Effect of Cerium Content on the Evolution of Inclusions and Formation of Acicular Ferrite in Ti-Mg-Killed EH36 Steel
Abstract
:1. Introduction
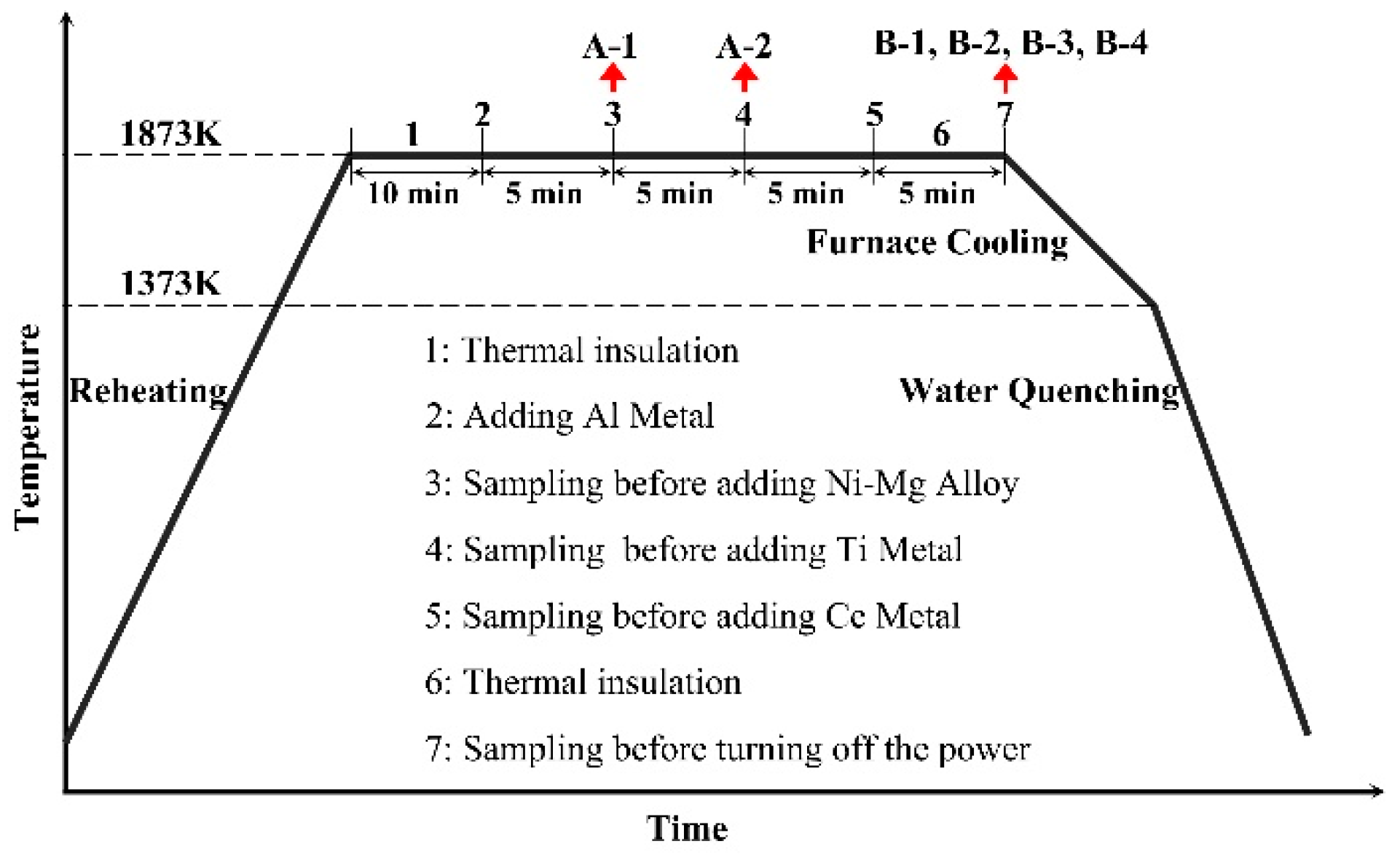
2. Materials and Methods
3. Results and Discussion
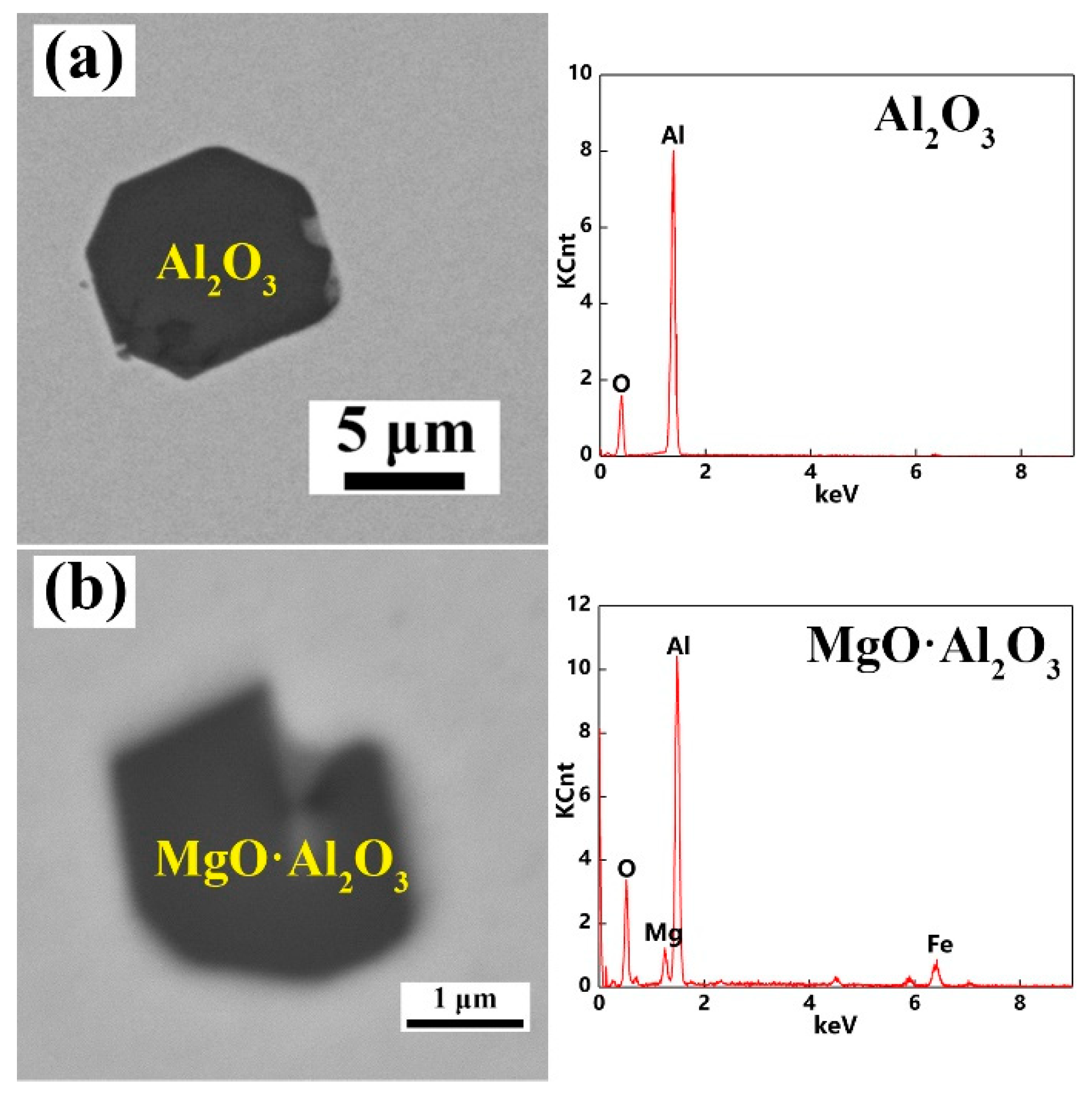
3.1. Composition, Size and Number Density of Total Inclusions
3.2. Microstructure of Tested Steels
3.3. Composition, Size and Number Density of Effective Inclusions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shome, M.; Gupta, O.P.; Mohanty, O.N. A Modified Analytical Approach for Modelling Grain Growth in the Coarse Grain HAZ of HSLA Steels. Scr. Mater. 2004, 50, 1007–1010. [Google Scholar] [CrossRef]
- Cao, R.; Li, J.; Liu, D.S.; Ma, J.Y.; Chen, J.H. Micromechanism of Decrease of Impact Toughness in Coarse-Grain Heat-Affected Zone of HSLA Steel with Increasing Welding Heat Input. Metall. Mater. Trans. A 2015, 46, 2999–3014. [Google Scholar] [CrossRef]
- Kitani, Y.; Ikeda, R.; Yasuda, K.; Oi, K.; Ichimiya, K. Improvement of HAZ Toughness for High Heat Input Welding by using Boron Diffusion from Weld Metal. Weld. World 2007, 51, 31–36. [Google Scholar] [CrossRef]
- Kundu, A. Austenite Grain Boundary Pinning during Reheating by Mixed AlN and Nb(C,N) Particles. ISIJ Int. 2014, 54, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, S.; Takamura, J. Control of Oxides as Inoculants. In Proceedings of the 6th International Iron and Steel Congress, ISIJ, Nagoya, Japan, 21–26 October 1990; The Iron and Steel Institute of Japan: Tokyo, Japan, 1989. [Google Scholar]
- Takemura, J.; Mizoguchi, S. Roles of Oxides in Steel Performance. In Proceedings of the 6th International Iron and Steel Congress, ISIJ, Nagoya, Japan, 21–26 October 1990; The Iron and Steel Institute of Japan: Tokyo, Japan, 1989. [Google Scholar]
