Mixed Oxides NiO/ZnO/Al2O3 Synthesized in a Single Step via Ultrasonic Spray Pyrolysis (USP) Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
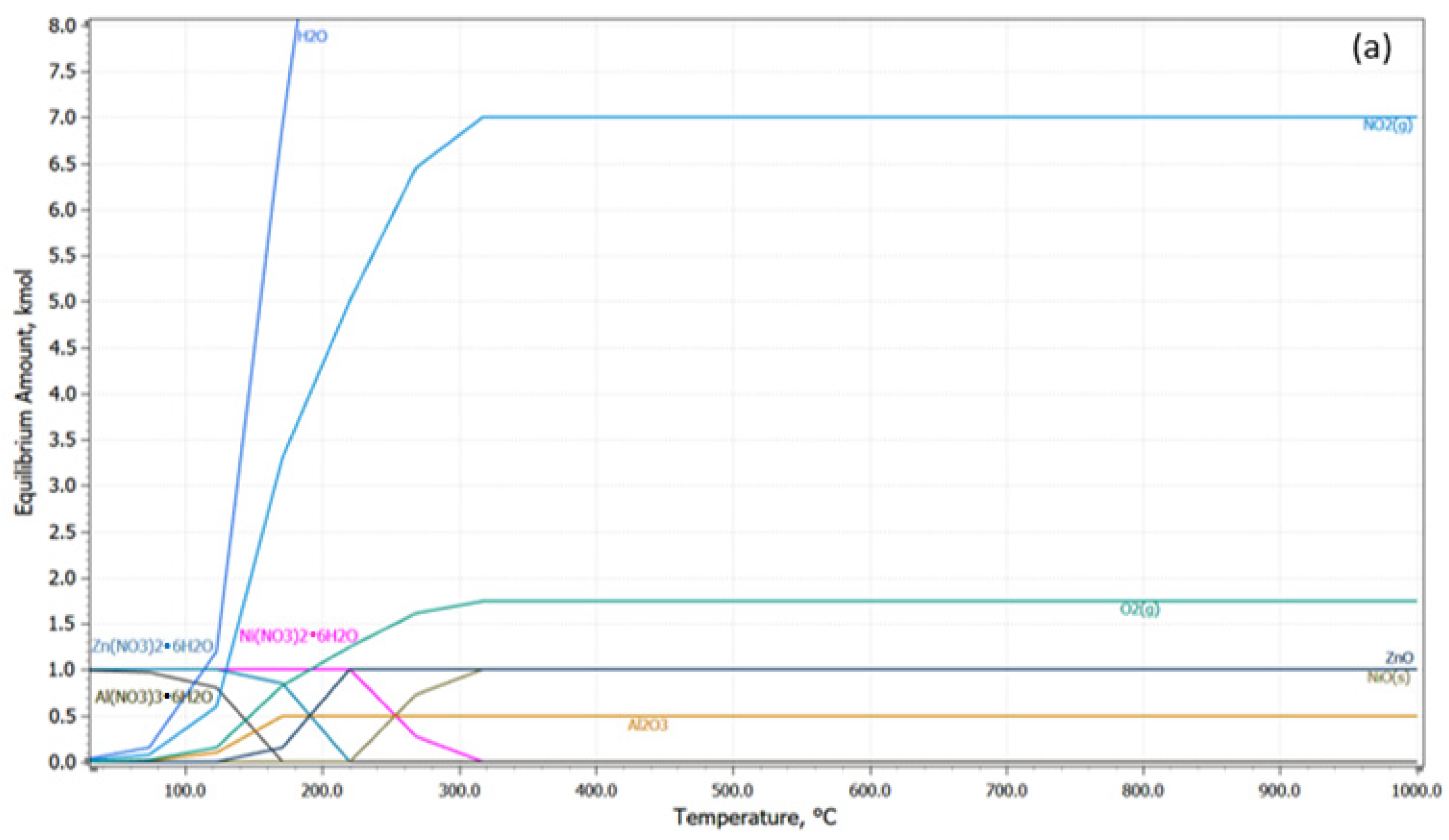
3.1. Thermodynamic Analysis
3.2. XRD Analysis
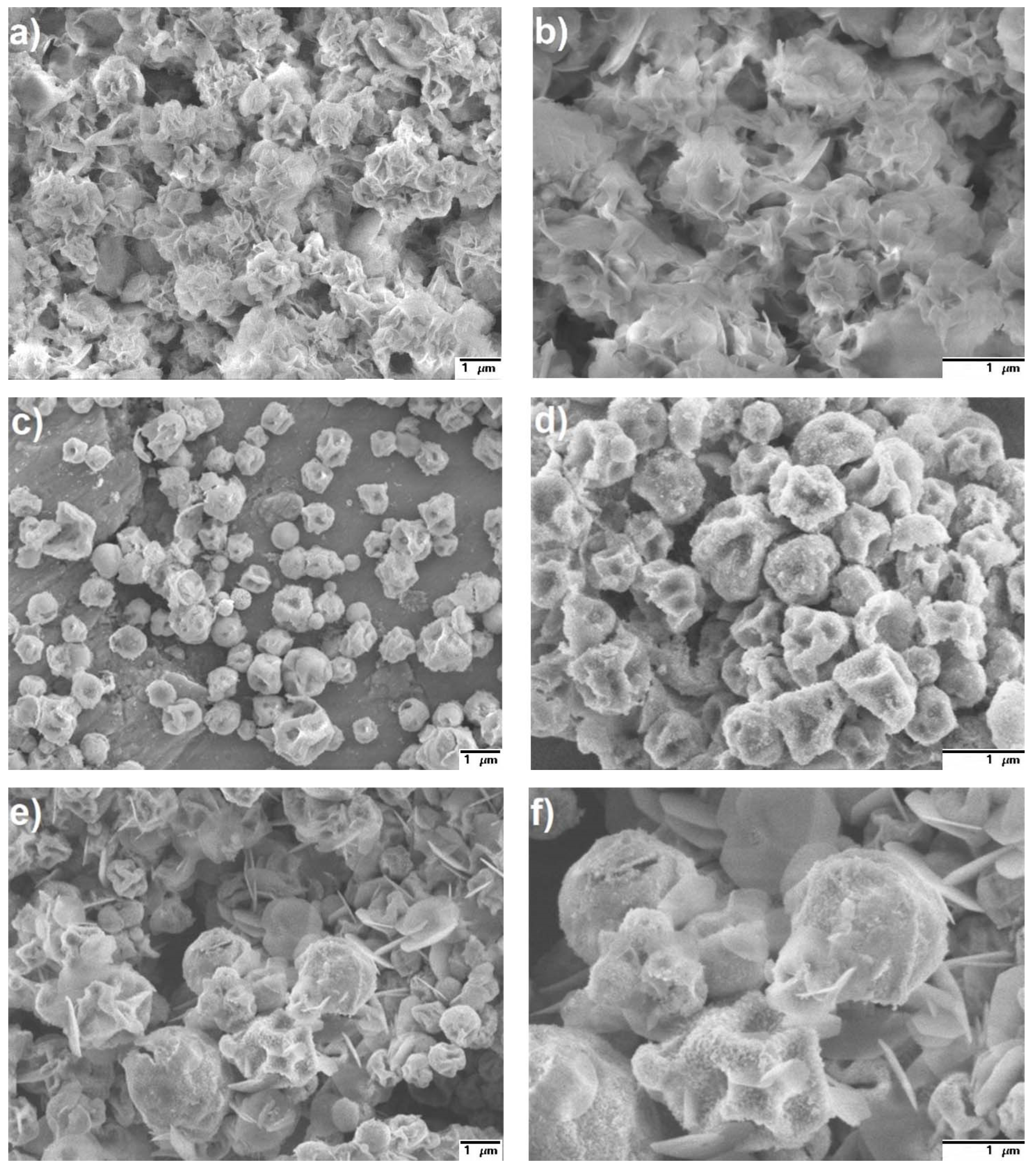
3.3. SEM Analysis
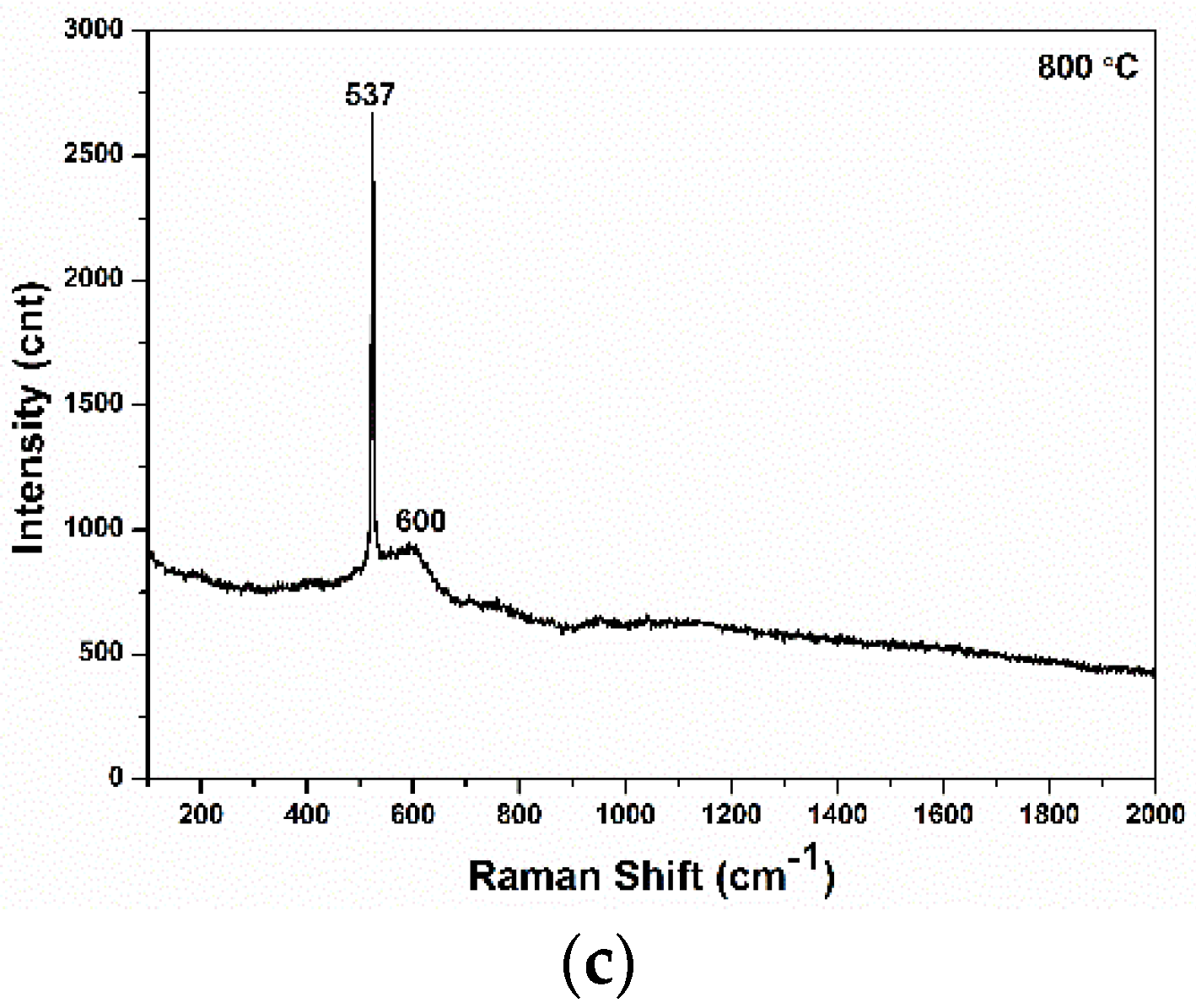
3.4. Raman Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix Nanocomposites, Processing, Manufacturing, and Application: An Overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Ravichandran, K.; Praseetha, P.K.; Arun, T.; Gobalakrishnan, S. Chapter 6—Synthesis of Nanocomposites, Synthesis of Inorganic Nanomaterials Advances and Key Technologies. In Micro and Nano Technologies; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Swaston, UK, 2018; pp. 141–168. [Google Scholar] [CrossRef]
- Juma, A.O.; Matibini, A. Synthesis and structural analysis of ZnO-NiO mixed oxide nanocomposite prepared by homogeneous precipitation. Ceram. Int. 2017, 43, 15424–15430. [Google Scholar] [CrossRef]
- Juma, A.O.; Arbab, E.A.; Muiva, C.; Lepodise, L.M.; Mola, G.T. Synthesis and characterization of CuO-NiO-ZnO mixed metal oxide nanocomposite. J. Alloy. Compd. 2017, 723, 866–872. [Google Scholar] [CrossRef]
- Loos, M. Nanoscience and Nanotechnology. In Carbon Nanotube Reinforced Composites; CNR Polymer Science and Technology; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 1–36. [Google Scholar]
- El-Nabarawy, T.; Attia, A.; Alaya, M. Effect of thermal treatment on the structural, textural and catalytic properties of the ZnO-Al2O3 system. Mater. Lett. 1995, 24, 319–325. [Google Scholar] [CrossRef]
- Sajid, S.; Elseman, A.M.; Huang, H.; Ji, J.; Dou, S.; Jiang, H.; Liu, X.; Wei, D.; Cui, P.; Li, M. Breakthroughs in NiOx-HTMs towards stable, low-cost and efficient perovskite solar cells. Nano Energy 2018, 51, 408–424. [Google Scholar] [CrossRef]
