Influence of Cooling Conditions on Long-Period Stacking-Ordered Phase Evolution and Corrosion Behavior of As-Cast Resoloy®
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
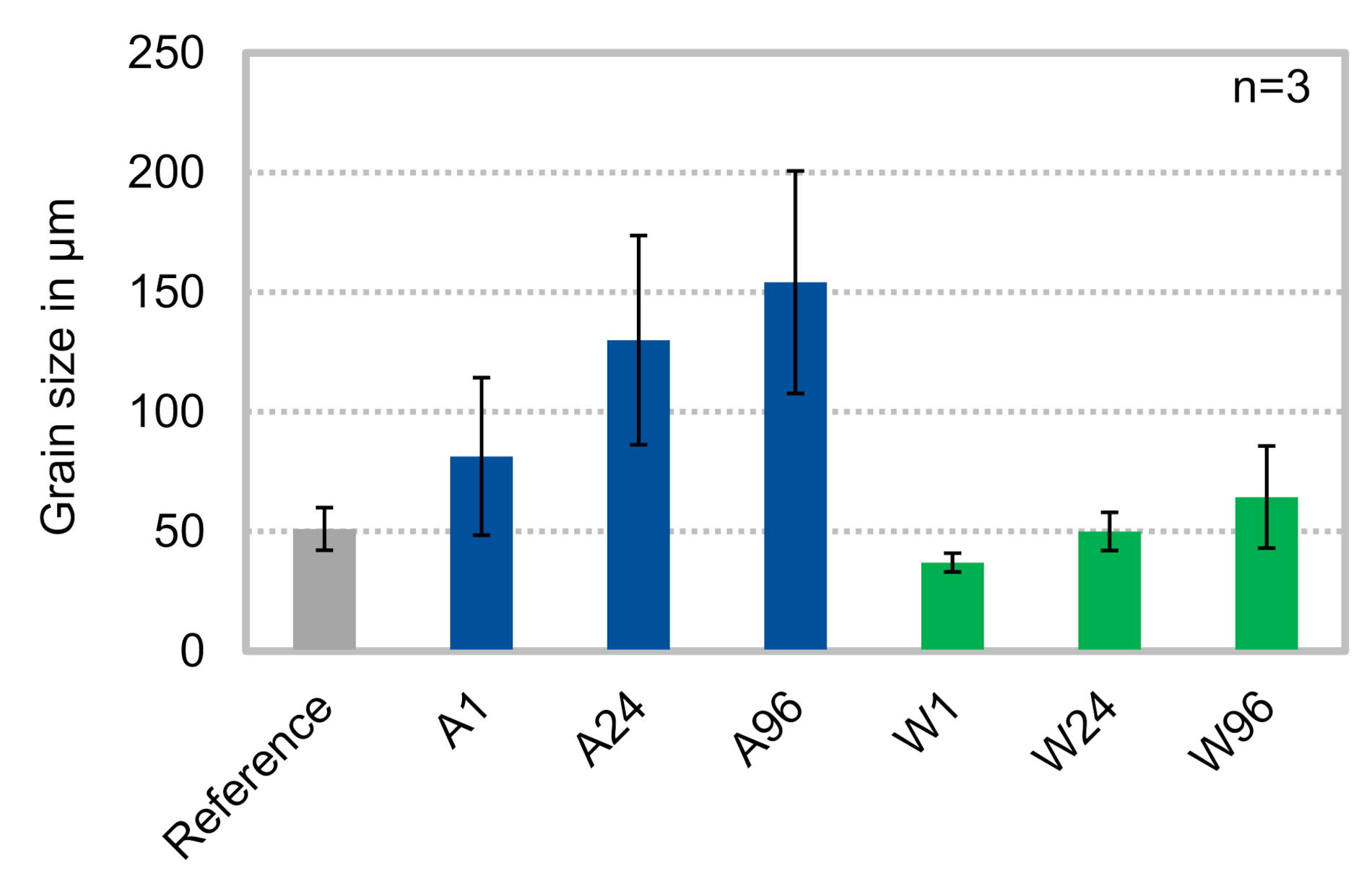
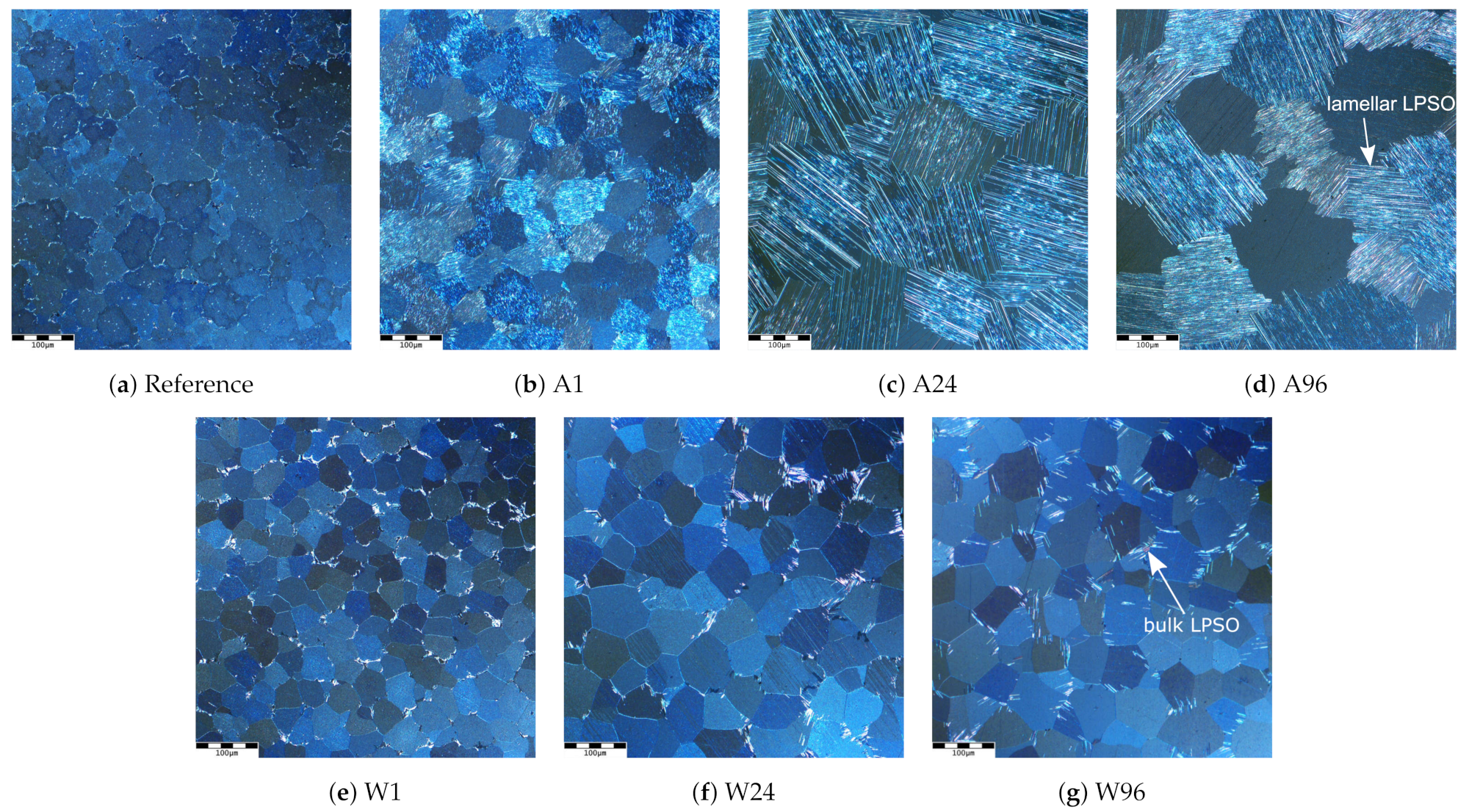
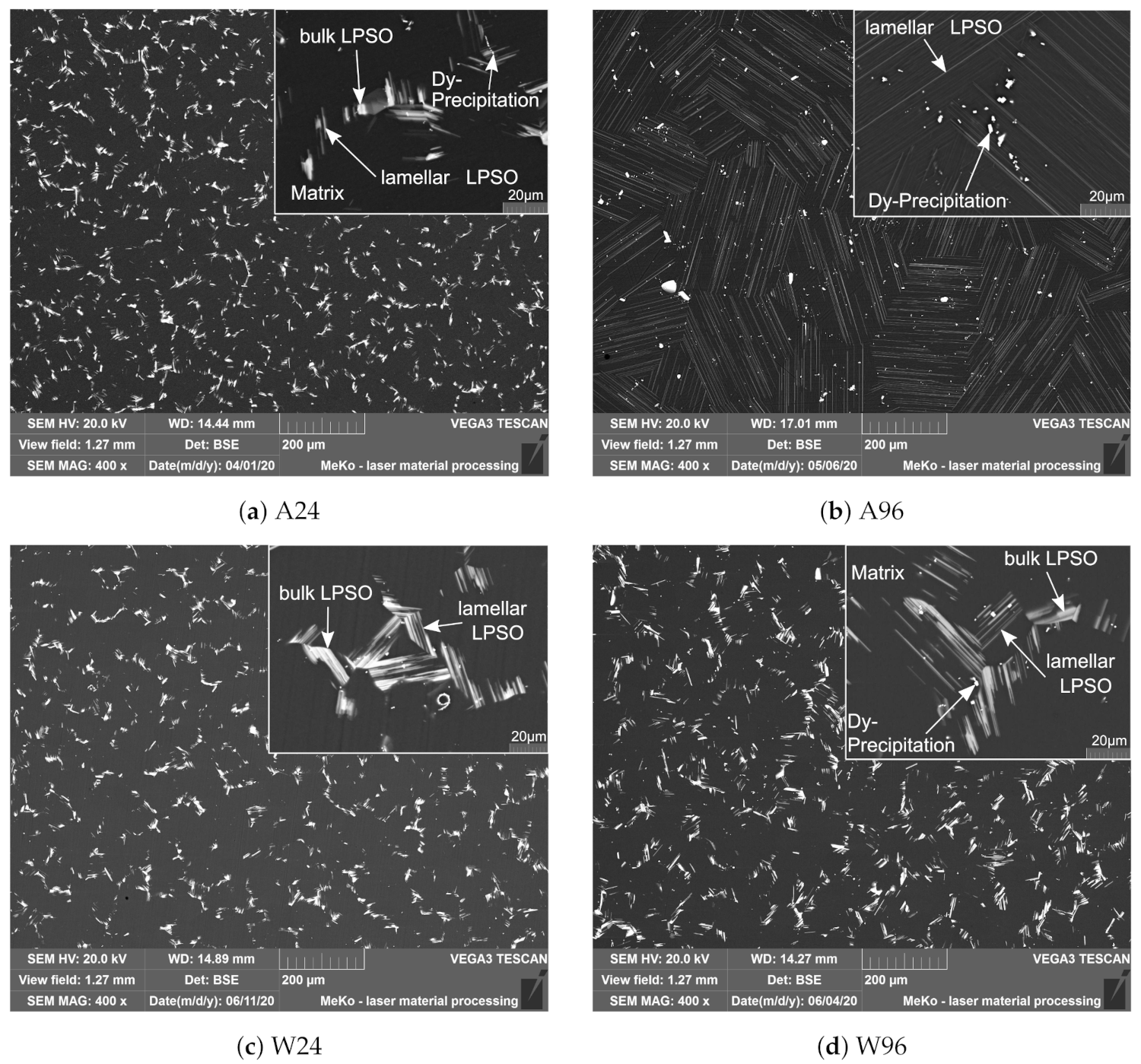
3.1. Microstructure
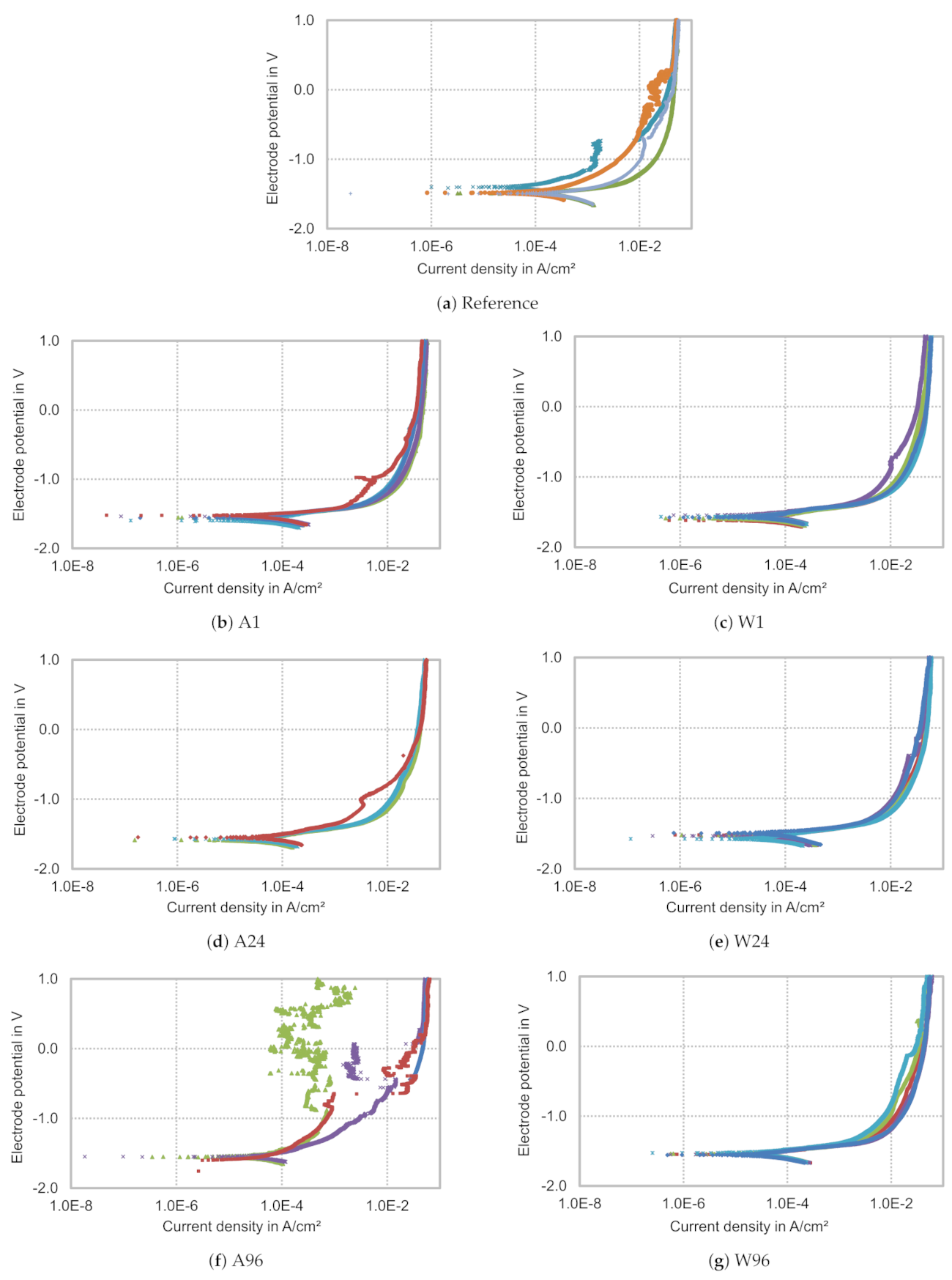
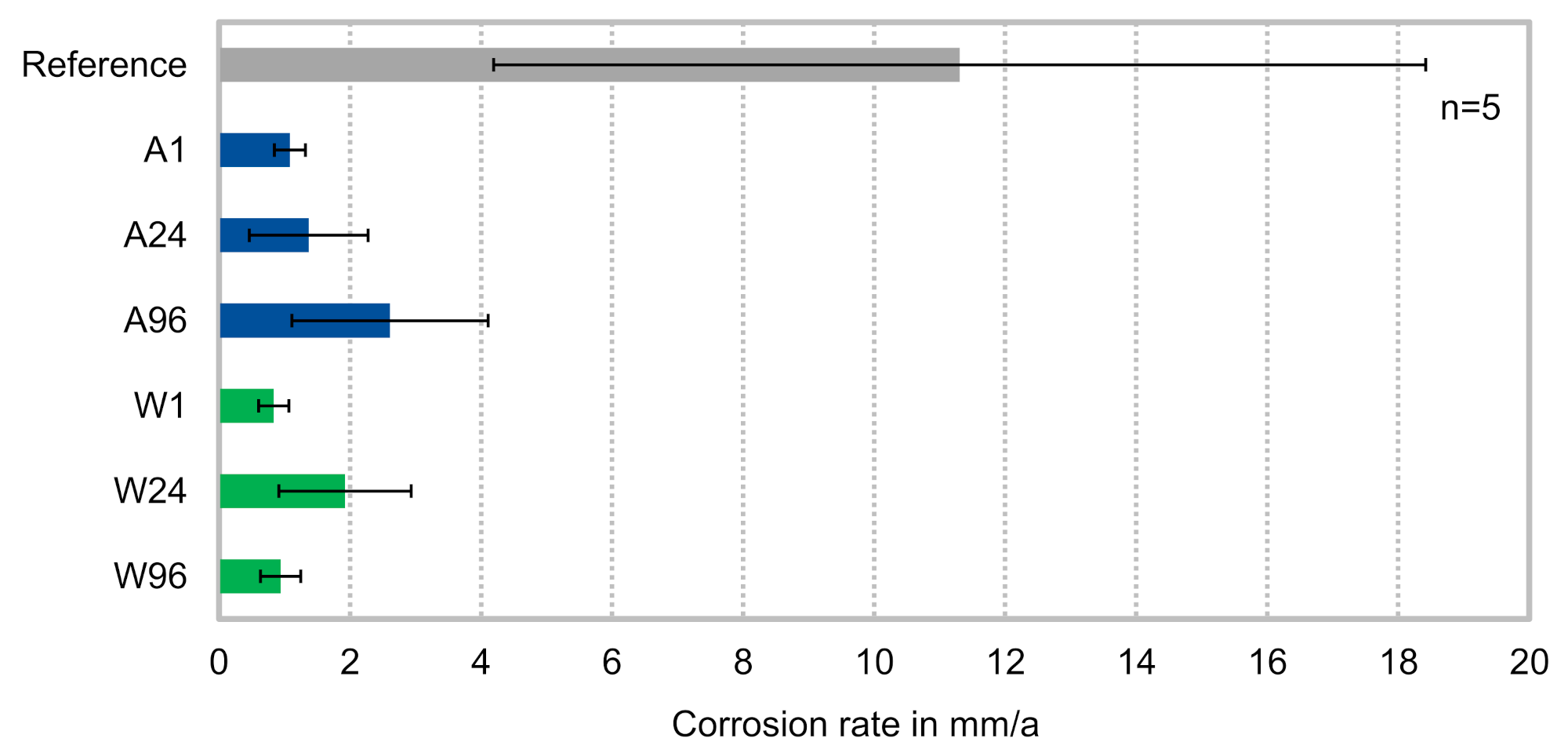
3.2. Corrosion
4. Conclusions
- (1)
- Cooling in air leads to larger grains than quenching in water and an increasing heat treatment duration leads to increasing grain sizes;
- (2)
