Dissolution Kinetics of Chlorine from Iron Ore Sintering Dust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. X-ray Diffraction Analysis
2.3. Granularity Analysis
2.4. In-Situ Monitor Leaching Setup
2.5. Scanning Electron Microscopy Investigation
2.6. Stumm’s Kinetic Model
2.7. Transition State Theory Analysis
3. Results and Discussion
3.1. Properties of Iron Ore Sintering Dust
3.1.1. Elemental Composition Analysis
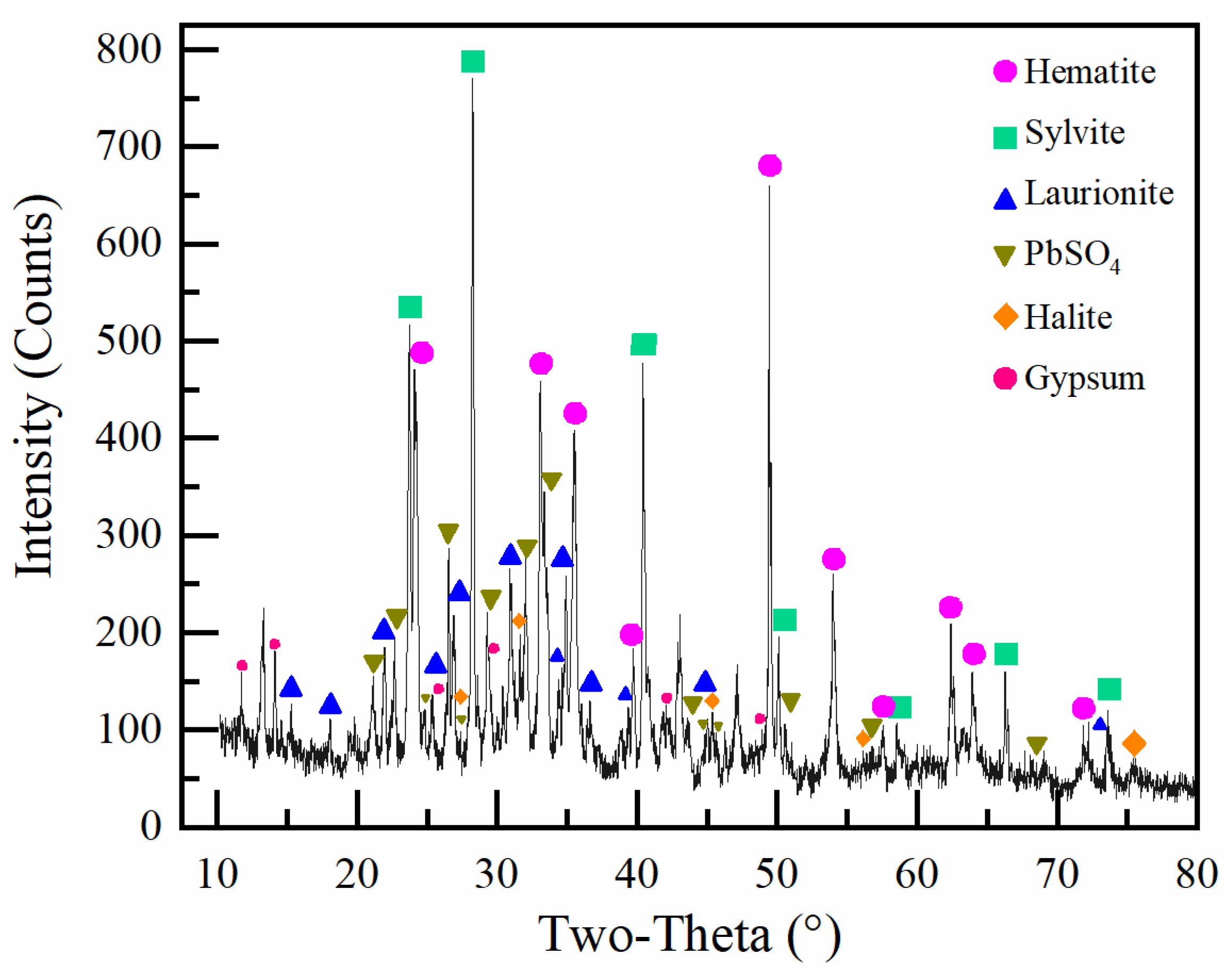
3.1.2. Phase Analysis
3.1.3. Surface Morphology
3.2. Effects of Temperature and S/L Ratio
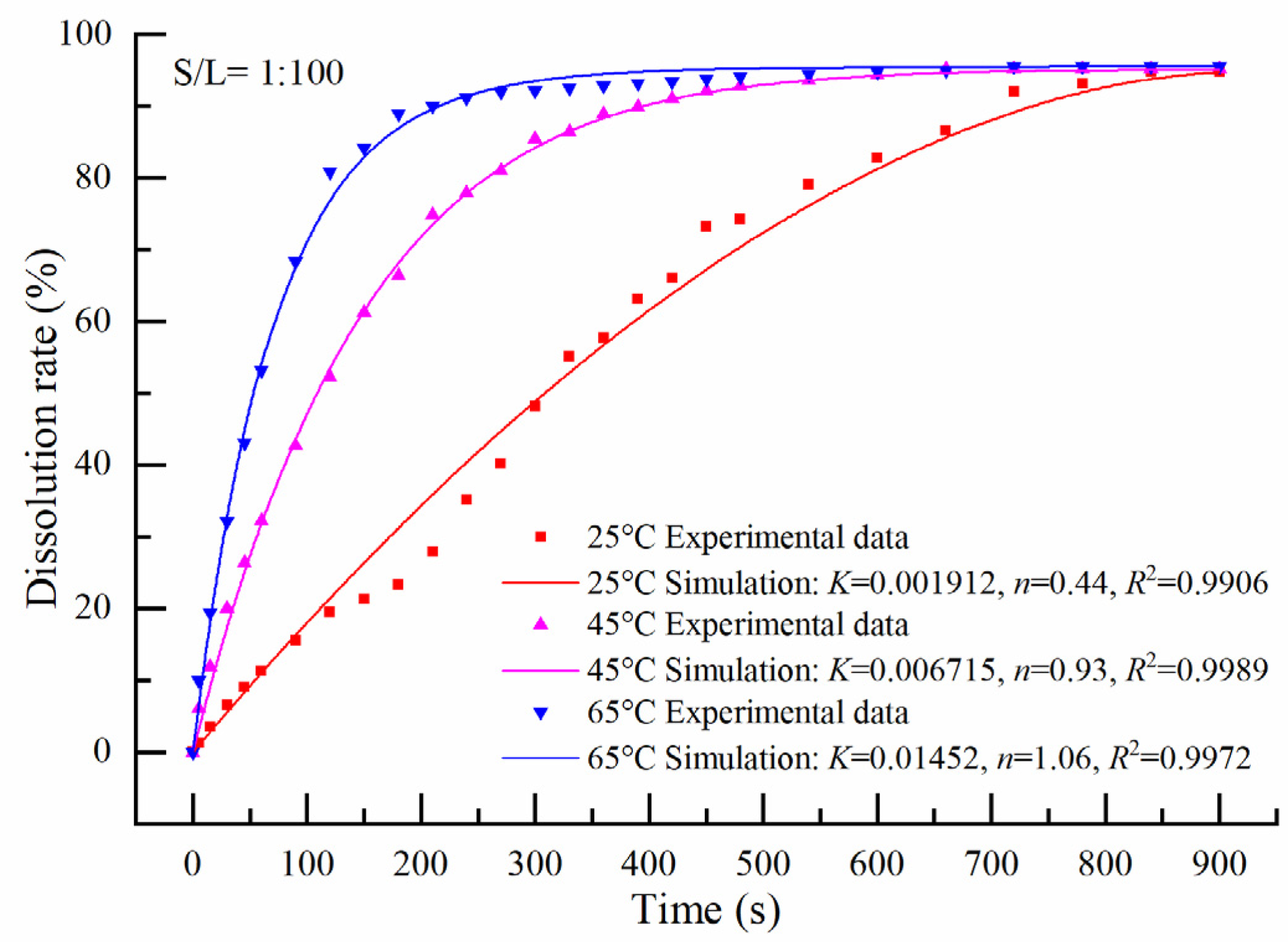
3.2.1. Solid to Liquid = 1:100
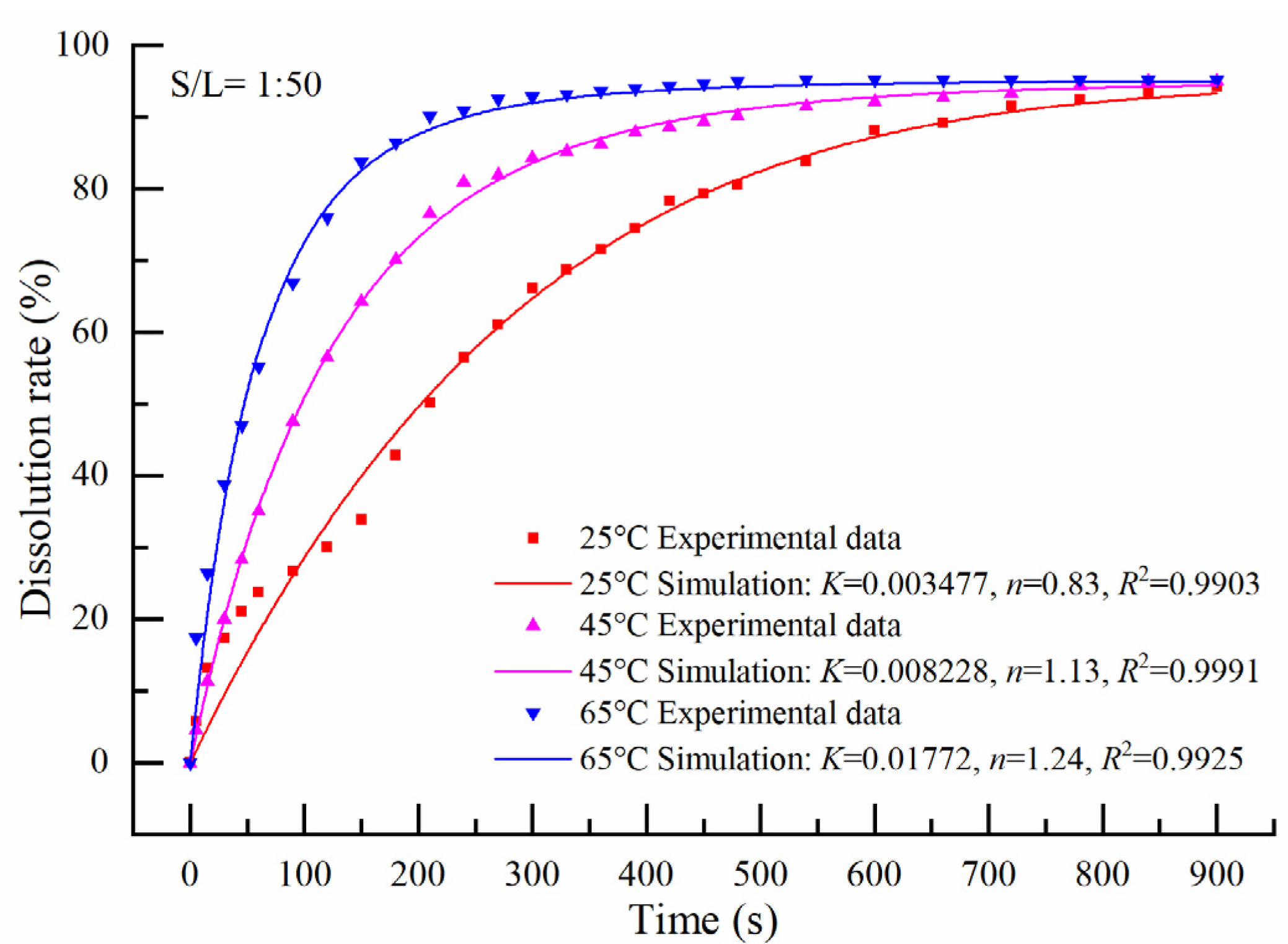
3.2.2. Solid to Liquid = 1:50
3.2.3. Solid to Liquid = 1:25
3.2.4. Apparent Activation Energy Calculation
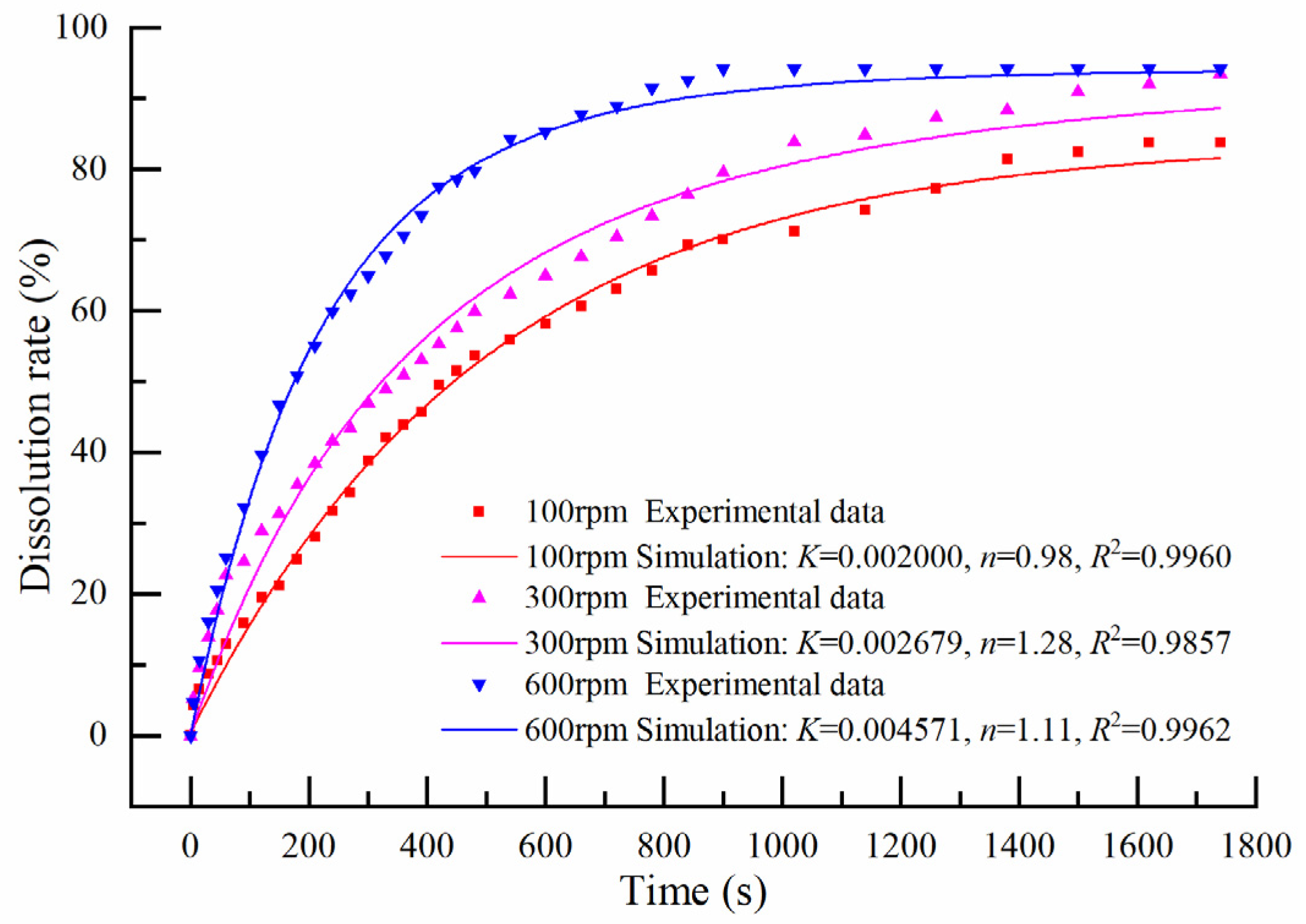
3.3. Effects of Stirring Speed
3.4. Effects of Particle Size
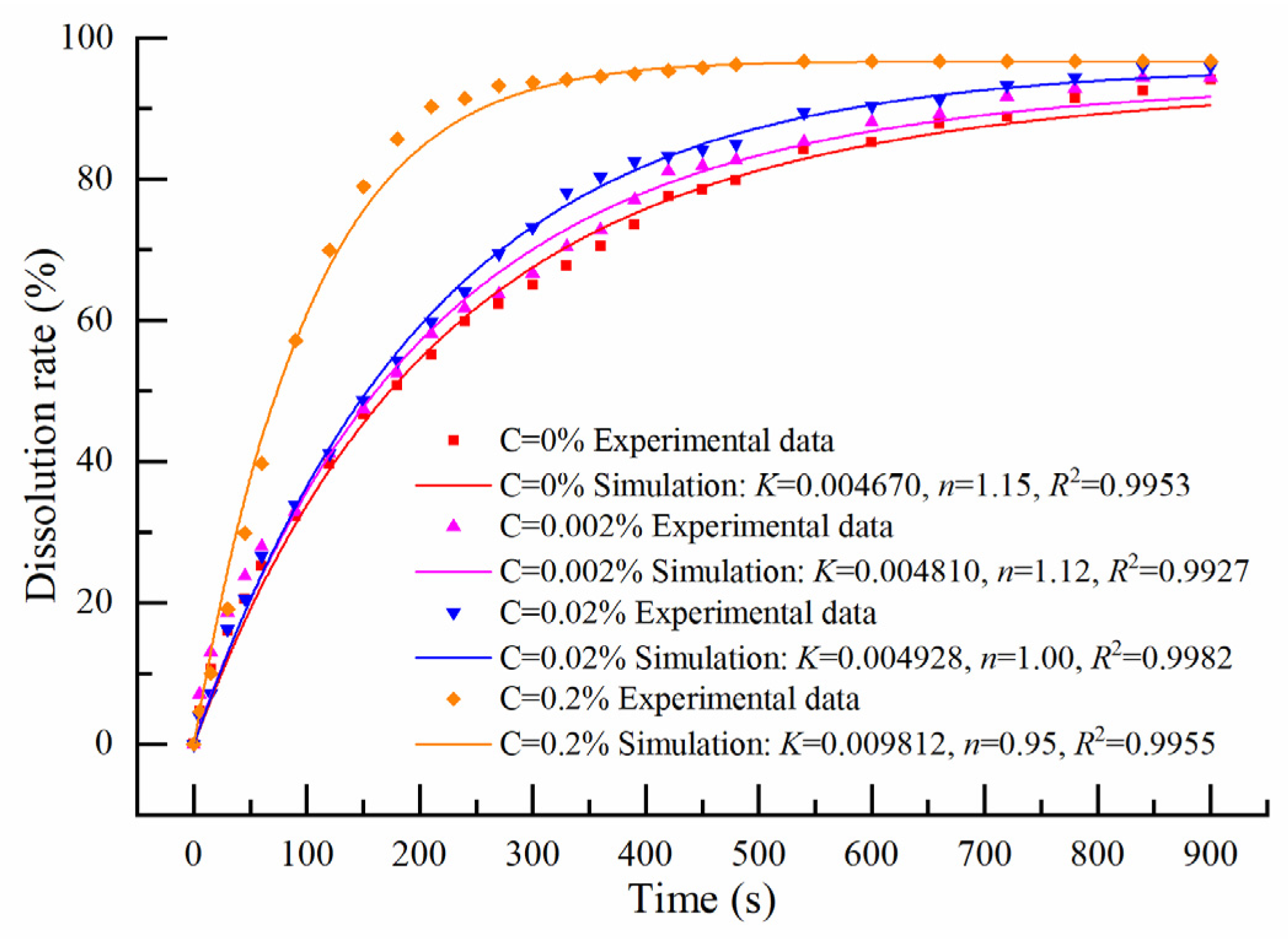
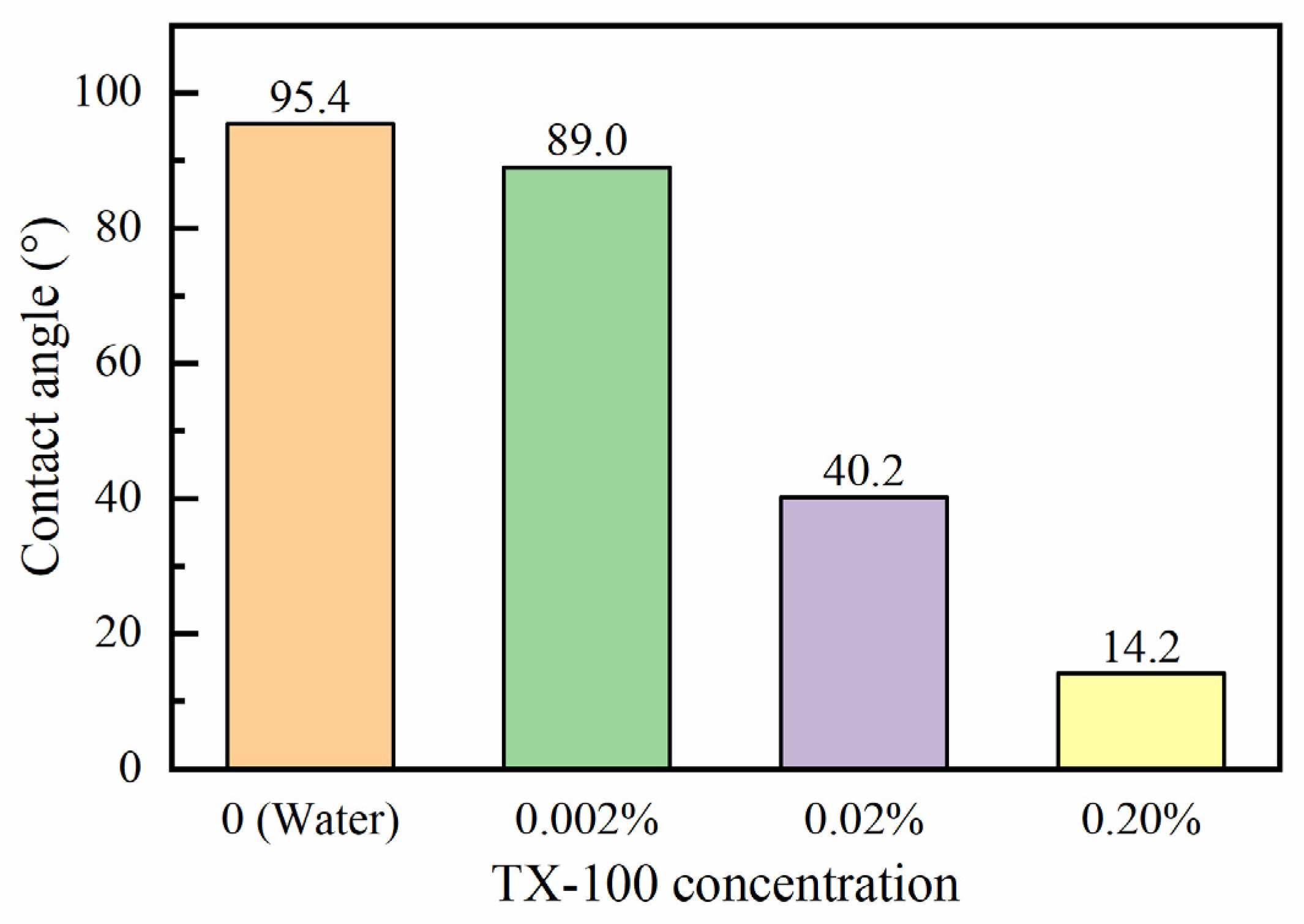
3.5. Effects of Surfactant Addition
3.6. SEM Examination of Insoluble Chlorides
4. Conclusions
- (1)
- Most of the sintering dust particles are porous agglomerates and the major chlorine-bearing substances in it were KCl, NaCl and PbOHCl. An in-situ monitor leaching system based on chloride ion selective electrode was designed to achieve real-time detection on the chloride ion leaching behaviors, while the chlorine dissolution data exhibited a good fit to Stumm’s kinetic models.
- (2)