- Goel, R.; Jha, R.; Bhushan, M.; Bhardwaj, R.; Ravikant, C. Hydrothermally synthesized nickel oxide (NiO) nano petals. In Materials Today: Proceedings; Elsevier BV: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Sharma, R.K.; Kumar, D.; Ghose, R. Synthesis of nanocrystalline ZnO–NiO mixed metal oxide powder by homogeneous precipitation method. Ceram. Int. 2016, 42, 4090–4098. [Google Scholar] [CrossRef]
- Khudiar, S.S.; Mutlak, F.A.-H.; Nayef, U.M. Synthesis of ZnO nanostructures by hydrothermal method deposited on porous silicon for photo-conversion application. Optik 2021, 247, 167903. [Google Scholar] [CrossRef]
- Tari, O.; Aronne, A.; Addonizio, M.L.; Daliento, S.; Fanelli, E.; Pernice, P. Sol–gel synthesis of ZnO transparent and conductive films: A critical approach. Sol. Energy Mater. Sol. Cells 2012, 105, 179–186. [Google Scholar] [CrossRef]
- Tawale, J.; Dey, K.; Pasricha, R.; Sood, K.; Srivastava, A. Synthesis and characterization of ZnO tetrapods for optical and antibacterial applications. Thin Solid Films 2010, 519, 1244–1247. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Khodair, Z.T.; Khadom, A.A. Preparation and investigation of the structural properties of α-Al2O3 nanoparticles using the sol-gel method. Chem. Data Collect. 2020, 29, 100531. [Google Scholar] [CrossRef]
- Yadav, S.K.; Jeevanandam, P. Synthesis of NiO–Al2O3 nanocomposites by sol–gel process and their use as catalyst for the oxidation of styrene. J. Alloy. Compd. 2014, 610, 567–574. [Google Scholar] [CrossRef]
- Li, X.; Mu, Z.; Hu, J.; Cui, Z. Gas sensing characteristics of composite NiO/Al2O3 for 2-chloroethanol at low temperature. Sens. Actuators B Chem. 2016, 232, 143–149. [Google Scholar] [CrossRef]
- Saedy, S.; Haghighi, M.; Amirkhosrow, M. Hydrothermal synthesis and physicochemical characterization of CuO/ZnO/Al2O3 nanopowder. Part I: Effect of crystallization time. Particuology 2012, 10, 729–736. [Google Scholar] [CrossRef]
