Preparation of Ag0 Nanoparticles by EDM Method as Catalysts for Oxygen Reduction
Abstract
:1. Introduction
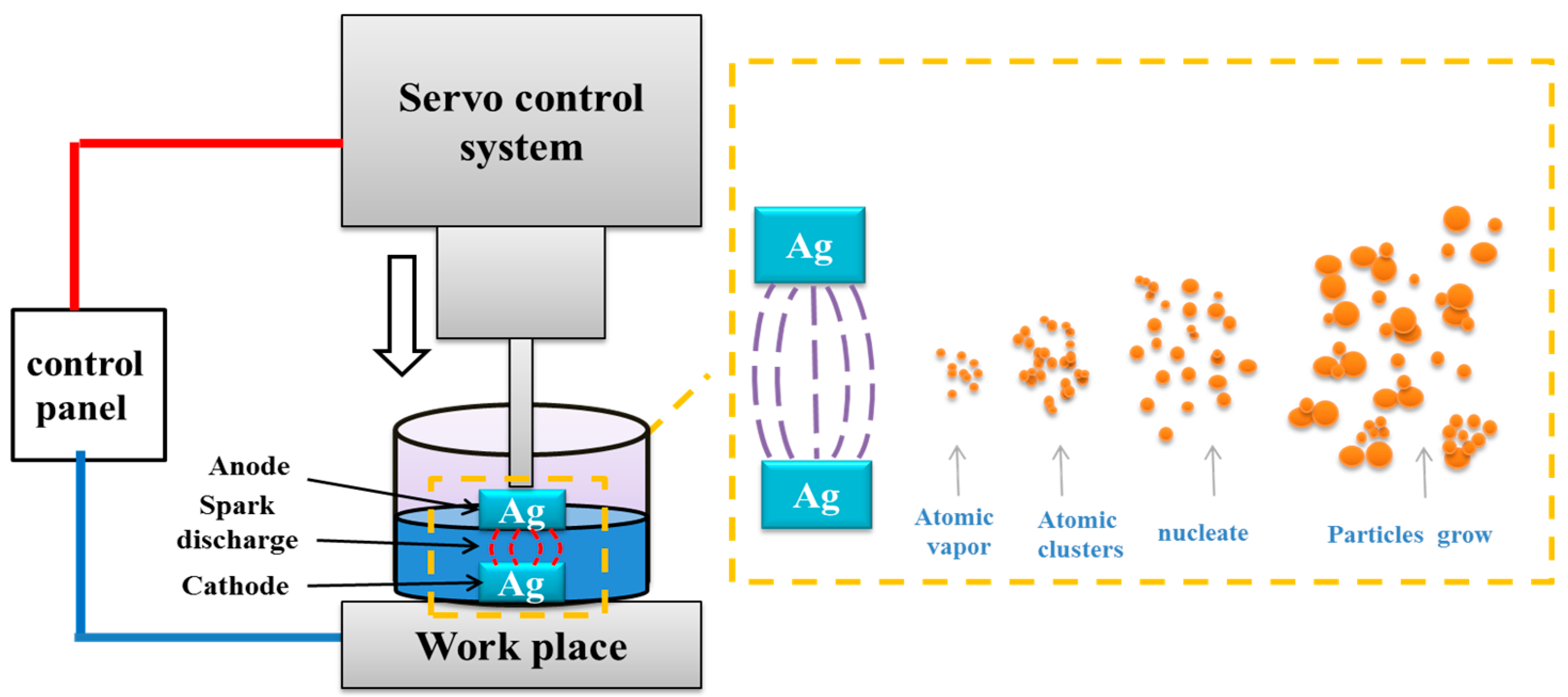
2. Materials and Methods
3. Results and Discussion
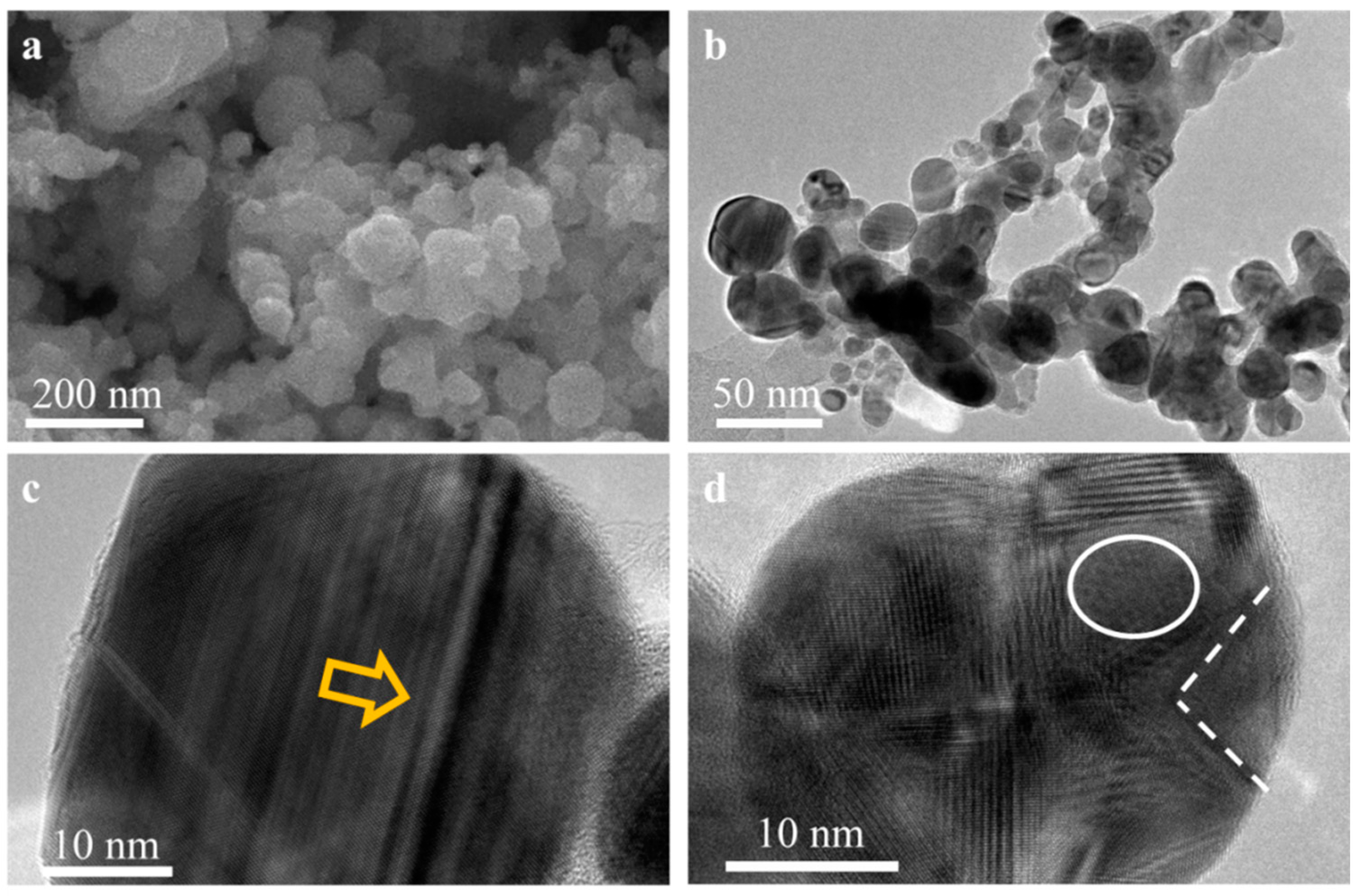
3.1. Synthesis and Characterization
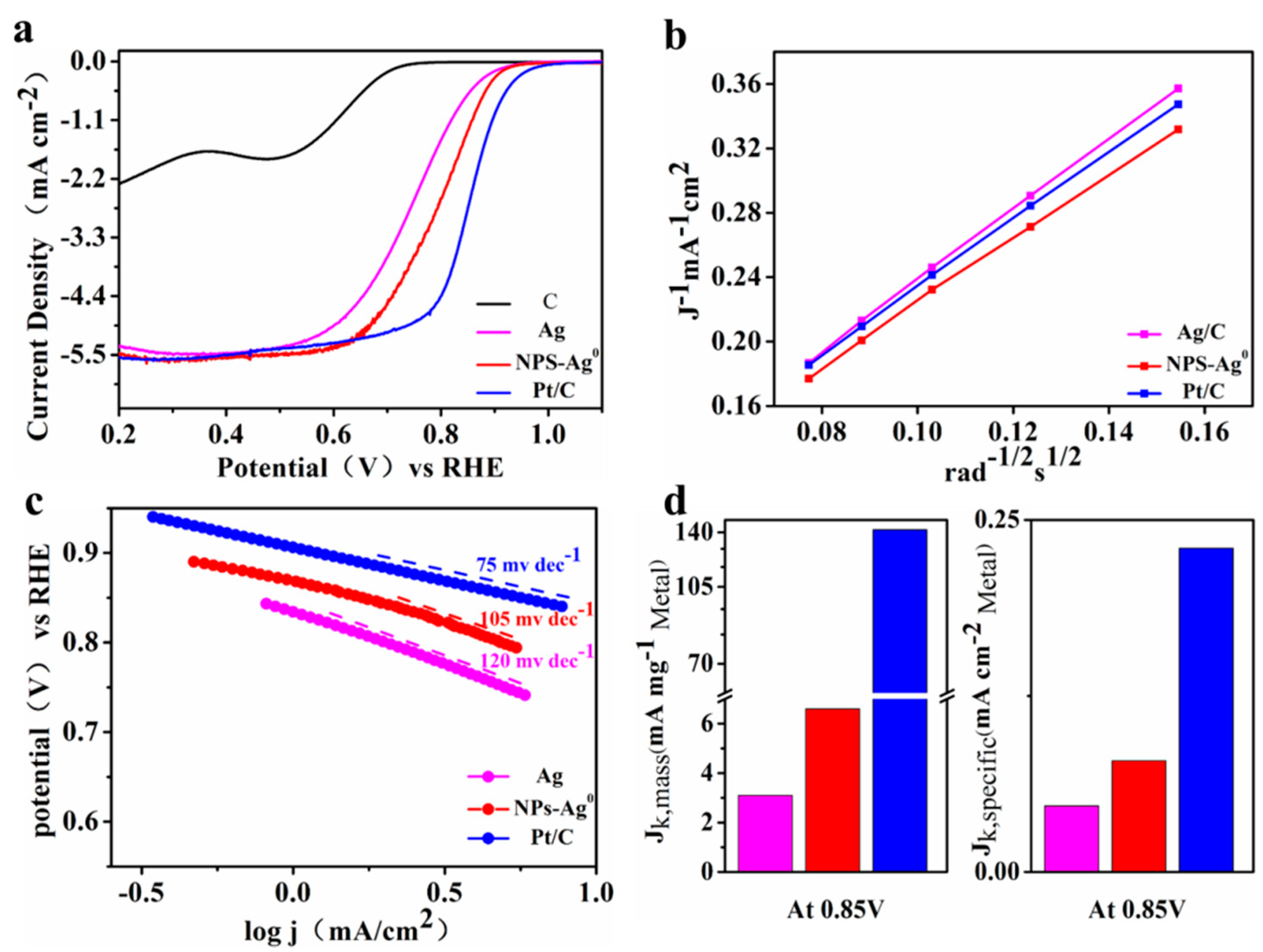
3.2. Electrochemical Measurements
3.3. The d-Band Center of NPS-Ag0 and Ag
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.A.; Wang, X.; Wen, C. A review of high energy density lithium-air battery technology. J. Appl. Electrochem. 2014, 44, 5–22. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Risch, M.; Han, B.; Shao-Horn, Y. Recent Insights into Manganese Oxides in Catalyzing Oxygen Reduction Kinetics. Acs Catal. 2015, 5, 6021–6031. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Li, L.; Lu, Z.; Yu, X.; Zhang, X.; Yang, X. OMS-2 nanorods filled with Co-ion in the tunnels as efficient electron conduits and regulatory substance for oxygen reduction. Appl. Catal. B-Environ. 2020, 279, 119373. [Google Scholar] [CrossRef]
- Luo, J.; Tian, X.; Zeng, J.; Li, Y.; Song, H.; Liao, S. Limitations and Improvement Strategies for Early-Transition-Metal Nitrides as Competitive Catalysts toward the Oxygen Reduction Reaction. ACS Catal. 2016, 6, 6165–6174. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Adimi, S.; Shen, H.; Thomas, T.; Ma, R.; Attfield, J.P.; Yang, M. Zirconium nitride catalysts surpass platinum for oxygen reduction. Nat. Mater. 