- Based on the kinetics analysis and transition state theory calculation, the apparent activation energy of chlorine dissolution in sintering dust with an S/L ratio of 1:100, 1:50 and 1:25 were 42.60, 34.10 and 16.59 kJ/mol, respectively. It demonstrated that the dissolution was controlled by diffusion at low S/L ratio, while changed to controlled by surface chemical reaction as the S/L ratio increased. Meanwhile, increasing both temperature and stirring speed would accelerate the chlorine dissolving speed when the reaction was totally or partly controlled by diffusion.
- (3)
- Reducing the particle size and addition of 0.2% TX-100 would be a benefit for removing chlorine in sintering dust since they could cause a reduction in surface energy and acceleration of surface chemical reaction. Besides, the SEM-EDS examination inferred that the existence of laurionite limited the dissolution rate of chlorine less than 97%, while beneficiation or hydrometallurgy treatment was needed to further reduce the chlorine content of sintering dust to meet the demand of a sinter.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanzerstorfer, C.; Steiner, D. Characterization of sintering dust collected in the various fields of the electrostatic precipitator. Environ. Technol. 2016, 37, 1559–1567. [Google Scholar] [CrossRef]
- Min, Y.; Liu, C.; Shi, P.; Qin, C.; Feng, Y.; Liu, B. Effects of the addition of municipal solid waste incineration fly ash on the behavior of polychlorinated dibenzo-p-dioxins and furans in the iron ore sintering process. Waste Manag. 2018, 77, 287–293. [Google Scholar] [CrossRef]
- Chun, T.; Li, D.; Ning, C.; Wang, Z.; Long, H. Characteristics and Control Technology of Fine Particulate Matter (PM) in Iron Ore Sintering; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Lanzerstorfer, C.; Bamberger-Strassmayr, B.; Pilz, K. Recycling of Blast Furnace Dust in the Iron Ore Sintering Process: Investigation of Coke Breeze Substitution and the Influence on Off-gas Emissions. ISIJ Int. 2015, 55, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Sun, W.; Han, H. A novel method for comprehensive utilization of sintering dust. Trans. Nonferrous Met. Soc. China 2015, 25, 4192–4200. [Google Scholar] [CrossRef]
- Chang, F.; Wu, S.; Zhang, J.; Kou, M.; Lu, H.; Wang, L. Chemical, Physical and Morphological Changes of Sintering Dust by Mechanical Activation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 501–509. [Google Scholar]
- Yu, Y.; Wang, Z.; Wei, H.; Li, Y.; Song, Q.; Zheng, Z. Separation and recovery of potassium chloride from sinter dust of a steel plant. Ironmak. Steelmak. 2019, 46, 193–198. [Google Scholar] [CrossRef]
- Gan, M.; Ji, Z.; Fan, X.; Chen, X.; Zhou, Y.; Wang, G.; Tian, Y.; Jiang, T. Clean recycle and utilization of hazardous iron-bearing waste in iron ore sintering process. J. Hazard. Mater. 2018, 353, 381–392. [Google Scholar] [CrossRef]