- Sarma, D.S.; Karasev, A.V.; Jonsson, P.G. On the Role of Non-metallic Inclusions in the Nucleation of Acicular Ferrite in Steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zou, X.; Matsuura, H.; Wang, C. Effect of Heat Input on Inclusion Evolution Behavior in Heat-Affected Zone of EH36 Shipbuilding Steel. JOM 2018, 70, 946–950. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, D.; Sun, J.; Wang, C.; Matsuura, H. An Integrated Study on the Evolution of Inclusions in EH36 Shipbuilding Steel with Mg Addition: From Casting to Welding. Metall. Mater. Trans. B 2018, 49, 481–489. [Google Scholar] [CrossRef]
- Zou, X.; Sun, J.; Matsuura, H.; Wang, C. In Situ Observation of the Nucleation and Growth of Ferrite Laths in the Heat-Affected Zone of EH36-Mg Shipbuilding Steel Subjected to Different Heat Inputs. Metall. Mater. Trans. B 2018, 49, 2168–2173. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Zhu, L. Effect of Inclusion Size and Type on the Nucleation of Acicular Ferrite in High Strength Ship Plate Steel. ISIJ Int. 2018, 58, 965–969. [Google Scholar] [CrossRef] [Green Version]
- Chai, F.; Yang, C.; Su, H.; Zhang, Y.; Xu, Z. Effect of Magnesium on Inclusion Formation in Ti-killed Steels and Microstructural Evolution in Welding Induced Coarse-grained Heat Affected Zone. J. Iron Steel Res. Int. 2009, 16, 69–74. [Google Scholar] [CrossRef]
- Song, M.; Song, B.; Hu, C.; Xin, W.; Song, G. Formation of Acicular Ferrite in Mg Treated Ti-bearing C-Mn Steel. ISIJ Int. 2015, 55, 1468–1473. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.; Lin, H.; Yue, Q.; Cai, Z. Effects of Ti-Mg Complex Inclusions on Acicular Ferrite Nucleation. High Temp. Mater. Process. 2017, 36, 459–465. [Google Scholar] [CrossRef]
- Lou, H.; Wang, C.; Wang, B.; Wang, Z.; Li, Y.; Chen, Z. Inclusion Evolution Behavior of Ti-Mg Oxide Metallurgy Steel and Its Effect on a High Heat Input Welding HAZ. Metals 2018, 8, 534. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, D.; Liu, C.; Jiang, M.; Lv, M.; Wang, B.; Zhang, S. Modification of Inclusions in Liquid Iron by Mg Treatment. J. Iron Steel Res. Int. 2014, 21, 99–103. [Google Scholar] [CrossRef]
