Manufacturing and Characterization of Tube-Filled ZA27 Metal Foam Heat Exchangers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Thermodynamic Analysis
3. Results and Discussion
4. Conclusions
- Comparison of the testing data revealed a heat transfer enhancement of up to 71%.
- Overall performance was found to be limited by the inefficient heat transfer between the internal mass stream and the copper tube, due to a relatively small contact area.
- For future research, we will, therefore, consider compact heat exchangers containing heat-transfer-enhancing foams that are located both inside and outside a tube.
- In addition, ZA27 foam may be replaced with aluminium foam due to the higher thermal conductivity of this metal, and to prevent the formation of a zinc hydroxite layer (see Figure 1c) that may decrease heat transfer over time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Mass | |
| Energy | |
| Local temperature | |
| Local enthalpy | |
| Internal mass stream | |
| External mass stream | |
| Internal heat transfer rate | |
| Dissipated thermal energy rate | |
| Velocity | |
| Elevation | |
| Power | |
| Heat transfer rate of metal foam | |
| Heat transfer rate of metal tube |
References
- Ghiani, C.; Linul, E.; Porcu, M.; Marsavina, L.; Movahedi, N.; Aymerich, F. Metal Foam-Filled Tubes as Plastic Dissipaters in Earthquake-Resistant Steel Buildings. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012051. [Google Scholar] [CrossRef]
- Kim, D.Y.; Sung, T.H.; Kim, K.C. Application of metal foam heat exchangers for a high-performance liquefied natural gas regasification system. Energy 2016, 105, 57–69. [Google Scholar] [CrossRef]
- Vainoris, M.; Cesiulis, H.; Tsyntsaru, N. Metal Foam Electrode as a Cathode for Copper Electrowinning. Coatings 2020, 10, 822. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, X.F.; Wei, X.; Han, F.S.; Wang, X.L. Sound absorption of open celled aluminium foam fabricated by investment casting method. Mater. Sci. Technol. 2011, 27, 800–804. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, W.; Tassou, S. Thermal analysis on metal-foam filled heat exchangers. Part II: Tube heat exchangers. Int. J. Heat Mass Transf. 2006, 49, 2762–2770. [Google Scholar] [CrossRef]
- T’Joen, C.; De Jaeger, P.; Huisseune, H.; Van Herzeele, S.; Vorst, N.; De Paepe, M. Thermo-hydraulic study of a single row heat exchanger consisting of metal foam covered round tubes. Int. J. Heat Mass Transf. 2010, 53, 3262–3274. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Lu, T.; Zhuang, D.; Ding, G. Experimental study of condensation heat transfer characteristics on metal foam wrapped tubes under sloshing conditions. Int. J. Heat Mass Transf. 2021, 176, 121394. [Google Scholar] [CrossRef]
- Bamorovat Abadi, G.; Kim, K.C. Experimental heat transfer and pressure drop in a metal-foam-filled tube heat exchanger. Exp. Therm. Fluid Sci. 2017, 82, 42–49. [Google Scholar] [CrossRef]
- Kotresha, B.; Gnanasekaran, N. Numerical Simulations of Fluid Flow and Heat Transfer through Aluminum and Copper Metal Foam Heat Exchanger—A Comparative Study. Heat Transf. Eng. 2020, 41, 637–649. [Google Scholar] [CrossRef]
- Chen, T.; Shu, G.; Tian, H.; Zhao, T.; Zhang, H.; Zhang, Z. Performance evaluation of metal-foam baffle exhaust heat exchanger for waste heat recovery. Appl. Energy 2020, 266, 114875. [Google Scholar] [CrossRef]
- Ho, J.Y.; Leong, K.C.; Wong, T.N. Additively-manufactured metallic porous lattice heat exchangers for air-side heat transfer enhancement. Int. J. Heat Mass Transf. 2020, 150, 119262. [Google Scholar] [CrossRef]
- Ferfera, R.S.; Madani, B. Thermal characterization of a heat exchanger equipped with a combined material of phase change material and metallic foams. Int. J. Heat Mass Transf. 2020, 148, 119162. [Google Scholar] [CrossRef]
- Lai, Z.; Hu, H.; Ding, G. Influence of pore density on heat transfer and pressure drop characteristics of wet air in hydrophilic metal foams. Appl. Therm. Eng. 2019, 159, 113897. [Google Scholar] [CrossRef]
- Movahedi, N.; Murch, G.E.; Belova, I.V.; Fiedler, T. Manufacturing and compressive properties of tube-filled metal syntactic foams. J. Alloy. Compd. 2020, 822, 153465. [Google Scholar] [CrossRef]
- Al-Sahlani, K.; Kisi, E.; Fiedler, T. Impact of particle strength and matrix ductility on the deformation mechanism of metallic syntactic foam. J. Alloy. Compd. 2019, 786, 292–299. [Google Scholar] [CrossRef]
- Holmgren, M. X Stream, Thermodynamic Properties of Water and Steam. MATLAB Central File Exchange. Retrieved January 2021. Available online: https://www.mathworks.com/matlabcentral/fileexchange/9817-x-stream-thermodynamic-properties-of-water-and-steam (accessed on 20 June 2021).