- Long, H.; Shi, Q.; Zhang, H.; Wei, R.; Chun, T.; Li, J. Application status and comparison of dioxin removal technologies for iron ore sintering process. J. Iron Steel Res. Int. 2018, 25, 357–365. [Google Scholar] [CrossRef]
- Zhan, G.; Guo, Z. Basic properties of sintering dust from iron and steel plant and potassium recovery. J. Environ. Sci. 2013, 25, 1226–1234. [Google Scholar] [CrossRef]
- She, X.; Wang, J.; Wang, G.; Xue, Q.; Zhang, X. Removal Mechanism of Zn, Pb and Alkalis from Metallurgical Dusts in Direct Reduction Process. J. Iron Steel Res. Int. 2014, 21, 488–495. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, F.; Guo, Z. Separation and Recovery of Potassium Chloride from Sintering Dust of Ironmaking Works. ISIJ Int. 2009, 49, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Şahin, F.Ç.; Derin, B.; Yücel, O. Chloride removal from zinc ash. Scandinavian J. Metall. 2000, 29, 224–230. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, L.; Sun, W.; Hu, Y.; Ji, X.; Han, H.; Guan, Q. Extraction of rubidium from respirable sintering dust. Hydrometallurgy 2018, 175, 144–149. [Google Scholar] [CrossRef]
- Peng, C.; Guo, Z.; Zhang, F. Discovery of Potassium Chloride in the Sintering Dust by Chemical and Physical Characterization. ISIJ Int. 2008, 48, 1398–1403. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Chang, F.; Shen, Y.; Tsai, M.; Ko, C. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012, 237–238, 116–120. [Google Scholar] [CrossRef]
