The Effect of Ultraviolet Treatment on TiO2 Nanotubes: A Study of Surface Characteristics, Bacterial Adhesion, and Gingival Fibroblast Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Field Emission Scanning Electron Microscopy (FESEM)
2.3. Surface Roughness Measurements
2.4. Contact Angle Measurements
2.5. Surface Free Energy Calculations
2.6. Bacterial Response
2.6.1. Cultivation of Streptococcus mutans and Preparation of Cell Suspensions
2.6.2. Adhesion Tests
2.6.3. Biofilm Formation Test
2.7. Cell Proliferation
2.8. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy
3.2. Surface Roughness Parameters
3.3. Contact Angle Measurements
3.4. Surface Free Energy Calculations
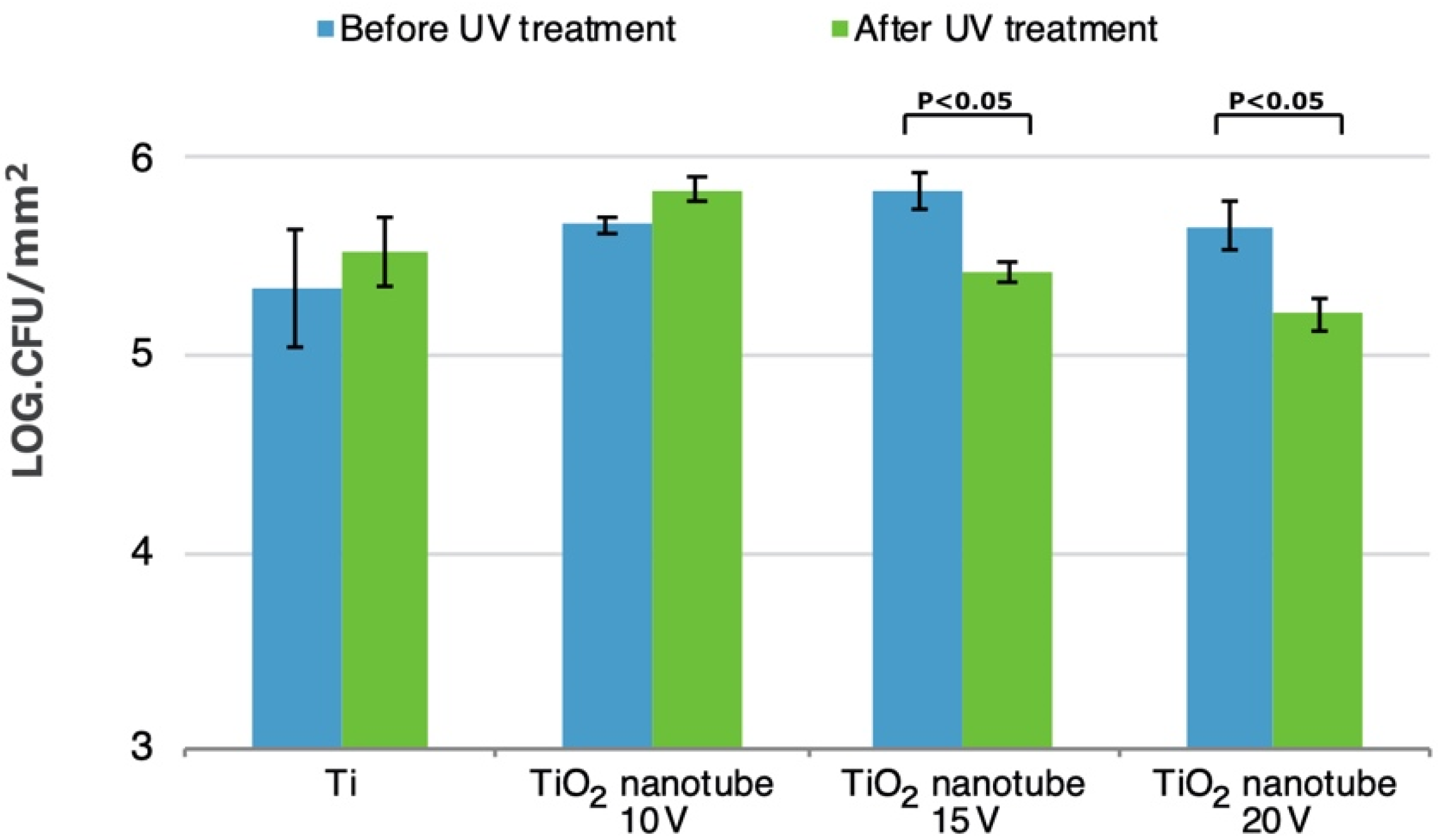
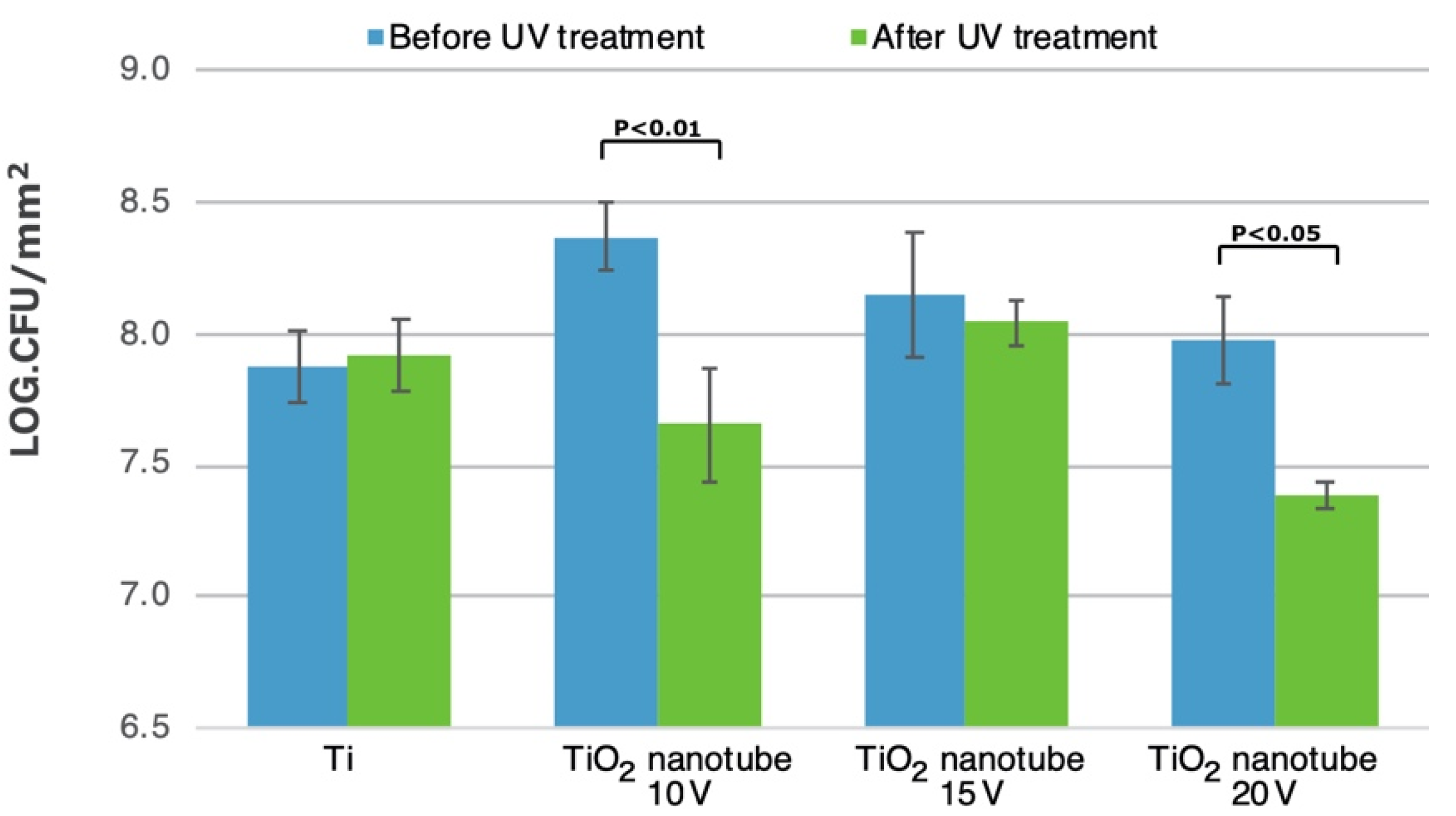
3.5. Bacterial Adhesion and Biofilm Formation
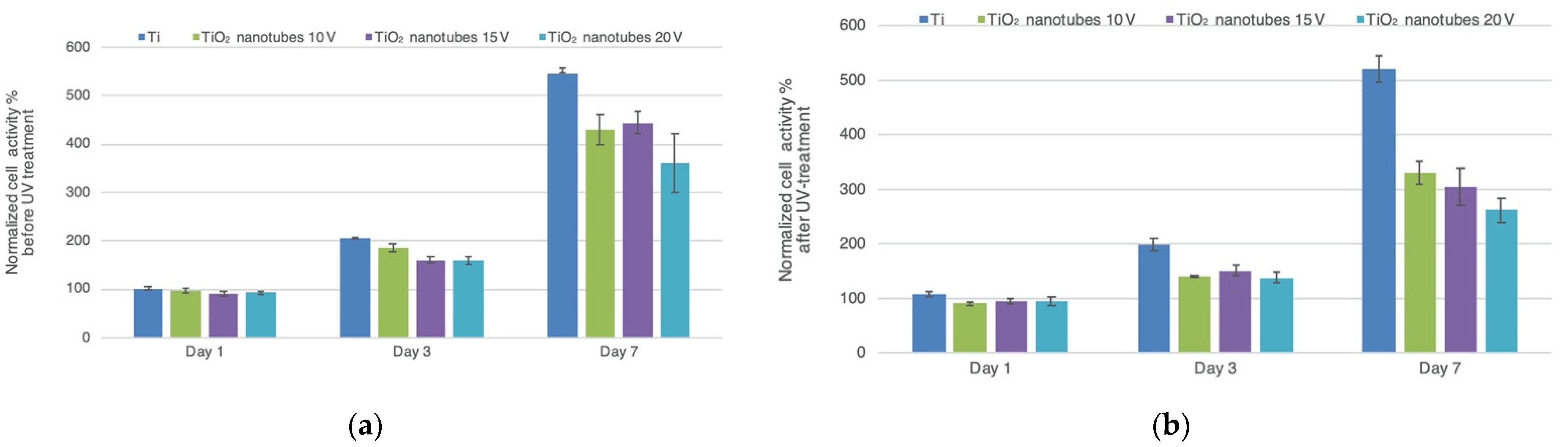
3.6. Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abrahamsson, I.; Berglundh, T.; Glantz, P.-O.; Lindhe, J. The mucosal attachment at different abutments. J. Clin. Periodontol. 1998, 25, 721–727. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Abrahamsson, I.; Berglundh, T.; Lindhe, J. Soft tissue reactions to plaque formation at implant abutments with different surface topography. J. Clin. Periodontol. 2002, 29, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Van Brakel, R.; Cune, M.S.; Van Winkelhoff, A.J.; De Putter, C.; Verhoeven, J.W.; Van Der Reijden, W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: An in vivo study in man. Clin. Oral Implants Res. 2010, 22, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Shahramian, K.; Gasik, M.; Kangasniemi, I.; Walboomers, X.F.; Willberg, J.; Abdulmajeed, A.; Närhi, T. Zirconia implants with improved attachment to the gingival tissue. J. Periodontol. 2019, 91, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P. Influence of abutment material on stability of peri-implant tissues: A systematic review. Int. J. Oral Maxillofac. Implants 2008, 23, 449–456. [Google Scholar] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Bürgers, R.; Gerlach, T.; Hahnel, S.; Schwarz, F.; Handel, G.; Gosau, M. In vivoandin vitrobiofilm formation on two different titanium implant surfaces. Clin. Oral Implants Res. 2010, 21, 156–164. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Faust, J.; Bächle, M.; Follo, M.; Wolkewitz, M.; Hannig, C.; Hellwig, E.; Carvalho, C.; Kohal, R. Biofilm formation and composition on different implant materials in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 101–109. [Google Scholar] [CrossRef]