2020, 19, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-W.; Song, B.-Y. Integrated Three-Dimensional Carbon Nanopolyhedron/Metal Sulfides: An Efficient Electrocatalyst Toward Oxygen Reduction Reaction. Front. Energy Res. 2021, 9, 189. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, L. High-Throughput Approach Exploitation: Two-Dimensional Double-Metal Sulfide (M2S2) of Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Energy Fuels 2020, 34, 5006–5015. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, K.; Zhang, Q.; Wu, H.; Gu, L.; Yao, K.; Shen, Y.; Shao, Y. In-situ synthesis, operation and regeneration of nanoporous silver with high performance toward oxygen reduction reaction. Nano Energy 2019, 58, 69–77. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, S.-Y. The plasma-induced formation of silver nanocrystals in aqueous solution and their catalytic activity for oxygen reduction. Nanotechnology 2018, 29, 085602. [Google Scholar] [CrossRef]
- Dong, J.; Sun, T.; Li, S.; Shan, N.; Chen, J.; Yan, Y.; Xu, L. 3D ordered macro-/mesoporous carbon supported Ag nanoparticles for efficient electrocatalytic oxygen reduction reaction. J. Colloid Interface Sci. 2019, 554, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.J.; Raji, A.-R.O.; Lambert, T.N.; Vigil, J.A.; Li, L.; Nan, K.; Tour, J.M. Silver-Graphene Nanoribbon Composite Catalyst for the Oxygen Reduction Reaction in Alkaline Electrolyte. Electroanalysis 2014, 26, 164–170. [Google Scholar] [CrossRef]