- Peng, C.; Guo, Z.; Zhang, F. Existing state of potassium chloride in agglomerated sintering dust and its water leaching kinetics. Trans. Nonferrous Met. Soc. China 2011, 21, 1847–1854. [Google Scholar] [CrossRef]
- Zhan, G.; Guo, Z. Water leaching kinetics and recovery of potassium salt from sintering dust. Trans. Nonferrous Met. Soc. China 2013, 23, 3770–3779. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, L.; Sun, W.; Hu, Y.; Han, H. Surface characteristics and wettability enhancement of respirable sintering dust by nonionic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 323–333. [Google Scholar] [CrossRef]
- Jin, J.; Liu, L.; Liu, R.; Wei, H.; Qian, G.; Zheng, J.; Xie, W.; Lin, F.; Xie, J. Preparation and thermal performance of binary fatty acid with diatomite as form-stable composite phase change material for cooling asphalt pavements. Constr. Build. Mater. 2019, 226, 616–624. [Google Scholar] [CrossRef]
- Guan, Q.; Sun, W.; Guan, C.; Yu, W.; Zhu, X.; Khoso, S.A.; Wang, P.; Peng, W. Promotion of conversion activity of flue gas desulfurization gypsum into α-hemihydrate gypsum by calcination-hydration treatment. J. Cent. South Univ. 2019, 26, 3213–3224. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry An Introduction Emphasizing Chemical Equilibria in Natural Waters; Wiley-Interscience: New York, NY, USA, 1970. [Google Scholar]