- Pritchard, P.J. Fox and Mc Donalds Introduction to Fluid Mechanics, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]

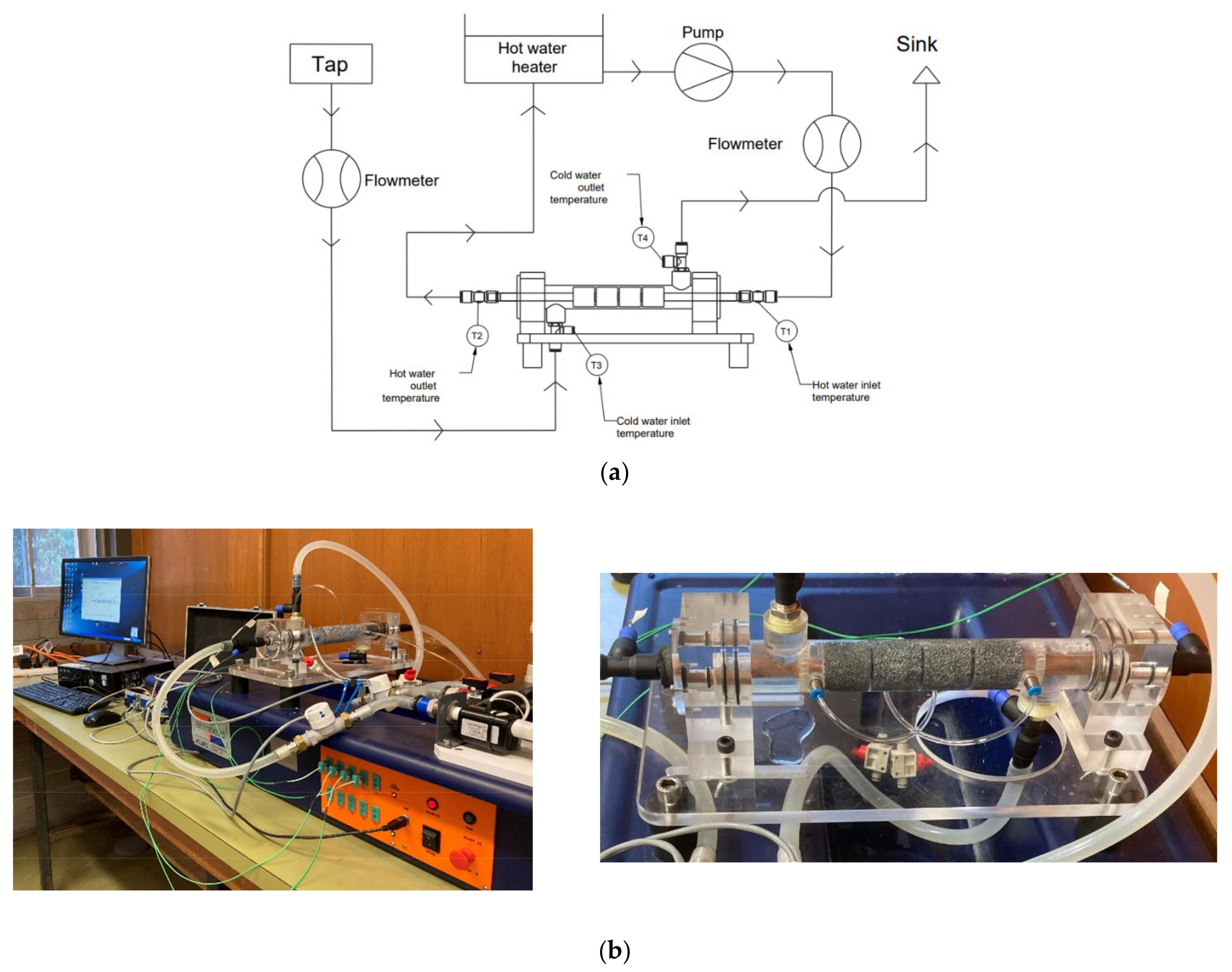
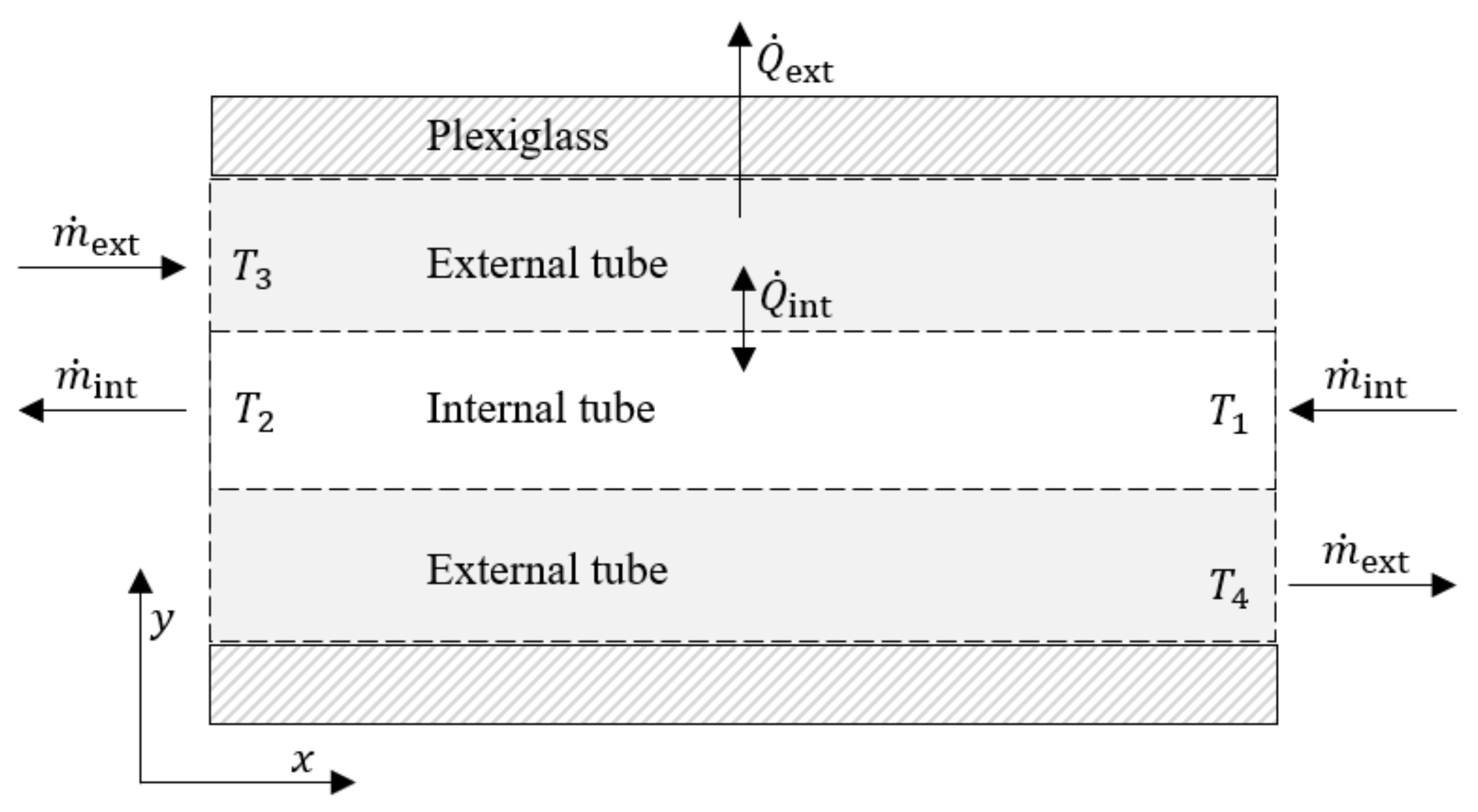

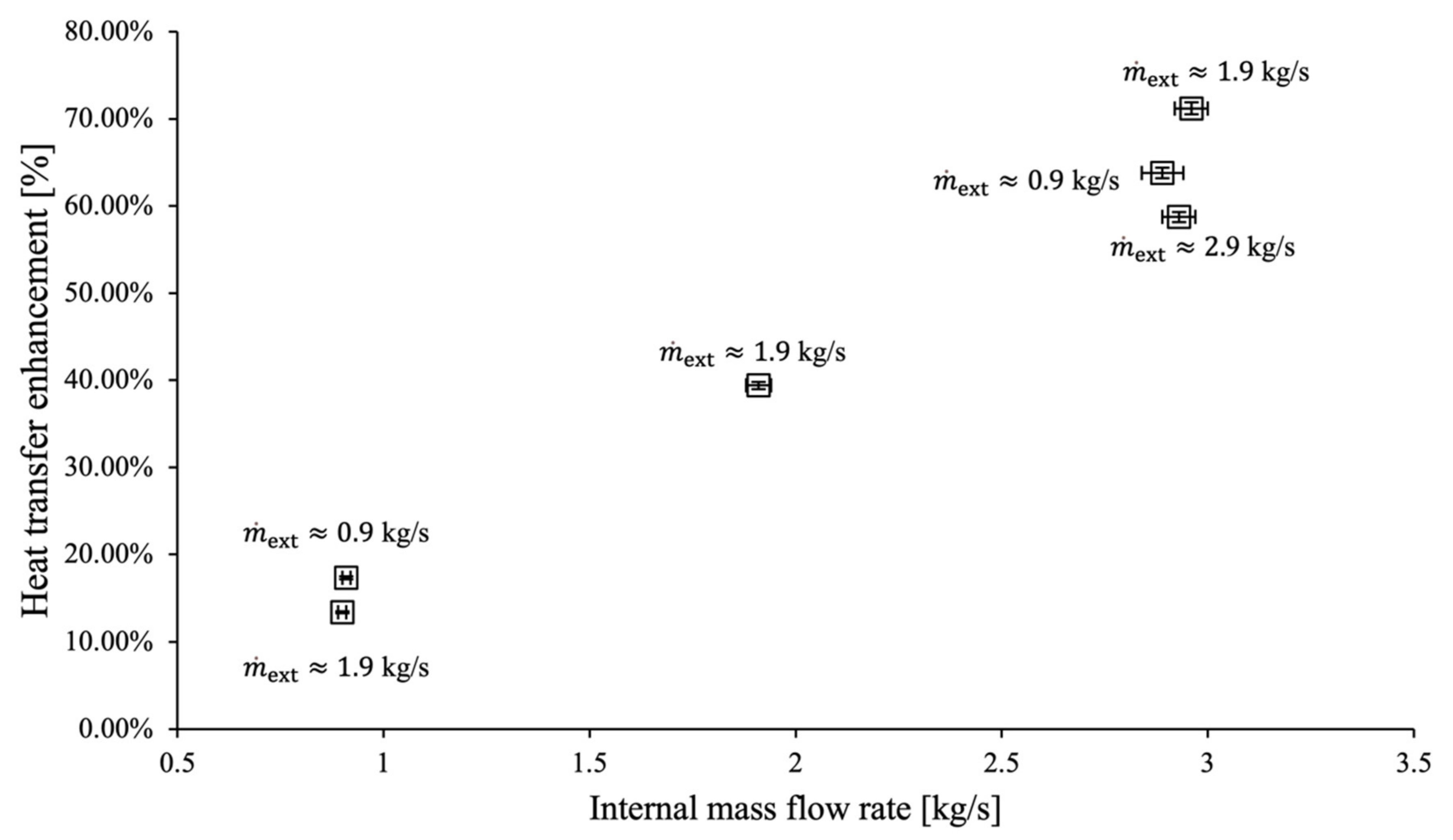
| Element | Al | Cu | Mg | Fe | Pb | Cd | Zn |
|---|---|---|---|---|---|---|---|
| Wt% | Balance |
| [kg/s] | [kg/s] | [°C] | [°C] | [°C] | [°C] | [kW] | |
|---|---|---|---|---|---|---|---|
| 0.93 | 0.91 | 59.48 | 54.71 | 18.70 | 23.79 | 18.16 | |
| #1 | 0.91 | 0.90 | 59.89 | 55.18 | 18.83 | 24.07 | 17.93 |
| 0.90 | 0.92 | 60.11 | 55.20 | 18.58 | 23.96 | 18.89 | |
| 1.91 | 0.92 | 59.98 | 54.44 | 18.52 | 21.56 | 21.32 | |
| #2 | 1.90 | 0.89 | 60.20 | 54.63 | 18.49 | 21.47 | 20.73 |
| 1.92 | 0.88 | 60.40 | 54.65 | 18.32 | 21.33 | 21.16 | |
| 0.90 | 2.92 | 60.01 | 57.08 | 18.57 | 28.68 | 35.79 | |