- Größner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.-D.; Briedigkeit, H.; Göbel, U.B.; Lange, K.-P. Plaque formation on surface modified dental implants. An in vitro study. Clin. Oral Implants Res. 2001, 12, 543–551. [Google Scholar] [CrossRef]
- Quirynen, M.; Van Der Mei, H.C.; Bollen, C.M.L.; Bossche, L.H.V.D.; Doornbusch, G.I.; van Steenberghe, D.; Busscher, H.J. The Influence of Surface-Free Energy on Supra- and Subgingival Plaque Microbiology. An In Vivo Study on Implants. J. Periodontol. 1994, 65, 162–167. [Google Scholar] [CrossRef]
- Hamdan, M.; Blanco, L.; Khraisat, A.; Tresguerres, I.F. Influence of Titanium Surface Charge on Fibroblast Adhesion. Clin. Implants Dent. Relat. Res. 2006, 8, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Moses, R.L.; Jacobs, J.J. Evaluation of Metallic and Polymeric Biomaterial Surface Energy and Surface Roughness Characteristics for Directed Cell Adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Schakenraad, J.M.; Busscher, H.J.; Wildevuur, C.R.H.; Arends, J. Thermodynamic aspects of cell spreading on solid substrata. Cell Biophys. 1988, 13, 75–91. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ozawa, S.; Shih, J.-H.; Ryu, K.; Sukotjo, C.; Yang, J.-M.; Nishimura, I. Biomechanical Evaluation of Osseous Implants Having Different Surface Topographies in Rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef]
- Shalabi, M.; Gortemaker, A.; Hof, M.V.; Jansen, J.; Creugers, N. Implant Surface Roughness and Bone Healing: A Systematic Review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Moon, S.; Oh, T.; Park, I.; Lee, M.; Bae, T. The effect of APH treatment on surface bonding and osseointegration of Ti-6Al-7Nb implants: An in vitro and in vivo study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schupbach, P.; Kim, D.M. Connective tissue attachment to laser-microgrooved abutments: A human histologic case report. Int. J. Periodontics Restor. Dent. 2012, 32, 385–392. [Google Scholar]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef]
- Butt, A.; Hamlekhan, A.; Patel, S.; Royhman, D.; Sukotjo, C.; Mathew, M.; Shokuhfar, T.; Takoudis, C. A Novel Investigation of the Formation of Titanium Oxide Nanotubes on Thermally Formed Oxide of Ti-6Al-4V. J. Oral Implants 2015, 41, 523–531. [Google Scholar] [CrossRef]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of oral streptococci to nanostructured titanium surfaces. Dent. Mater. 2015, 31, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef] [PubMed]