- Cheng, P.; Chen, D.; Liu, H.; Zou, X.; Wu, Z.; Xie, J.; Qing, C.; Kong, D.; Chen, T. Synergetic effects of anhydrite and brucite-periclase materials on phosphate removal from aqueous solution. J. Mol. Liq. 2018, 254, 145–153. [Google Scholar] [CrossRef]
- Alvarado-Macías, G.; Fuentes-Aceituno, J.C.; Nava-Alonso, F.; Lee, J. Silver leaching with the nitrite–copper novel system: A kinetic study. Hydrometallurgy 2016, 160, 98–105. [Google Scholar] [CrossRef]
- Barsa, C.S.; Normand, M.D.; Peleg, M. On Models of the Temperature Effect on the Rate of Chemical Reactions and Biological Processes in Foods. Food Eng. Rev. 2012, 4, 191–202. [Google Scholar] [CrossRef]
- Tang, H.; Jiang, F.; Hu, Y.; Han, H.; Wang, L.; Sun, W. Flotability of laurionite and its response to sulfidization flotation. Miner. Eng. 2020, 148, 106183. [Google Scholar] [CrossRef]
- Zhan, G.; Guo, Z. Preparation of potassium salt with joint production of spherical calcium carbonate from sintering dust. Trans. Nonferrous Met. Soc. China 2015, 25, 628–639. [Google Scholar] [CrossRef]
- Li, X.; Ye, Y.; Xue, S.; Jiang, J.; Wu, C.; Kong, X.; Hartley, W.; Li, Y. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue. Trans. Nonferrous Met. Soc. China 2018, 28, 1248–1255. [Google Scholar] [CrossRef]
