Thermal Decomposition Kinetics of Rare Earth Minerals in Tailings with Addition of MgO
Abstract
:1. Introduction
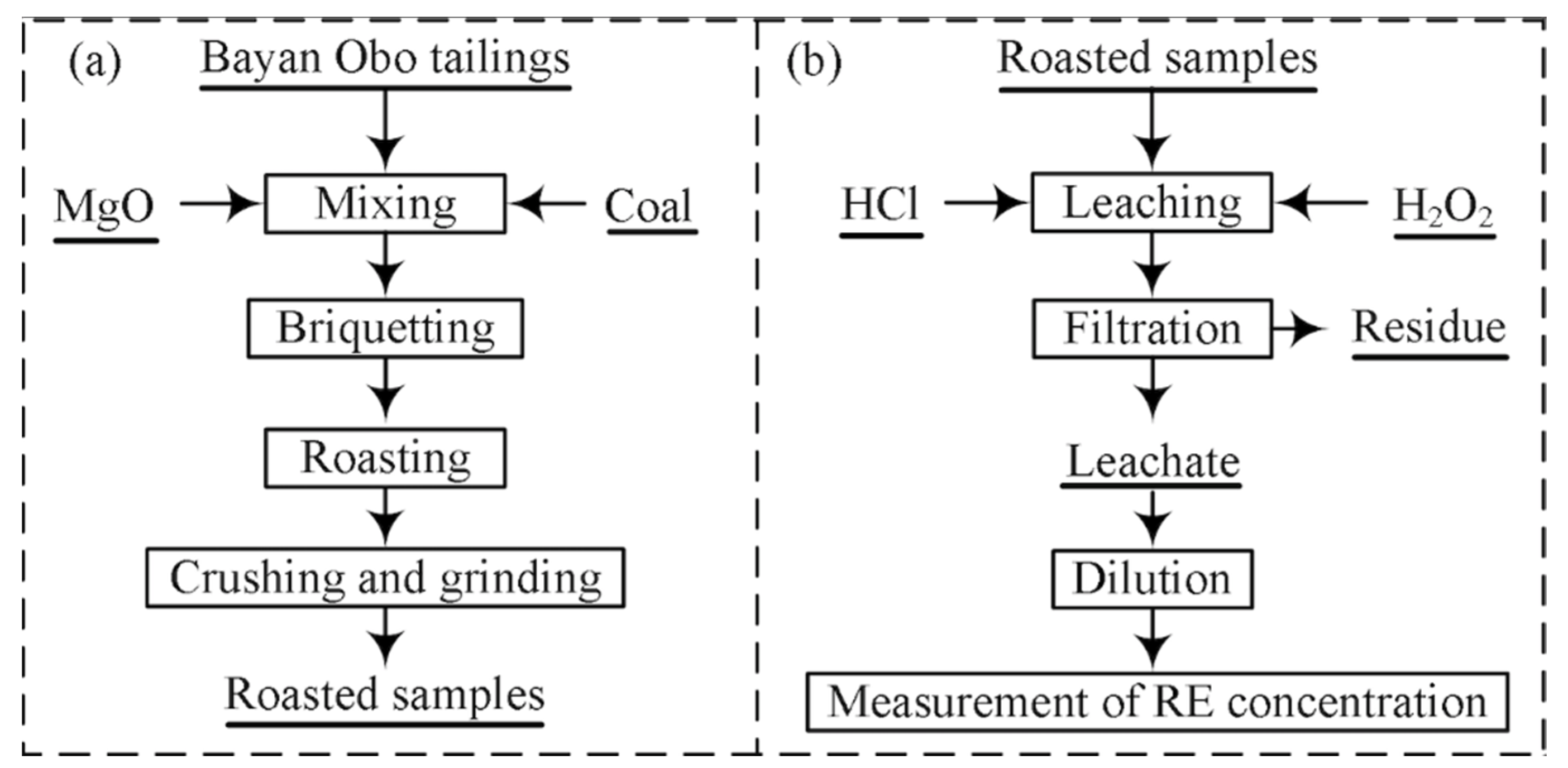
2. Experimental
2.1. Materials
2.2. Procedures and Characterization
3. Results and Discussion
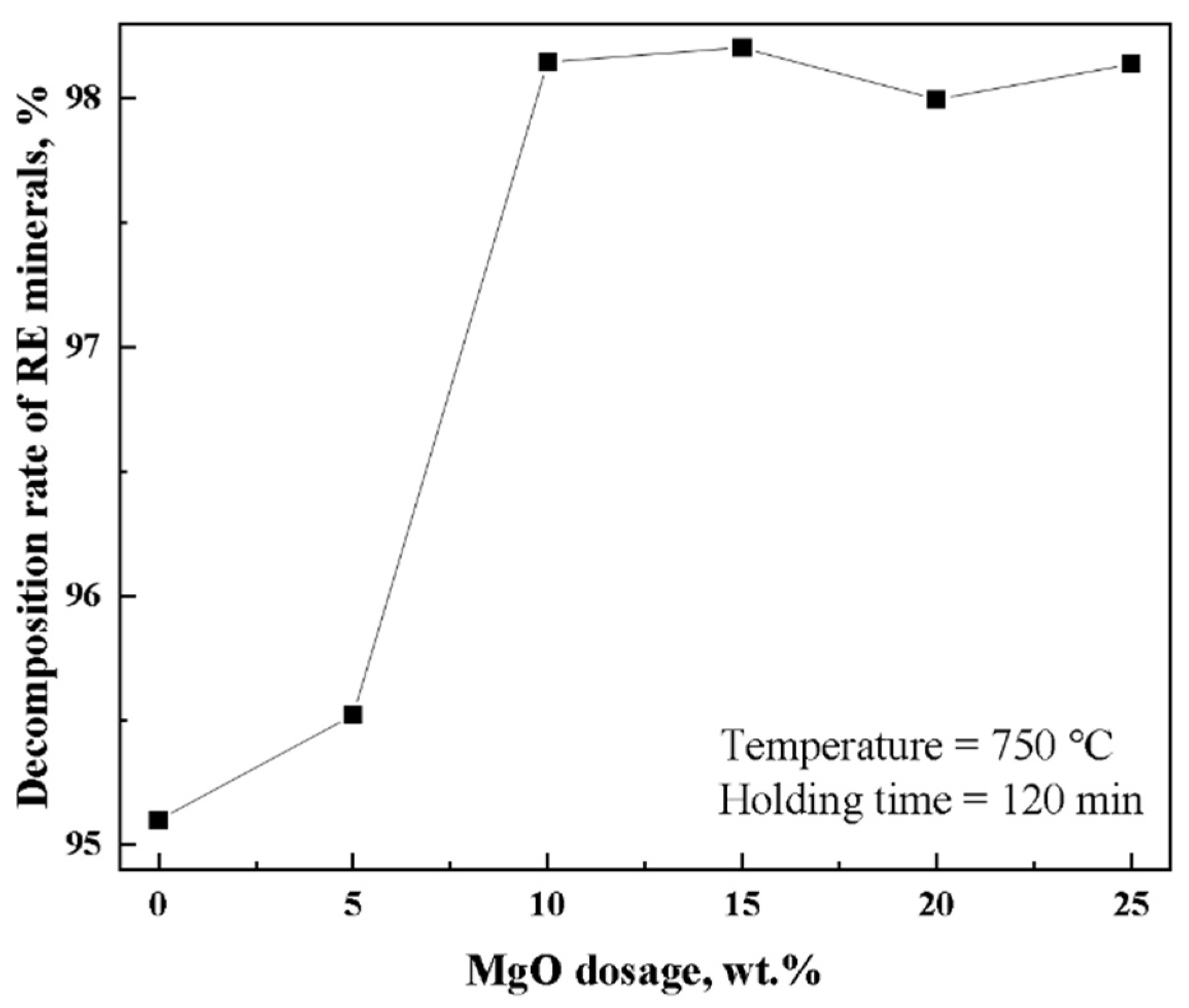
3.1. Effect of MgO Dosage on Decomposition Rate
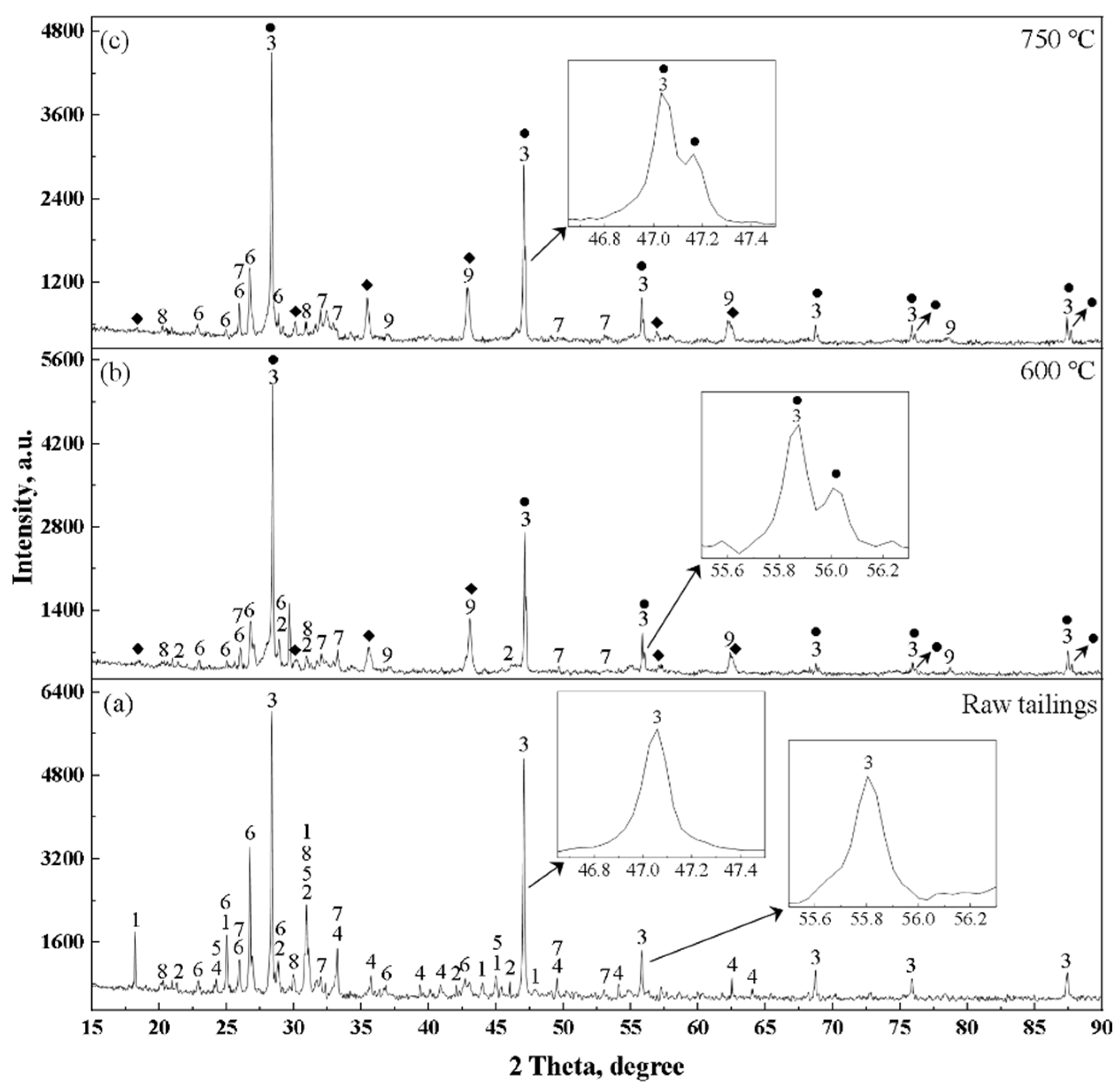
3.2. Phase Characterization
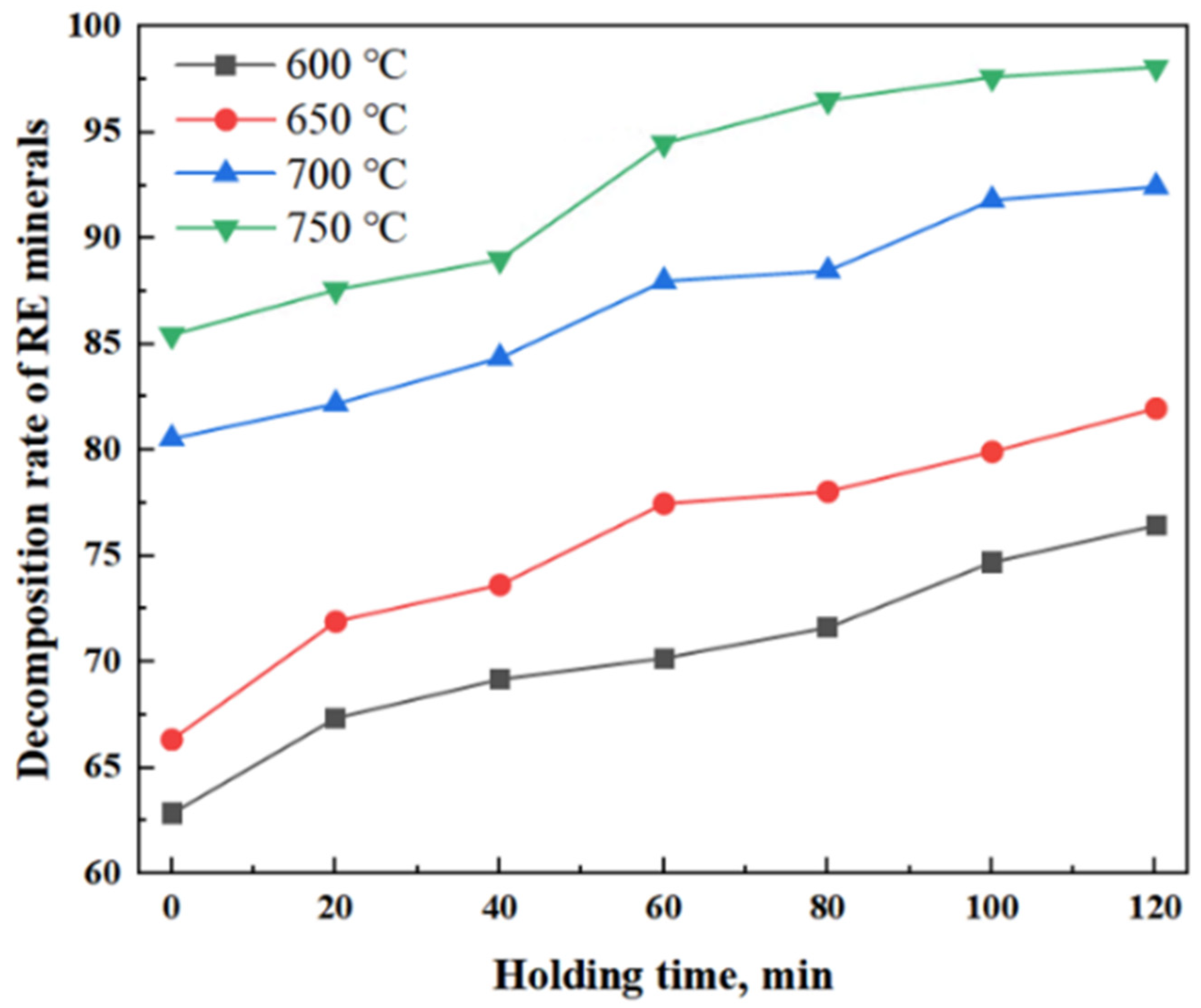
3.3. Effect of Roasting Temperature on Decomposition Rate
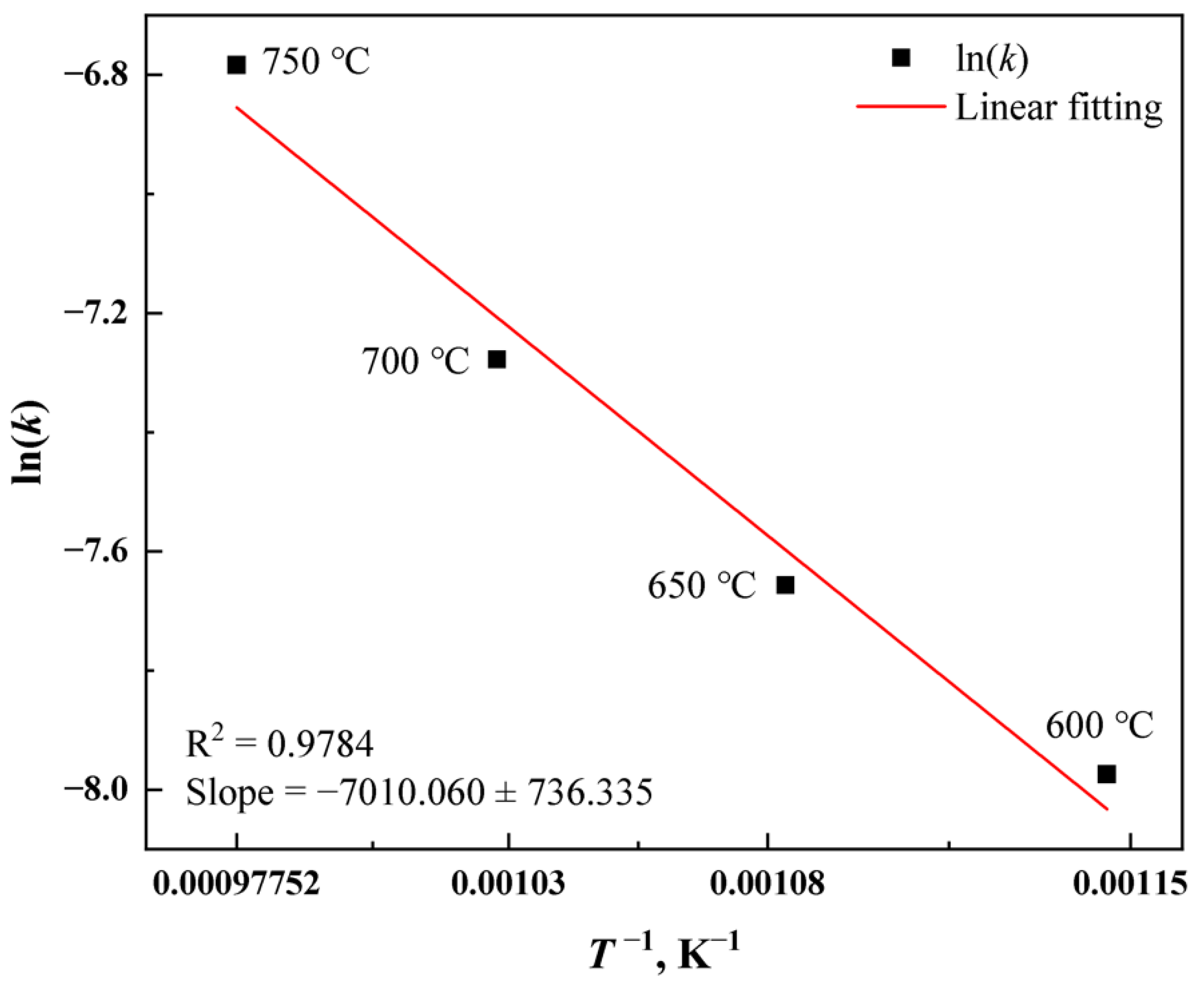
3.4. Kinetics Analysis of Mixed RE Mineral Decomposition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Zhang, Y.; Li, H. Development Trend and Research Progress of Rare Earth Extraction in China. Bull. Natl Nat. Sci. Found. China 2011, 25, 134–137. [Google Scholar]
- Xu, G.X. Rare Earths, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2012; Volume 1, ISBN 7-5024-1678-1. [Google Scholar]