- Zygmuntowicz, J.; Wiecińska, P.; Miazga, A.; Konopka, K. Characterization of composites containing NiAl2O4 spinel phase from Al2O3/NiO and Al2O3/Ni systems. J. Therm. Anal. Calorim. 2016, 125, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Macedo, H.P.D.; Medeiros, R.L.B.D.A.; Medeiros, A.L.D.; Oliveira, Â.A.S.D.; Figueredo, G.P.D.; Melo, M.A.D.F.; Melo, D.M.D.A. Characterization of ZnAl2O4 Spinel Obtained by Hydrothermal and Microwave Assisted Combustion Method: A Comparative Study. Mater. Res. 2017, 20, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yan, J.; Zhou, M.; Yang, Y.; Liu, Y.-N. Fabrication and photocatalytic properties of spheres-in-spheres ZnO/ZnAl2O4 composite hollow microspheres. Appl. Surf. Sci. 2013, 268, 237–245. [Google Scholar] [CrossRef]
- Crisan, M.; Zaharescu, M.; Kumari, V.D.; Subrahmanyam, M.; Crişan, D.; DrĂgan, N.; Răileanu, M.; Jitianu, M.; Rusu, A.; Sadanandam, G.; et al. Sol–gel based alumina powders with catalytic applications. Appl. Surf. Sci. 2011, 258, 448–455. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, F.; Li, G.; Li, X. Effect of Zn/Al ratio of Ni/ZnO-Al2O3 catalysts on the catalytic deoxygenation of oleic acid into alkane. Appl. Catal. A Gen. 2017, 529, 175–184. [Google Scholar] [CrossRef]
- Lin, F.; Nordlund, D.; Weng, T.-C.; Moore, R.G.; Gillaspie, D.T.; Dillon, A.C.; Richards, R.M.; Engtrakul, C. Hole Doping in Al-Containing Nickel Oxide Materials to Improve Electrochromic Performance. ACS Appl. Mater. Interfaces 2013, 5, 301–309. [Google Scholar] [CrossRef]
- Sonawane, G.H.; Patil, S.P.; Sonawane, S.H. Chapter 1-Nanocomposites and Its Applications, Applications of Nanomaterials. In Micro and Nano Technologies; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 1–22. [Google Scholar]
- Messing, G.L.; Zhang, S.-C.; Jayanthi, G.V. Ceramic Powder Synthesis by Spray Pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Hsieh, H.-C.; Yu, J.; Rwei, S.-P.; Lin, K.-F.; Shih, Y.-C.; Wang, L. Ultra-compact titanium oxide prepared by ultrasonic spray pyrolysis method for planar heterojunction perovskite hybrid solar cells. Thin Solid Films 2018, 659, 41–47. [Google Scholar] [CrossRef]