- Zang, L.; Rodgers, M.A.J. Diffusion-Controlled Charge Transfer from Excited Ru(bpy)32+ into Nanosized TiO2 Colloids Stabilized with EDTA. J. Phys. Chem. B 2000, 104, 468–474. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, G.Q.; Zhao, D.; Ma, J.H.; Yang, J. Dephosphorization of high phosphorus oolitic hematite by acid leaching and the leaching kinetics. Hydrometallurgy 2017, 171, 61–68. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, T.; Wen, J.; Gao, H.; Wang, J.; Xue, X. Leaching kinetics of mechanically activated boron concentrate in a NaOH solution. Hydrometallurgy 2018, 179, 60–72. [Google Scholar] [CrossRef]
- Ho, Y.; Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C. Kinetics and model building of leaching of water-soluble compounds of Tilia sapwood. Sep. Purif. Technol. 2005, 45, 169–173. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, Y.; Lv, W.; Liu, J.; Song, X.; Chen, L.; Wang, C.; Sels, B.F.; Ma, L. Complementing Vanillin and Cellulose Production by Oxidation of Lignocellulose with Stirring Control. ACS Sustain. Chem. Eng. 2020, 8, 2361–2374. [Google Scholar] [CrossRef]
- Guan, R.; Yuan, X.; Wu, Z.; Wang, H.; Jiang, L.; Li, Y.; Zeng, G. Functionality of surfactants in waste-activated sludge treatment: A review. Sci. Total Environ. 2017, 609, 1433–1442. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.J.; Sun, F.; Liu, C. Catalytic Effect of a Combined Silver and Surfactant Catalyst on Cobalt Ore Bioleaching. JOM 2018, 70, 2819–2824. [Google Scholar] [CrossRef]
- Ito, R.; Fujita, T.; Sadaki, J.; Matsumoto, Y.; Ahn, J. Removal of Chloride in Bottom Ash from the Industrial and Municipal Solid Waste Incinerators. Int. J. Soc. Mater. Eng. Resour. 2006, 13, 70–74. [Google Scholar] [CrossRef]