- Resende, L.V.; Morais, C.A. Study of the Recovery of Rare Earth Elements from Computer Monitor Scraps–Leaching Experiments. Miner. Eng. 2010, 23, 277–280. [Google Scholar] [CrossRef]
- Savel’eva, I.L. The Rare-Earth Metals Industry of Russia: Present Status, Resource Conditions of Development. Geogr. Nat. Resour. 2011, 32, 65–71. [Google Scholar] [CrossRef]
- Li, K.; Liang, T.; Wang, L.; Yang, Z. Contamination and Health Risk Assessment of Heavy Metals in Road Dust in Bayan Obo Mining Region in Inner Mongolia, North China. J. Geogr. Sci. 2015, 25, 1439–1451. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, H.; Xue, X.; Yuan, S. Separation and Recovery of Iron and Rare Earth from Bayan Obo Tailings by Magnetizing Roasting and (NH4)2SO4 Activation Roasting. Metals 2017, 7, 195. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-L.; Bai, L.; Wang, Q.-C.; Liu, J.; Chi, M.-Y.; Wang, Z.-C. Recovery of Rare Earths, Niobium, and Thorium from the Tailings of Giant Bayan Obo Ore in China. Metall. Materi. Trans. B 2012, 43, 485–493. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Accumulation and Fractionation of Rare Earth Elements in Atmospheric Particulates around a Mine Tailing in Baotou, China. Atmos. Environ. 2014, 88, 23–29. [Google Scholar] [CrossRef]
- Xiaowei, H. Development Status and Research Progress in Rare Earth Hydrometallurgy in China. J. Chin. Rare Earth Soc. Chin. Ed. 2006, 24, 129. [Google Scholar]
- Yuan, S.; Yang, H.; Xue, X.-X.; Zhou, Y. Kinetics of Roasting Decomposition of the Rare Earth Elements by CaO and Coal. Metals 2017, 7, 213. [Google Scholar] [CrossRef]