- Ouhaibi, A.; Ghamnia, M.; Dahamni, M.; Heresanu, V.; Fauquet, C.; Tonneau, D. The effect of strontium doping on structural and morphological properties of ZnO nanofilms synthesized by ultrasonic spray pyrolysis method. J. Sci. Adv. Mater. Devices 2018, 3, 29–36. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Ma, S.; Yan, S. Hierarchical heterostructure of porous NiO nanosheets on flower-like ZnO assembled by hexagonal nanorods for high-performance gas sensor. Ceram. Int. 2017, 43, 7508–7515. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Ferroni, M.; Poli, N.; Campanini, M.; Negrea, R.; Comini, E. Branch-like NiO/ZnO heterostructures for VOC sensing. Sens. Actuators B Chem. 2018, 262, 477–485. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W.; Yang, J.; Li, Y. One-step hydrothermal fabrication of nanosheet-assembled NiO/ZnO microflower and its ethanol sensing property. Ceram. Int. 2018, 44, 19825–19830. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Lu, T.; Yao, Y.; Chua, D.H.; Pan, L. Metal-organic frameworks derived yolk-shell ZnO/NiO microspheres as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 2018, 335, 579–589. [Google Scholar] [CrossRef]
- Kim, K.H.; Yoshihara, Y.; Abe, Y.; Kawamura, M.; Kiba, T. Morphological characterization of sphere-like structured ZnO-NiO nanocomposites with annealing temperatures. Mater. Lett. 2016, 186, 364–367. [Google Scholar] [CrossRef]
- Mahajan, A.; Deshpande, P.; Butee, S. Synthesis and characterization of NiO/ZnO composite prepared by solid-state reaction method. In Materials Today: Proceedings; Elsevier BV: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Xu, D.; Jiang, C.; Cheng, B. Fabrication of hierarchical porous ZnO-Al2O3 microspheres with enhanced adsorption performance. Appl. Surf. Sci. 2017, 426, 360–368. [Google Scholar] [CrossRef]