- Xie, X.; Wei, M.; Du, L.; Nie, Y.; Qi, X.; Shao, Y.; Wei, Z. Enhancement in kinetics of the oxygen reduction on a silver catalyst by introduction of interlaces and defect-rich facets. J. Mater. Chem. A 2017, 5, 15390–15394. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, Y.-I.; Kim, Y.H.; Park, M.; Jang, K.Y.; Song, H.; Nam, K.M. Preparation and phase transition of FeOOH nanorods: Strain effects on catalytic water oxidation. Nanoscale 2017, 9, 4751–4758. [Google Scholar] [CrossRef]
- Xia, Z.; Guo, S. Strain engineering of metal-based nanomaterials for energy electrocatalysis. Chem. Soc. Rev. 2019, 48, 3265–3278. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Kuehl, S.; Strasser, P.; Roldan Cuenya, B. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Ling, T.; Yan, D.-Y.; Jiao, Y.; Wang, H.; Zheng, Y.; Zheng, X.; Mao, J.; Du, X.-W.; Hu, Z.; Jaroniec, M.; et al. Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chattot, R.; Le Bacq, O.; Beermann, V.; Kuehl, S.; Herranz, J.; Henning, S.; Kuhn, L.; Asset, T.; Guetaz, L.; Renou, G.; et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis. Nat. Mater. 2018, 17, 827–833. [Google Scholar] [CrossRef]
- Cheng, W.; Zhao, X.; Su, H.; Tang, F.; Che, W.; Zhang, H.; Liu, Q. Lattice-strained metal-organic-framework arrays for bifunctional oxygen electrocatalysis. Nat. Energy 2019, 4, 115–122. [Google Scholar] [CrossRef]