- Von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In vivoevaluation of anodic TiO2 nanotubes: An experimental study in the pig. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef]
- Funato, A.; Yamada, M.; Ogawa, T. Success Rate, Healing Time, and Implant Stability of Photofunctionalized Dental Implants. Int. J. Oral Maxillofac. Implants 2013, 28, 1261–1271. [Google Scholar] [CrossRef]
- Att, W.; Ogawa, T. Biological aging of implant surfaces and their restoration with ultraviolet light treatment: A novel understanding of osseointegration. Int. J. Oral Maxillofac. Implants 2012, 27, 753–761. [Google Scholar]
- Hori, N.; Ueno, T.; Minamikawa, H.; Iwasa, F.; Yoshino, F.; Kimoto, K.; Lee, M.C.-I.; Ogawa, T. Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater. 2010, 6, 4175–4180. [Google Scholar] [CrossRef] [PubMed]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, X.; Li, B.; Cao, C.; Dong, Y.; Ding, C.; Chu, P.K. UV-irradiation-induced bioactivity on TiO2 coatings with nanostructural surface. Acta Biomater. 2008, 4, 544–552. [Google Scholar] [CrossRef]
- Viitaniemi, L.; Abdulmajeed, A.; Sulaiman, T.; Söderling, E.; Närhi, T. Adhesion and Early Colonization of S. Mutans on Lithium Disilicate Reinforced Glass-Ceramics, Monolithic Zirconia and Dual Cure Resin. Eur. J. Prosthodont. Restor. Dent. 2017, 25, 228–234. [Google Scholar] [CrossRef]
- Macak, J.; Tsuchiya, H.; Ghicov, A.; Schmuki, P. Dye-sensitized anodic TiO2 nanotubes. Electrochem. Commun. 2005, 7, 1133–1137. [Google Scholar] [CrossRef]
- De Jong, H.; Van Pelt, A.; Arends, J. Contact Angle Measurements on Human Enamel—An in vitro Study of Influence of Pellicle and Storage Period. J. Dent. Res. 1982, 61, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, P.; Giese, R.; Van Oss, C. Determination of the acid-base characteristics of clay mineral surfaces by contact angle measurements-implications for the adsorption of organic solutes from aqueous media. J. Adhes. Sci. Technol. 1990, 4, 267–275. [Google Scholar] [CrossRef]
- Busscher, H.J. Wettability of Surfaces in the Oral Cavity. Mod. Approaches Wettability 1992, 1992, 249–261. [Google Scholar] [CrossRef]