- Cha, W.; Nagasaka, T.; Miki, T.; Sasak, Y.; Hino, M. Equilibrium between titanium and oxygen in liquid Fe–Ti alloy coexisted with titanium oxides at 1873 K. ISIJ Int. 2006, 46, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zou, X.; Matsuura, H.; Wang, C. Evolution of Inclusions During 1473 K Heating Process in EH36 Shipbuilding Steel with Mg Addition. JOM 2018, 70, 521–526. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of Adding Cerium on Microstructure and morphology of Ce-Based Inclusion Formed in Low-Carbon Steel. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Song, M.; Song, B.; Xin, W.; Sun, G.; Song, G.; Hu, C. Effects of Rare Earth Addition on Microstructure of C-Mn Steel. Ironmak. Steelmak. 2015, 42, 594–599. [Google Scholar] [CrossRef]
- Appelberg, J.; Nakajima, K.; Shibata, H.; Tilliander, A.; Jönsson, P. In Situ Studies of Misch-metal Particle Behavior on a Molten Stainless Steel Surface. Mater. Sci. Eng. A 2008, 495, 330–334. [Google Scholar] [CrossRef]
- Brarin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 159–162. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L. Effect of Cerium Content on Inclusions in an Ultra-Low-Carbon Aluminum-Killed Steel. Metall. Mater. Trans. B 2020, 51, 589–600. [Google Scholar] [CrossRef]
- Díaz-Fuentes, M.; Iza-Mendia, A.; Gutiérrez, I. Analysis of Different Acicular Ferrite Microstructures in Low-carbon Steels by Electron Backscattered Diffraction. Study of Their Toughness Behavior. Metall. Mater. Trans. A 2003, 34, 2505–2516. [Google Scholar] [CrossRef]
- Deng, X.; Jiang, M.; Wang, X. Mechanisms of Inclusion Evolution and Intra-granular Acicular Ferrite Formation in Steels Containing Rare Earth Elements. Acta Metall. Sin. (Engl. Lett.) 2012, 25, 241–248. [Google Scholar] [CrossRef]
- Shim, J.; Oh, Y.; Suh, J.; Cho, Y.; Shim, J.; Byun, J.; Lee, D. Ferrite Nucleation Potency of Non-metallic Inclusions in Medium Carbon Steels. Acta Mater. 2001, 49, 2115–2122. [Google Scholar] [CrossRef]
- Furuhara, T.; Shinyoshi, T.; Miyamoto, G.; Yamaguchi, J.; Sugita, N.; Kimura, N.; Takemura, N.; Maki, T. Multiphase Crystallography in the Nucleation of Intragranular Ferrite on MnS+V(C, N) Complex Precipitate in Austenite. ISIJ Int. 2003, 12, 2028–2037. [Google Scholar] [CrossRef] [Green Version]