- Chi, R.; Zhang, X.; Zhu, G.; Zhou, Z.A.; Wu, Y.; Wang, C.; Yu, F. Recovery of Rare Earth from Bastnasite by Ammonium Chloride Roasting with Fluorine Deactivation. Miner. Eng. 2004, 17, 1037–1043. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, G.; Hua, J.; Xu, S.; Chi, R. Recovery of RE from Baotou rare earth concentrate with chlorination roasting. Trans. Nonferrous Metals Soc. China 2003, 13, 438–442. [Google Scholar]
- Zhu, G.; Chi, R.; Shi, W.; Xu, Z. Chlorination Kinetics of Fluorine-Fixed Rare Earth Concentrate. Miner. Eng. 2003, 16, 671–674. [Google Scholar] [CrossRef]
- Cen, P.; Wu, W.; Bian, X. Thermodynamic Mechanism Analysis of Calcification Roasting Process of Bastnaesite Concentrates. Metall. Mater. Trans. B 2017, 48, 1539–1546. [Google Scholar] [CrossRef]
- Xue, B.; Fengyun, Z.; Wenyuan, W. Kinetics of Mixed Rare Earths Minerals Decomposed by CaO with NaCl-CaCl2 Melting Salt. J. Rare Earths 2010, 28, 5. [Google Scholar]