- The lamellar LPSO phase forms during cooling by diffusion processes and re-segregation of solute atoms into the matrix;
- (3)
- Lamellar LPSO structures develop from bulk LPSO structures during cooling;
- (4)
- The corrosion rate of solution heat treated and air-cooled Resoloy® increases with increasing grain size, but is significantly lower than the corrosion rate of the as-cast condition;
- (5)
- Solution heat treatment at 500 °C shifts the corrosion potential towards slightly less nobler potentials and results in a more homogenous anodic part of the polarisation curve;
- (6)
- Corrosion starts at the matrix between the LPSO lamellae. These lamellae hinder further corrosion, if they are uniformly distributed over the matrix of the entire grain. The layered structure of the surface makes the more resistant cathodic structures visible.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stekker, M. Magnesium Alloy and Resorbable Stents Containing the Same. Patent No. EP2744531B1, 15 August 2012. [Google Scholar]
- Maier, P.; Steinacker, A.; Clausius, B.; Hort, N. Influence of Solution Heat Treatment on the Microstructure, Hardness and Stress Corrosion Behavior of Extruded Resoloy. JOM 2020, 72, 1870–1879. [Google Scholar] [CrossRef] [Green Version]
- Heiden, M.; Walker, E.; Stancui, L. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation—A Review. J. Biotechnol. Biomater. 2015, 5, 2–9. [Google Scholar]
- Peng, Q.; Guo, J.; Fu, H.; Cai, X.; Wang, Y.; Lui, B.; Xu, Z. Degradation behavior of Mg-based biomaterials containing different long-period stacking ordered phases. Sci. Rep. 2014, 4, 3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinacker, A.; Mendis, C.; Mohedano, M.; Feyerabend, F.; Stekker, M.; Maier, P.; Kainer, K.U.; Hort, N. Microstructure evolution and corrosion behaviour of the biodegradable EZK1110 alloy. Eur. Cells Mater. 2016, 32, 11. [Google Scholar]
- Xu, C.; Nakata, T.; Xiaoguang, Q.; Mingyi, Z.; Kun, W.; Kamado, S. Effect of LPSO and SFs on microstructure evolution and mechanical properties of Mg-Gd-Y-Zn-Zr alloy. Sci. Rep. 2017, 7, 40846. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, Y.; Feyerabend, F.; Willumeit, R.; Kainer, K.U.; Hort, N. Influence of ageing treatment on microstructure, mechanical and bio-corrosion properties of Mg–Dy alloys. J. Mech. Behav. Biomed. Mater. 2012, 13, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Smola, B.; Stulikova, I.; Cerna, J.; Cizek, J.; Vlach, M. Phase transformations in MgTbNd alloy. Phys. Status Solidi A 2011, 208, 2741–2748. [Google Scholar] [CrossRef]
- Elsayed, F.R.; Hort, N.; Ordorica, M.A.; Kainer, K.U. Magnesium Permanent Mold Castings Optimization. Mater. Sci. Forum 2011, 690, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Getting Started with Electrochemical Corrosion Measurement. Available online: https://www.gamry.com/application-notes/corrosion-coatings/basics-of-electrochemical-corrosion-measurements/ (accessed on 13 October 2020).