- Ullah, R.; Bai, P.; Wu, P.; Etim, U.; Zhang, Z.; Han, D.; Subhan, F.; Ullah, S.; Rood, M.; Yan, Z. Superior performance of freeze-dried Ni/ZnO-Al2O3 adsorbent in the ultra-deep desulfurization of high sulfur model gasoline. Fuel Process. Technol. 2017, 156, 505–514. [Google Scholar] [CrossRef]
- Li, B.; Yuan, H.; Yang, P.; Yi, B.; Zhang, Y. Fabrication of the composite nanofibers of NiO/γ-Al2O3 for potential application in photocatalysis. Ceram. Int. 2016, 42, 17405–17409. [Google Scholar] [CrossRef]
- Battiston, S.; Rigo, C.; Severo, E.D.C.; Mazutti, M.A.; Kuhn, R.C.; Gundel, A.; Foletto, E.L. Synthesis of zinc aluminate (ZnAl2O4) spinel and its application as photocatalyst. Mater. Res. 2014, 17, 734–738. [Google Scholar] [CrossRef]
- Du, X.; Li, L.; Zhang, W.; Chen, W.; Cui, Y. Morphology and structure features of ZnAl2O4 spinel nanoparticles prepared by matrix-isolation-assisted calcination. Mater. Res. Bull. 2015, 61, 64–69. [Google Scholar] [CrossRef]
- Ragupathi, C.; Vijaya, J.J.; Kennedy, L.J. Preparation, characterization and catalytic properties of nickel aluminate nanoparticles: A comparison between conventional and microwave method. J. Saudi Chem. Soc. 2017, 21, S231–S239. [Google Scholar] [CrossRef] [Green Version]
- Benrabaa, R.; Barama, A.; Boukhlouf, H.; Guerrero-Caballero, J.; Rubbens, A.; Bordes-Richard, E.; Löfberg, A.; Vannier, R.-N. Physico-chemical properties and syngas production via dry reforming of methane over NiAl2O4 catalyst. Int. J. Hydrog. Energy 2017, 42, 12989–12996. [Google Scholar] [CrossRef]
- García-Gómez, N.; Valecillos, J.; Remiro, A.; Valle, B.; Bilbao, J.; Gayubo, A.G. Effect of reaction conditions on the deactivation by coke of a NiAl2O4 spinel derived catalyst in the steam reforming of bio-oil. Appl. Catal. B Environ. 2021, 297, 120445. [Google Scholar] [CrossRef]
- Rajkumar, T.; Sápi, A.; Ábel, M.; Farkas, F.; Gómez-Pérez, J.F.; Kukovecz, Á.; Konya, Z. Ni–Zn–Al-Based Oxide/Spinel Nanostructures for High Performance, Methane-Selective CO2 Hydrogenation Reactions. Catal. Lett. 2019, 150, 1527–1536. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Mohaček-Grošev, V.; Vrankić, M.; Maksimović, A.; Mandić, V. Influence of titanium doping on the Raman spectra of nanocrystalline ZnAl2O4. J. Alloy. Compd. 2017, 697, 90–95. [Google Scholar] [CrossRef]