- Sadri, F.; Nazari, A.M.; Ghahreman, A. A Review on the Cracking, Baking and Leaching Processes of Rare Earth Element Concentrates. J. Rare Earths 2017, 35, 739–752. [Google Scholar] [CrossRef]
- Carrillo García, A.; Latifi, M.; Chaouki, J. Kinetic Study of Calcination of a Rare Earth Ore. Hydrometallurgy 2021, 200, 105557. [Google Scholar] [CrossRef]
- WU, W.; BIAN, X.; WU, Z.; SUN, S.; TU, G. Reaction Process of Monazite and Bastnaesite Mixed Rare Earth Minerals Calcined by CaO-NaCl-CaCl2. Trans. Nonferrous Metals Soc. China 2007, 17, 864–868. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, X.; Huang, X.; Yang, H.; Chen, G. Study on Recycling and Leaching Valuable Elements from Bayan Obo Tailings. Metall. Res. Technol. 2019, 116, 114. [Google Scholar] [CrossRef]
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms; Citeseer: Princeton, NJ, USA, 1995; Volume 102. [Google Scholar]






| Fetotal | REO | Nb2O5 | Sc | CaO | SiO2 | BaO | MgO |
| 13.06 | 7.09 | 0.11 | 0.01 | 30.34 | 11.71 | 4.13 | 3.32 |
| Al2O3 | MnO | TiO2 | F | S | P | Na2O | K2O |
| 1.39 | 1.15 | 0.80 | 12.50 | 1.66 | 1.39 | 1.03 | 0.52 |
| Element | Point A | Point B | Point C | Point D | Point E | Point F |
|---|---|---|---|---|---|---|
| C | 3.49 | 4.68 | 1.23 | 5.64 | 3.90 | 4.40 |
| O | 8.10 | 24.56 | 5.25 | 30.06 | 40.18 | 34.51 |
| F | 42.98 | 4.05 | 3.57 | - | - | - |
| Mg | 3.38 | 9.85 | 1.97 | 10.87 | 47.81 | 17.26 |
| Si | - | - | - | 1.23 | - | 5.89 |
| P | - | - | - | - | 1.18 | 1.55 |
| S | - | - | - | - | - | 2.12 |
| Ca | 37.95 | 6.04 | 10.50 | 5.62 | 3.05 | 10.76 |
| Fe | 0.63 | 11.78 | 1.07 | 14.74 | 1.76 | 6.64 |
| Mn | - | - | - | - | - | 0.57 |
| Ba | - | - | - | - | 0.99 | 3.86 |
| La | - | 19.41 | 23.01 | 14.75 | - | 5.42 |
| Ce | - | 14.12 | 40.05 | 7.86 | 1.15 | 3.05 |
| Pr | - | 2.19 | 3.70 | 3.19 | - | 1.21 |