- Fumin, L.; Aibin, M.; Jinghua, J.; Donghuib, Y.; Qi, Z. Review on long-period stacking-ordered structures in Mg-Zn-RE alloys. Rare Met. 2012, 31, 303–310. [Google Scholar]
- Wu, Y.; Zeng, X.; Lin, D.; Peng, L.; Ding, W. The microstructure evolution with lamellar 14H-type LPSO structure in an Mg96.5Gd2.5Zn1 alloy during solid solution heat treatment at 773K. J. Alloys Compd. 2008, 477, 193–197. [Google Scholar] [CrossRef]
- Song, G.-L. Corrosion Prevention of Magnesium Alloys, 1st ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 509–517. [Google Scholar]
- Li, C.Q.; Xu, D.K.; Zeng, Z.R.; Wang, B.J.; Sheng, L.Y.; Chen, X.-B.; Han, E.H. Effect of volume fraction of LPSO phases on corrosion and mechanical properties of Mg–Zn–Y alloys. Mater. Des. 2017, 121, 430–441. [Google Scholar] [CrossRef]

| Heat Treatment Duration | |||
|---|---|---|---|
| 1 h | 24 h | 96 h | |
| Cooled in air | A1 | A24 | A96 |
| Quenched in water | W1 | W24 | W96 |
| Element | Matrix | Bulk LPSO | Lamellar LPSO | Segregation |
|---|---|---|---|---|
| Mg | 88.4 ± 0.3 | 65.7 ± 0.3 | 86.8 ± 1.2 | 24.7 ± 1.3 |
| Zn | 1.0 ± 0.1 | 7.1 ± 0.2 | 1.4 ± 0.4 | 1.7 ± 1.2 |
| Zr | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Nd | 0.8 ± 0.2 | 2.0 ± 0.1 | 0.9 ± 0.0 | 1.2 ± 0.3 |
| Dy | 9.7 ± 0.1 | 25.0 ± 0.3 | 10.8 ± 0.8 | 72.4 ± 0.1 |
| Element | Matrix | Bulk LPSO | Lamellar LPSO | Segregation |
|---|---|---|---|---|
| Mg | 88.6 ± 0.2 | 63.4 ± 0.5 | 72.2 ± 0.2 | 12.2 ± 0.2 |
| Zn | 0.8 ± 0.0 | 7.9 ± 0.1 | 5.3 ± 0.2 | 0.7 ± 0.3 |
| Zr | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Nd | 0.8 ± 0.1 | 2.2 ± 0.0 | 1.7 ± 0.1 | 1.1 ± 0.1 |
| Dy | 9.7 ± 0.3 | 26.5 ± 0.4 | 20.7 ± 0.2 | 85.9 ± 5.4 |
| Element | Matrix | Eutectics |
|---|---|---|
| Mg | 94.3 ± 0.5 | 56.1 ± 5.0 |
| Zn | 0.3 ± 0.0 | 7.5 ± 1.4 |
| Zr | 0.2 ± 0.1 | 0.0 ± 0.0 |
| Nd | 0.2 ± 0.1 | 5.8 ± 1.2 |
| Dy | 5.1 ± 0.6 | 30.6 ± 2.7 |
| Element | Lamellar LPSO | Aligned Structure |
|---|---|---|
| Mg | 86.8 ± 1.2 | 86.6 ± 1.0 |
| Zn | 1.4 ± 0.4 | 1.3 ± 0.4 |
| Zr | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Nd | 0.9 ± 0.0 | 0.7 ± 0.1 |
| Dy | 10.8 ± 0.8 | 11.2 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahlers, S.; Bittner, B.; Maier, P. Influence of Cooling Conditions on Long-Period Stacking-Ordered Phase Evolution and Corrosion Behavior of As-Cast Resoloy®. Metals 2021, 11, 1372. https://doi.org/10.3390/met11091372
Ahlers S, Bittner B, Maier P. Influence of Cooling Conditions on Long-Period Stacking-Ordered Phase Evolution and Corrosion Behavior of As-Cast Resoloy®. Metals. 2021; 11(9):1372. https://doi.org/10.3390/met11091372
Chicago/Turabian StyleAhlers, Sandra, Benjamin Bittner, and Petra Maier. 2021. "Influence of Cooling Conditions on Long-Period Stacking-Ordered Phase Evolution and Corrosion Behavior of As-Cast Resoloy®" Metals 11, no. 9: 1372. https://doi.org/10.3390/met11091372