- Babu, S.S. The Mechanism of Acicular Ferrite in Weld Deposits. Curr. Opin. Solid State Mater. Sci. 2004, 8, 267–278. [Google Scholar] [CrossRef]
- Bramfitt, L.B. The Effect of Carbide and Nitride Additions on the Heterogeneous Nucleation Behavior of Liquid Iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Wen, B.; Song, B.; Pan, N.; Hu, Q.Y.; Mao, J.H. Effect of SiMg alloy on inclusions and microstructures of 16Mn steel. Ironmak. Steelmak. 2011, 38, 577–583. [Google Scholar] [CrossRef]
- Wen, B.; Song, B.; Pan, N.; Hu, Q.; Mao, J. Effect of Austenitizing Temperature on Microstructure in 16Mn Steel Treated by Cerium. Int. J. Miner. Metall. Mater. 2011, 18, 652–658. [Google Scholar] [CrossRef]









| No. | C 1 | Mn 2 | Si 2 | P 2 | S 1 | Al 2 | Mg 2 | Ti 2 | Ce 3 | T.O 1 | N 4 | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 0.053 | 1.46 | 0.24 | 0.01 | 0.0082 | 0.0087 | 0.0044 | 0.016 | 0 | 0.0040 | 0.0037 | Bal. |
| #2 | 0.053 | 1.44 | 0.24 | 0.01 | 0.0081 | 0.0086 | 0.0028 | 0.013 | 0.014 | 0.0055 | 0.0038 | Bal. |
| #3 | 0.053 | 1.46 | 0.24 | 0.01 | 0.0082 | 0.0086 | 0.0029 | 0.014 | 0.024 | 0.0047 | 0.0037 | Bal. |
| #4 | 0.052 | 1.46 | 0.24 | 0.01 | 0.0083 | 0.0088 | 0.0028 | 0.013 | 0.037 | 0.0041 | 0.0034 | Bal. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Song, B.; Yang, Z.; Cui, X.; Li, L.; Wang, L.; Song, Z. Effect of Cerium Content on the Evolution of Inclusions and Formation of Acicular Ferrite in Ti-Mg-Killed EH36 Steel. Metals 2020, 10, 863. https://doi.org/10.3390/met10070863
Liu Z, Song B, Yang Z, Cui X, Li L, Wang L, Song Z. Effect of Cerium Content on the Evolution of Inclusions and Formation of Acicular Ferrite in Ti-Mg-Killed EH36 Steel. Metals. 2020; 10(7):863. https://doi.org/10.3390/met10070863
Chicago/Turabian StyleLiu, Zhen, Bo Song, Zhanbing Yang, Xiaokang Cui, Longfei Li, Lei Wang, and Zirui Song. 2020. "Effect of Cerium Content on the Evolution of Inclusions and Formation of Acicular Ferrite in Ti-Mg-Killed EH36 Steel" Metals 10, no. 7: 863. https://doi.org/10.3390/met10070863