| #3 | 0.92 | 2.90 | 60.00 | 57.07 | 18.67 | 28.59 | 35.54 |
| 0.93 | 2.85 | 60.07 | 57.12 | 18.65 | 28.50 | 35.29 | |
| 1.93 | 1.92 | 60.05 | 55.67 | 18.61 | 23.39 | 35.18 | |
| #4 | 1.91 | 1.92 | 60.10 | 55.72 | 18.65 | 23.45 | 35.18 |
| 1.91 | 1.900.03 | 59.96 | 55.57 | 18.59 | 23.36 | 34.89 | |
| 1.88 | 2.940.04 | 60.01 | 56.45 | 18.44 | 24.51 | 43.78 | |
| #5 | 1.86 | 2.98 | 60.05 | 56.50 | 18.47 | 24.62 | 44.25 |
| 1.85 | 2.96 | 59.93 | 56.40 | 17.61 | 23.75 | 43.71 | |
| 2.85 | 2.91 | 60.02 | 56.000.84 | 17.84 | 22.25 | 48.93 | |
| #6 | 2.85 | 2.95 | 59.95 | 56.00 | 18.06 | 22.52 | 48.74 |
| 2.86 | 2.95 | 59.98 | 56.03 | 18.05 | 22.53 | 48.74 |
| [kg/s] | [kg/s] | [°C] | [°C] | [°C] | [°C] | [kW] | |
|---|---|---|---|---|---|---|---|
| 0.94 | 0.95 | 60.30 | 56.11 | 16.09 | 20.53 | 16.65 | |