- Olsson, J.; Carlen, A.; Holmberg, K. Inhibition of Streptococcus mutans Adherence to Hydroxyapatite with Combinations of Alkyl Phosphates and Nonionic Surfactants. Caries Res. 1991, 25, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Robinson, C.; Söderling, E.; Vallittu, P. Early plaque formation on fibre-reinforced composites in vivo. Clin. Oral Investig. 2005, 9, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Ebi, N.; Imazato, S.; Noiri, Y.; Ebisu, S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent. Mater. 2001, 17, 485–491. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Lu, R.; Gao, S.; Ling, Y.; Chen, S. Responses of human gingival fibroblasts to superhydrophilic hydrogenated titanium dioxide nanotubes. Colloids Surf. B Biointerfaces 2021, 198, 111489. [Google Scholar] [CrossRef] [PubMed]
- Singhatanadgit, W.; Toso, M.; Pratheepsawangwong, B.; Pimpin, A.; Srituravanich, W. Titanium dioxide nanotubes of defined diameter enhance mesenchymal stem cell proliferation via JNK- and ERK-dependent up-regulation of fibroblast growth factor-2 by T lymphocytes. J. Biomater. Appl. 2018, 33, 997–1010. [Google Scholar] [CrossRef]
- Tang, T.; Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; von der Mark, K. Synergistic Control of Mesenchymal Stem Cell Differentiation by Nanoscale Surface Geometry and Immobilized Growth Factors on TiO2 Nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 Nanotube Surfaces: 15 nm-An Optimal Length Scale of Surface Topography for Cell Adhesion and Differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochimica Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Donahue, H.J. Cell Sensing and Response to Micro- and Nanostructured Surfaces Produced by Chemical and Topographic Patterning. Tissue Eng. 2007, 13, 1879–1891. [Google Scholar] [CrossRef]
- Minagar, S.; Wang, J.; Berndt, C.; Ivanova, E.; Wen, C. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. Part A 2013, 101, 2726–2739. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Hori, N.; Ueno, T.; Suzuki, T.; Yamada, M.; Att, W.; Okada, S.; Ohno, A.; Aita, H.; Kimoto, K.; Ogawa, T. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int. J. Oral Maxillofac. Implants 2010, 25, 49–62. [Google Scholar]
- Dos Santos, E.A.; Farina, M.; Soares, G.A.; Anselme, K. Surface energy of hydroxyapatite and β-tricalcium phosphate ceramics driving serum protein adsorption and osteoblast adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2307–2316. [Google Scholar] [CrossRef]