- Gurmen, S.; Ebin, B.; Stopić, S.; Friedrich, B. Nanocrystalline spherical iron–nickel (Fe–Ni) alloy particles prepared by ultrasonic spray pyrolysis and hydrogen reduction (USP-HR). J. Alloy. Compd. 2009, 480, 529–533. [Google Scholar] [CrossRef]
- Ebin, B.; Gençer, Ö.; Gürmen, S. Simple preperation of CuO nanoparticles and submicron spheres via ultrasonic spray pyrolysis (USP). Int. J. Mater. Res. 2013, 104, 199–206. [Google Scholar] [CrossRef]





| Process Temperature (°C) | Ni(NO3)2 6H2O (M) | Zn(NO3)2 6H2O (M) | Al(NO3)3 9H2O (M) | Ultrasonic Frequency (MHz) | Air Flow Rate (L min−1) |
|---|---|---|---|---|---|
| 400 | 0.2 | 0.2 | 0.2 | 1.3 | 1 |
| 600 | 0.2 | 0.2 | 0.2 | 1.3 | 1 |
| 800 | 0.2 | 0.2 | 0.2 | 1.3 | 1 |
| Molarity (M)/Temperature (°C) | Element (Atomic %) | |||
|---|---|---|---|---|
| O | Al | Ni | Zn | |
| 0.2 M/400 °C | 63.5 | 7.8 | 18.0 | 10.7 |
| 0.2 M/600 °C | 59.2 | 10.2 | 18.3 | 12.3 |
| 0.2 M/800 °C | 58.7 | 9.1 | 17.1 | 15.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeşiltepe Özcelik, D.; Ebin, B.; Stopic, S.; Gürmen, S.; Friedrich, B. Mixed Oxides NiO/ZnO/Al2O3 Synthesized in a Single Step via Ultrasonic Spray Pyrolysis (USP) Method. Metals 2022, 12, 73. https://doi.org/10.3390/met12010073
Yeşiltepe Özcelik D, Ebin B, Stopic S, Gürmen S, Friedrich B. Mixed Oxides NiO/ZnO/Al2O3 Synthesized in a Single Step via Ultrasonic Spray Pyrolysis (USP) Method. Metals. 2022; 12(1):73. https://doi.org/10.3390/met12010073
Chicago/Turabian StyleYeşiltepe Özcelik, Duygu, Burçak Ebin, Srecko Stopic, Sebahattin Gürmen, and Bernd Friedrich. 2022. "Mixed Oxides NiO/ZnO/Al2O3 Synthesized in a Single Step via Ultrasonic Spray Pyrolysis (USP) Method" Metals 12, no. 1: 73. https://doi.org/10.3390/met12010073