| #1 | 0.92 | 0.98 | 60.04 | 56.18 | 15.86 | 19.94 | 15.82 |
| 0.93 | 0.96 | 59.99 | 56.29 | 15.81 | 19.51 | 14.39 | |
| 1.91 | 0.96 | 60.12 | 55.48 | 13.88 | 16.14 | 18.63 | |
| #2 | 1.91 | 0.96 | 60.03 | 55.44 | 14.14 | 16.40 | 18.43 |
| 1.90 | 0.98 | 59.96 | 55.40 | 14.63 | 16.90 | 18.69 | |
| 0.94 | 2.840.04 | 60.62 | 58.78 | 15.79 | 21.53 | 21.86 | |
| #3 | 0.96 | 2.84 | 60.00 | 58.20 | 15.81 | 21.39 | 21.39 |
| 0.96 | 2.84 | 59.98 | 58.14 | 15.87 | 21.61 | 21.86 | |
| 1.91 | 1.90 | 60.28 | 57.07 | 15.21 | 18.39 | 25.51 | |
| #4 | 1.89 | 1.88 | 60.00 | 56.82 | 15.27 | 18.42 | 25.01 |
| 1.88 | 1.89 | 59.98 | 56.82 | 15.26 | 18.46 | 24.98 | |
| 1.90 | 2.81 | 60.56 | 58.36 | 15.32 | 19.04 | 25.86 | |
| #5 | 1.89 | 2.83 | 60.04 | 57.89 | 15.33 | 18.83 | 25.45 |
| 1.90 | 2.89 | 59.98 | 57.86 | 15.39 | 18.95 | 25.63 | |
| 2.81 | 2.80 | 60.66 | 58.05 | 15.33 | 18.24 | 30.57 | |
| #6 | 2.84 | 2.83 | 60.03 | 57.44 | 15.29 | 18.12 | 30.66 |
| 2.84 | 2.84 | 60.00 | 57.39 | 15.30 | 18.23 | 31.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, T.; Moore, R.; Movahedi, N. Manufacturing and Characterization of Tube-Filled ZA27 Metal Foam Heat Exchangers. Metals 2021, 11, 1277. https://doi.org/10.3390/met11081277
Fiedler T, Moore R, Movahedi N. Manufacturing and Characterization of Tube-Filled ZA27 Metal Foam Heat Exchangers. Metals. 2021; 11(8):1277. https://doi.org/10.3390/met11081277
Chicago/Turabian StyleFiedler, Thomas, Ryan Moore, and Nima Movahedi. 2021. "Manufacturing and Characterization of Tube-Filled ZA27 Metal Foam Heat Exchangers" Metals 11, no. 8: 1277. https://doi.org/10.3390/met11081277