- Clark, A.J.; Kotlicki, A.; Haynes, C.A.; Whitehead, L.A. A New Model of Protein Adsorption Kinetics Derived from Simultaneous Measurement of Mass Loading and Changes in Surface Energy. Langmuir 2007, 23, 5591–5600. [Google Scholar] [CrossRef]
- Noh, H.; Vogler, E.A. Volumetric interpretation of protein adsorption: Mass and energy balance for albumin adsorption to particulate adsorbents with incrementally increasing hydrophilicity. Biomaterials 2006, 27, 5801–5812. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone–titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium–cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Papaioanno, W.; Van Eldere, J.; Schepers, E.; Quirynen, M.; Van Steenberghe, D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implants Res. 1996, 7, 201–211. [Google Scholar] [CrossRef]
- Patel, S.B.; Baker, N.; Marques, I.; Hamlekhan, A.; Mathew, M.T.; Takoudis, C.; Friedrich, C.; Sukotjo, C.; Shokuhfar, T. Transparent TiO2 nanotubes on zirconia for biomedical applications. RSC Adv. 2017, 7, 30397–30410. [Google Scholar] [CrossRef] [Green Version]
- Rosqvist, E.; Niemelä, E.; Venu, A.P.; Kummala, R.; Ihalainen, P.; Toivakka, M.; Eriksson, J.E.; Peltonen, J. Human dermal fibroblast proliferation controlled by surface roughness of two-component nanostructured latex polymer coatings. Colloids Surf. B Biointerfaces 2019, 174, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Lassila, L.V.; Garoushi, S.; Tanner, J.; Vallittu, P.K.; Söderling, E. Adherence of Streptococcus mutans to Fiber-Reinforced Filling Composite and Conventional Restorative Materials. Open Dent. J. 2009, 3, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet Treatment Overcomes Time-Related Degrading Bioactivity of Titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef]

| Liquid | Source | Surface Tension (mN/m) | |||
|---|---|---|---|---|---|
| γTOT | γD | γ+ | γ− | ||
| Distilled water, ultrapure water Milli-Q | Produced in-house | 72.8 | 21.8 | 25.5 | 25.5 |
| Diiodomethane > 99% purity | Sigma-Aldrich, St. Louis, CA, USA | 50.8 | 50.8 | 0 | 0 |
| Formamide, pro analysis | Merck, Darmstadt, Germany | 58 | 39 | 2.28 | 39.6 |
| Voltage (V) | Pore Diameter (nm) Mean (SD) | Tube Length (nm) Mean (SD) |
|---|---|---|
| TiO2 nanotube 10 V | 42.2 (0.6) a | 225 (21.2) a |
| TiO2 nanotube 15 V | 67.6 (4.0) b | 260.3 (4.5) b |
| TiO2 nanotube 20 V | 117 (2.6) c | 360 (4.3) c |