| Nd | - | 3.33 | 9.66 | 6.05 | - | 2.75 |
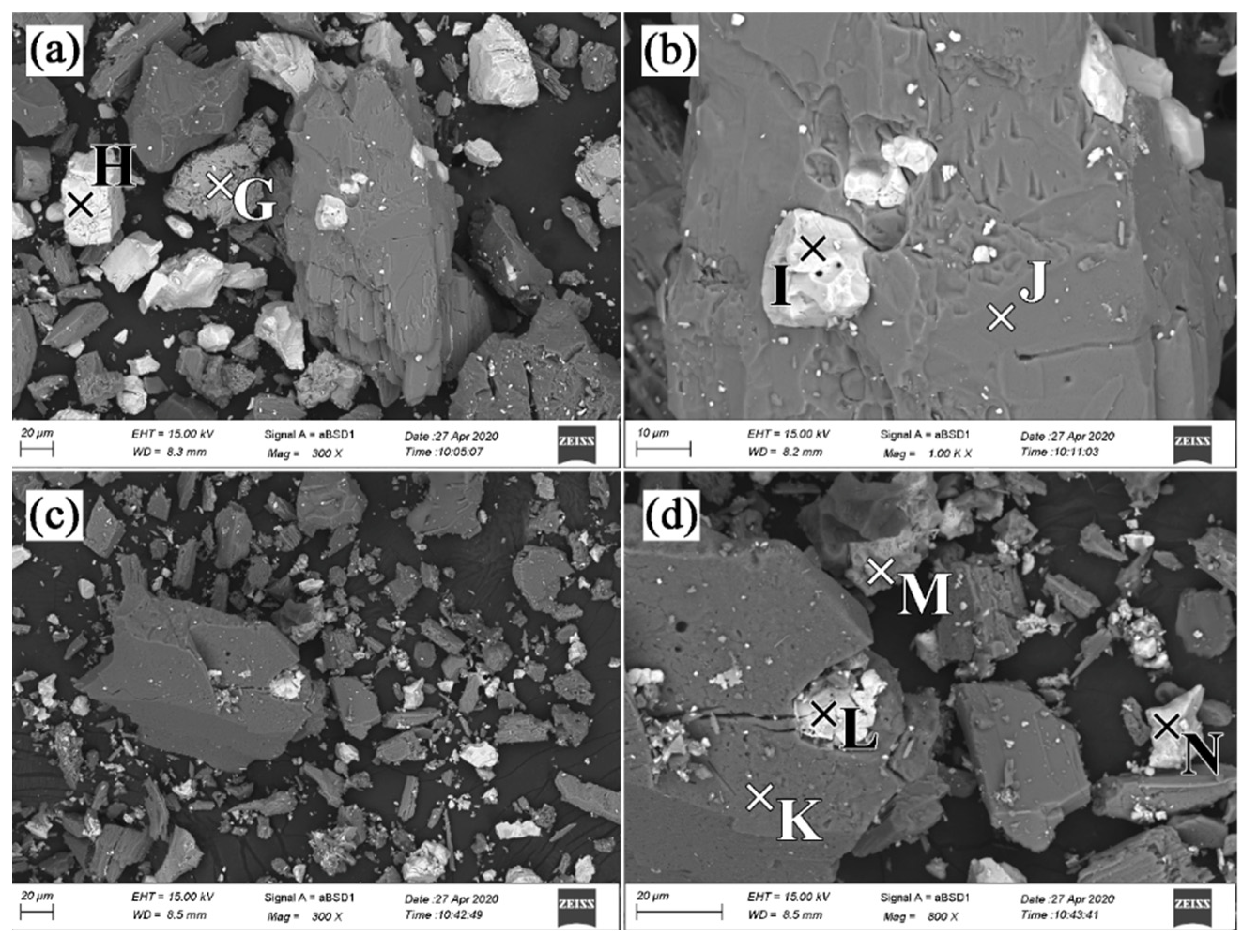
| Element | Point G | Point H | Point I | Point J | Point K | Point L | Point M | Point N |
|---|---|---|---|---|---|---|---|---|
| C | 1.38 | 4.63 | 3.60 | 3.78 | - | 0.86 | - | 2.38 |
| O | 10.78 | 25.00 | 17.32 | 25.65 | 45.41 | 5.56 | 27.28 | 17.92 |
| F | - | - | - | - | - | - | 2.69 | - |
| Na | - | - | - | 7.34 | 11.21 | 0.33 | 2.74 | 1.01 |
| Al | - | - | - | - | - | 0.59 | - | 0.29 |
| Si | 0.45 | - | - | 26.02 | 23.81 | 5.65 | 3.02 | 2.85 |
| P | - | - | 11.68 | - | - | - | - | - |
| S | - | 12.93 | - | - | - | 5.99 | - | 12.32 |
| Ca | - | - | - | 0.40 | 0.41 | - | 16.70 | - |
| Sc | - | - | - | - | 0.57 | - | - | - |
| Ti | 20.90 | - | - | - | - | 3.07 | - | |
| Fe | 64.39 | - | - | 36.80 | 18.59 | 0.69 | 0.73 | 1.23 |
| Nb | 2.11 | - | - | - | - | - | 41.29 | - |
| Ba | - | 57.39 | - | - | - | 80.32 | - | 62.01 |
| La | - | - | 13.80 | - | - | - | - | - |
| Ce | - | - | 36.37 | - | - | - | 1.59 | - |
| Pr | - | - | 4.91 | - | - | - | - | - |
| Nd | - | - | 12.32 | - | - | - | 0.89 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Song, S.; Liu, J.; Cheng, G.; Yang, H.; Xue, X. Thermal Decomposition Kinetics of Rare Earth Minerals in Tailings with Addition of MgO. Metals 2021, 11, 701. https://doi.org/10.3390/met11050701
Zhou Y, Song S, Liu J, Cheng G, Yang H, Xue X. Thermal Decomposition Kinetics of Rare Earth Minerals in Tailings with Addition of MgO. Metals. 2021; 11(5):701. https://doi.org/10.3390/met11050701
Chicago/Turabian StyleZhou, Yan, Shizhe Song, Jianxing Liu, Gongjin Cheng, He Yang, and Xiangxin Xue. 2021. "Thermal Decomposition Kinetics of Rare Earth Minerals in Tailings with Addition of MgO" Metals 11, no. 5: 701. https://doi.org/10.3390/met11050701






