, S.; Mayrhofer, K.J.J. Dissolution of Platinum in Presence of Chloride Traces. Electrochim. Acta 2015, 179, 24–31. [Google Scholar] [CrossRef]
- Bard, A.J. Standard Potentials in Aqueous Solution; IUPAC: Oxford, UK, 1985; pp. 353–354. [Google Scholar]
- Jerkiewicz, G.; Vatankhah, G.; Lessard, J.; Soriaga, M.P.; Park, Y.S. Surface-oxide growth at platinum electrodes in aqueous H2SO4: Reexamination of its mechanism through combined cyclic-voltammetry, electrochemical quartz-crystal nanobalance, and Auger electron spectroscopy measurements. Electrochim. Acta 2003, 49, 1451–1459. [Google Scholar] [CrossRef]
- Kinoshita, K.; Lundquist, J.; Stonehart, P. Hydrogen adsorption on high surface area platinum crystallites. J. Catal. 1973, 31, 325–334. [Google Scholar] [CrossRef]
- Mundy, G.R.; Potter, R.J.; Christensen, P.A.; Hamnett, A. A study of the electro-oxidation of methanol on platinum in 1 M Na2SO4 by electrochemical and in-situ FTIR techniques. J. Electroanal. Chem. 1990, 279, 257–272. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-esparza, A.T.; Takanabe, K. Mechanistic Switching by Hydronium Ion Activity for Hydrogen Evolution and Oxidation over Polycrystalline Platinum Disk and Platinum/Carbon Electrodes. ChemElectroChem 2014, 1, 1497–1507. [Google Scholar] [CrossRef]
- Nørgaard, C.F.; Stamatin, S.N.; Skou, E.M. Redeposition of electrochemically dissolved platinum as nanoparticles on carbon. Int. J. Hydrog. Energy 2014, 39, 17322–17326. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, V.; Huan, T.D. Exploring PtSO4 and PdSO4 phases: An evolutionary algorithm based investigation. Phys. Chem. Chem. Phys. 2015, 17, 18146–18151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topalov, A.A.; Katsounaros, I.; Auinger, M.; Cherevko, S.; Meier, J.C.; Klemm, S.O.; Mayrhofer, K.J.J. Dissolution of platinum: Limits for the deployment of electrochemical energy conversion? Angew. Chem. Int. Ed. Engl. 2012, 51, 12613–12615. [Google Scholar] [CrossRef] [Green Version]
- Hodnik, N.; Baldizzone, C.; Polymeros, G.; Geiger, S.; Grote, J.; Cherevko, S.; Mingers, A.; Zeradjanin, A.; Mayrhofer, K.J.J. Platinum recycling going green via induced surface potential alteration enabling fast and efficient dissolution. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- He, J.-J.; Wang, C.-X.; Zheng, T.-T.; Zhao, Y.-K. Thermally Induced Deactivation and the Corresponding Strategies for Improving Durability in Automotive Three-Way Catalysts: A review of latest developments and fundamentals. In Johnson Matthey Technology Review; Johnson Matthey Plc: Royston, UK, 2016; pp. 179–185. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, J.C.; Kim, M.S.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Satterfield, C.N. Heterogeneous Catalysis in Industrial Practice, 2nd ed.; Krieger Publishing: Melbourne, FL, USA, 1996. [Google Scholar]
- Bronshtein, I.; Feldman, Y.; Shilstein, S.; Wachtel, E.; Lubomirsky, I.; Kaplan, V. Efficient Chloride Salt Extraction of Platinum Group Metals from Spent Catalysts. J. Sustain. Metall. 2018, 4, 103–114. [Google Scholar] [CrossRef]
- Prasetyo, E.; Anderson, C. Platinum Group Elements Recovery from Used Catalytic Converters by Acidic Fusion and Leaching. Metals 2020, 10, 485. [Google Scholar] [CrossRef] [Green Version]
- Graedel, T.E.; Allwood, J.; Birat, J.-P.; Reck, B.K.; Sibley, S.F.; Sonnemann, G.; Buchert, M.; Hagelüken, C. UNEP Recycling Rates of Metals–A Status Report, A Report of the Working Group on the Global Metal Flows to the International Resource Panel. 2011. Available online: https://www.resourcepanel.org/reports/recycling-rates-metals (accessed on 12 April 2020).
- Shrestha, B.R.; Tada, E.; Nishikata, A. Effect of chloride on platinum dissolution. Electrochim. Acta 2014, 143, 161–167. [Google Scholar] [CrossRef]
- Sharma, R.; Gyergyek, S.; Andersen, S.M. Environmentally and Industrially Friendly Recycling of Platinum Nanoparticles Through Electrochemical Dissolution–Electrodeposition in Acid-Free/Dilute Acidic Electrolytes. ChemSusChem 2018, 11, 3742–3750. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, N.; Legeai, S.; Balva, M.; Hazotte, C.; Comel, J.; Lapicque, F.; Billy, E.; Meux, E. Recovery of Metals from Secondary Raw Materials by Coupled Electroleaching and Electrodeposition in Aqueous or Ionic Liquid Media. Metals 2018, 8, 556. [Google Scholar] [CrossRef] [Green Version]
- Balva, M.; Legeai, S.; Leclerc, N.; Billy, E.; Meux, E. Environmentally Friendly Recycling of Fuel-Cell Membrane Electrode Assemblies by Using Ionic Liquids. ChemSusChem 2014, 10, 2922–2935. [Google Scholar] [CrossRef]




| Reactions | Potential (V) vs. Standard Hydrogen Electrode (SHE) | Abbreviations |
|---|---|---|
| 1.188 | R1 | |
| ) | 0.758 | R2 |
| ) | 0.744 | R3 |
| 1.396 | R4 | |
| 1.229 | R5 | |
| 0.980 | R6 |
| Catalyst/μg | Initially/μg | At the End of Experiment/μg | Element Efficiency/% | Total Efficiency/% | |||
|---|---|---|---|---|---|---|---|
| Pt | Pd | Pt | Pd | Pt | Pd | Pt and Pd | |
| 7075 | 1.061 | 3.184 | 0.5 | 1.55 | 47.1 | 48.7 | 48.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diac, C.; Maxim, F.I.; Tirca, R.; Ciocanea, A.; Filip, V.; Vasile, E.; Stamatin, S.N. Electrochemical Recycling of Platinum Group Metals from Spent Catalytic Converters. Metals 2020, 10, 822. https://doi.org/10.3390/met10060822
Diac C, Maxim FI, Tirca R, Ciocanea A, Filip V, Vasile E, Stamatin SN. Electrochemical Recycling of Platinum Group Metals from Spent Catalytic Converters. Metals. 2020; 10(6):822. https://doi.org/10.3390/met10060822
Chicago/Turabian StyleDiac, Cornelia, Florentina Iuliana Maxim, Radu Tirca, Adrian Ciocanea, Valeriu Filip, Eugeniu Vasile, and Serban N. Stamatin. 2020. "Electrochemical Recycling of Platinum Group Metals from Spent Catalytic Converters" Metals 10, no. 6: 822. https://doi.org/10.3390/met10060822