- Huang, H.; Jia, H.; Liu, Z.; Gao, P.; Zhao, J.; Luo, Z.; Yang, J.; Zeng, J. Understanding of Strain Effects in the Electrochemical Reduction of CO2: Using Pd Nanostructures as an Ideal Platform. Angew. Chem.-Int. Ed. 2017, 56, 3594–3598. [Google Scholar] [CrossRef]
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T. Vacancy-Induced Ferromagnetism of MoS_2 Nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef]
- Jiao, S.L.; Fu, X.W.; Zhang, L.; Zeng, Y.J.; Huang, H.W. Point-defect-optimized electron distribution for enhanced electrocatalysis: Towards the perfection of the imperfections—ScienceDirect. Nano Today 2020, 31, 100833. [Google Scholar] [CrossRef]
- Ye, C.; Liu, J.; Zhang, Q.; Jin, X.; Zhao, Y.; Pan, Z.; Chen, G.; Qiu, Y.; Ye, D.; Gu, L.; et al. Activating Metal Oxides Nanocatalysts for Electrocatalytic Water Oxidation by Quenching-Induced Near-Surface Metal Atom Functionality. J. Am. Chem. Soc. 2021, 143, 169–177. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.-Y.; Feng, Y.; Dong, C.-K.; Liu, H.; Du, X.-W. A silver catalyst activated by stacking faults for the hydrogen evolution reaction. Nat. Catal. 2019, 2, 1107–1114. [Google Scholar] [CrossRef]
- Kuo-Hsiung, T.; Lin, Y.H.; Der-Chi, T.; Wu, T.C.; Leszek, S. Preparation of Ag Nanoparticles in Ammonia by Using EDM and a Study of the Relationships Between Ammonia and Silver Nanoparticles. J. Clust. Sci. 2018, 29, 1115–1122. [Google Scholar]