| Ra (µm) | Rp (µm) | Rq (µm) | Rt (µm) | Rv (µm) | |
|---|---|---|---|---|---|
| Ti | 0.45 (0.01) | 1.92 (0.18) | 0.54 (0.01) | 3.72 (0.16) | −1.80 (0.19) |
| TiO2 nanotube 10 V | 0.63 (0.04) | 2.19 (0.11) | 0.74 (0.03) | 4.82 (0.40) | −2.63 (0.50) |
| TiO2 nanotube 15 V | 0.27 (0.03) | 1.65 (0.13) | 0.34 (0.03) | 2.72 (0.25) | −1.07 (0.20) |
| TiO2 nanotube 20 V | 0.37 (0.03) | 1.91 (0.33) | 0.46 (0.04) | 3.58 (0.36) | −1.67 (0.22) |
| Groups | Before UV Treatment (θC) | After UV Treatment (θC) | ||||
|---|---|---|---|---|---|---|
| Water | Diiodomethane | Formamide | Water | Diiodomethane | Formamide | |
| Ti | 89.2 (2.1) | 52.1 (3.4) | 57.9 (8.0) | 83.0 (1.7) | 48.1 (1.6) | 59.2 (4.5) |
| TiO2 nanotube 10 V | 16.2 (2.1) | 9.4 (1.4) | 11.3 (0.6) | <0.1 | <0.1 | <0.1 |
| TiO2 nanotube 15 V | 22.4 (2.6) | 12.7 (1.2) | 17.5 (1.7) | <0.1 | <0.1 | <0.1 |
| TiO2 nanotube 20 V | 16.0 (2.0) | 8.9 (1.9) | 12.8 (3.1) | <0.1 | <0.1 | <0.1 |
| Groups | Before UV Treatment | After UV Treatment | ||||
|---|---|---|---|---|---|---|
| Dispersive SFE (γD mJ/m2) | Polar SFE (γP mJ/m2) | Total SFE (γTOT mJ/m2) | Dispersive SFE (γD mJ/m2) | Polar SFE (γP mJ/m2) | Total SFE (γTOT mJ/m2) | |
| Ti | 34.68 | 1.94 | 36.62 | 34.47 | 3.39 | 37.85 |
| TiO2 nanotube 10 V | 42.14 | 29.43 | 71.57 | N/A | N/A | N/A |
| TiO2 nanotube 15 V | 41.75 | 27.75 | 69.49 | N/A | N/A | N/A |
| TiO2 nanotube 20 V | 42.06 | 29.46 | 71.52 | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Abdulmajeed, A.A.; Moon, J.; Shahramian, K.; Punkkinen, R.; Shimada, J.; Vallittu, P.K.; Lassila, L.V. The Effect of Ultraviolet Treatment on TiO2 Nanotubes: A Study of Surface Characteristics, Bacterial Adhesion, and Gingival Fibroblast Response. Metals 2022, 12, 80. https://doi.org/10.3390/met12010080
Kobayashi M, Abdulmajeed AA, Moon J, Shahramian K, Punkkinen R, Shimada J, Vallittu PK, Lassila LV. The Effect of Ultraviolet Treatment on TiO2 Nanotubes: A Study of Surface Characteristics, Bacterial Adhesion, and Gingival Fibroblast Response. Metals. 2022; 12(1):80. https://doi.org/10.3390/met12010080
Chicago/Turabian StyleKobayashi, Masahiko, Aous A. Abdulmajeed, Jongyun Moon, Khalil Shahramian, Risto Punkkinen, Jun Shimada, Pekka K. Vallittu, and Lippo V. Lassila. 2022. "The Effect of Ultraviolet Treatment on TiO2 Nanotubes: A Study of Surface Characteristics, Bacterial Adhesion, and Gingival Fibroblast Response" Metals 12, no. 1: 80. https://doi.org/10.3390/met12010080