- Chen, W.; Shen, Y.; Tsai, M.; Chang, F. Removal of chloride from electric arc furnace dust. J. Hazard. Mater. 2011, 190, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Um, N.; Han, G.; You, K.; Cho, H. Effect of Carbonation in Removal of Chloride from Municipal Solid Waste Incineration Bottom Ash. Geosystem Eng. 2006, 9, 87–90. [Google Scholar] [CrossRef]






| Elements | Cl | K | Na | Fe | Pb | Zn | S | Ca | O |
|---|---|---|---|---|---|---|---|---|---|
| Content | 33.38 | 20.51 | 4.39 | 4.85 | 16.02 | 0.47 | 3.49 | 0.92 | 12.12 |
| S/L ratio | Ea (kJ/mol) | ΔHa (kJ/mol) | ΔSa (J/mol) |
|---|---|---|---|
| 1:100 | 42.60 | 39.96 | −162.37 |
| 1:50 | 34.10 | 31.46 | −186.41 |
| 1:25 | 16.59 | 13.96 | −239.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Zhao, L.; Yang, Y.; Han, H.; Wang, L.; Sun, W. Dissolution Kinetics of Chlorine from Iron Ore Sintering Dust. Metals 2021, 11, 1185. https://doi.org/10.3390/met11081185
Tang H, Zhao L, Yang Y, Han H, Wang L, Sun W. Dissolution Kinetics of Chlorine from Iron Ore Sintering Dust. Metals. 2021; 11(8):1185. https://doi.org/10.3390/met11081185
Chicago/Turabian StyleTang, Honghu, Lihua Zhao, Yue Yang, Haisheng Han, Li Wang, and Wei Sun. 2021. "Dissolution Kinetics of Chlorine from Iron Ore Sintering Dust" Metals 11, no. 8: 1185. https://doi.org/10.3390/met11081185