- Kiritani, M. Story of stacking fault tetrahedra. Mater. Chem. Phys. 1997, 50, 133–138. [Google Scholar] [CrossRef]
- Markovi, N.M.; Schmidt, T.J.; Stamenkovi, V.; Ross, P.N. Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Ma, L.; Wang, C.; Xia, B.Y.; Mao, K.; He, J.; Wu, X.; Xiong, Y.; Lou, X. Platinum Multicubes Prepared by Ni2+-Mediated Shape Evolution Exhibit High Electrocatalytic Activity for Oxygen Reduction. Angew. Chem. Int. Ed. 2015, 54, 5666–5671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qiao, J.; Lin, Y.; Zhang, J. A Review of Graphene-Based Nanostructural Materials for Both Catalyst Supports and Metal-Free Catalysts in PEM Fuel Cell Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1–25. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Mu, X.; Song, S.; Ren, Y.; Wang, K.; Lu, Z. Preparation of Ag0 Nanoparticles by EDM Method as Catalysts for Oxygen Reduction. Metals 2021, 11, 1491. https://doi.org/10.3390/met11091491
Guo J, Mu X, Song S, Ren Y, Wang K, Lu Z. Preparation of Ag0 Nanoparticles by EDM Method as Catalysts for Oxygen Reduction. Metals. 2021; 11(9):1491. https://doi.org/10.3390/met11091491
Chicago/Turabian StyleGuo, Jia, Xiaoming Mu, Shihao Song, Yanwei Ren, Kai Wang, and Zunming Lu. 2021. "Preparation of Ag0 Nanoparticles by EDM Method as Catalysts for Oxygen Reduction" Metals 11, no. 9: 1491. https://doi.org/10.3390/met11091491




