Diffusion Bonding of Ti6Al4V at Low Temperature via SMAT
Abstract
:1. Introduction
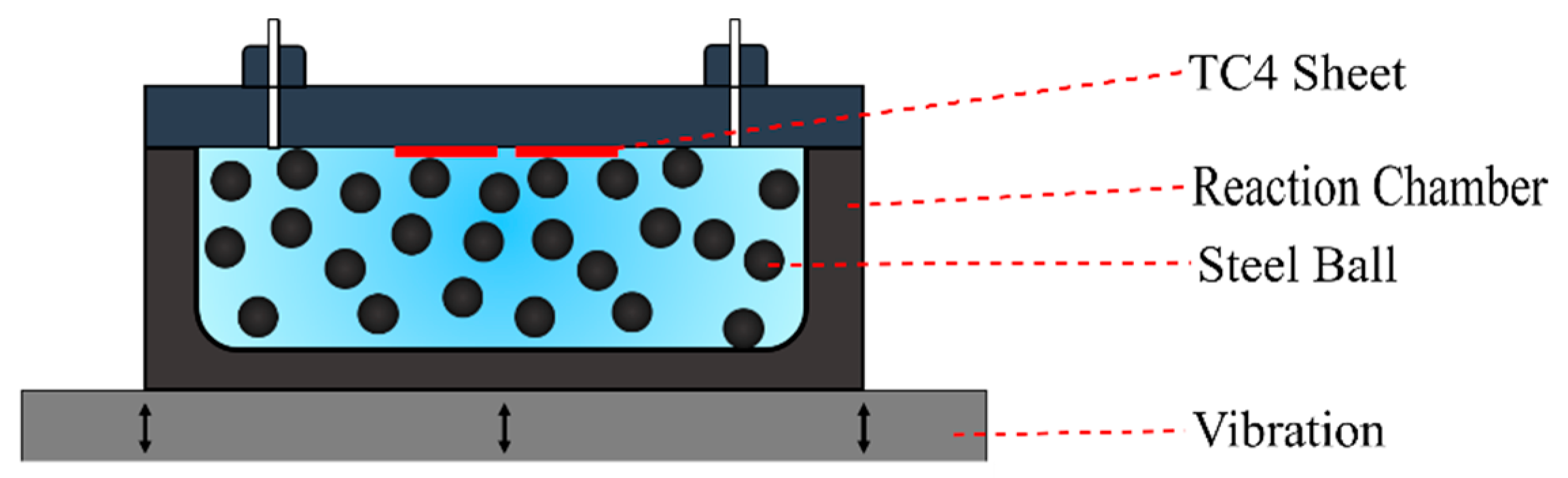
2. Materials and Methods
3. Results and Discussion
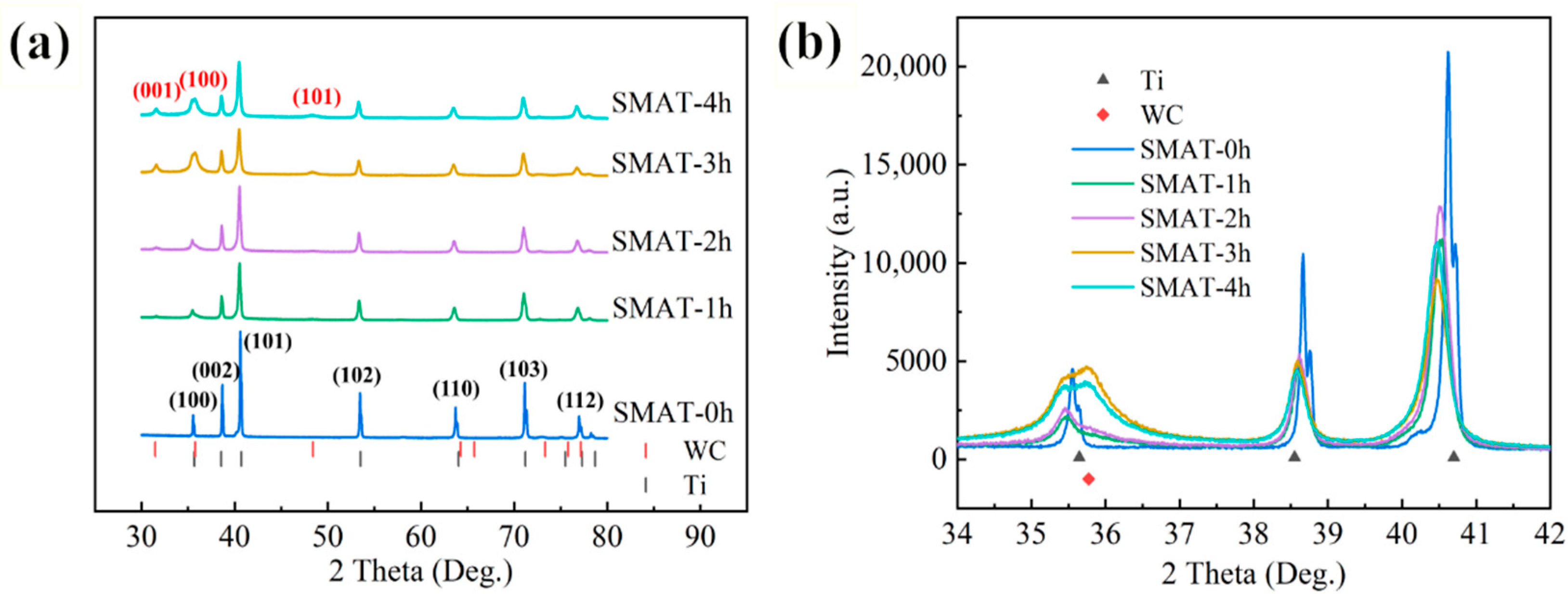
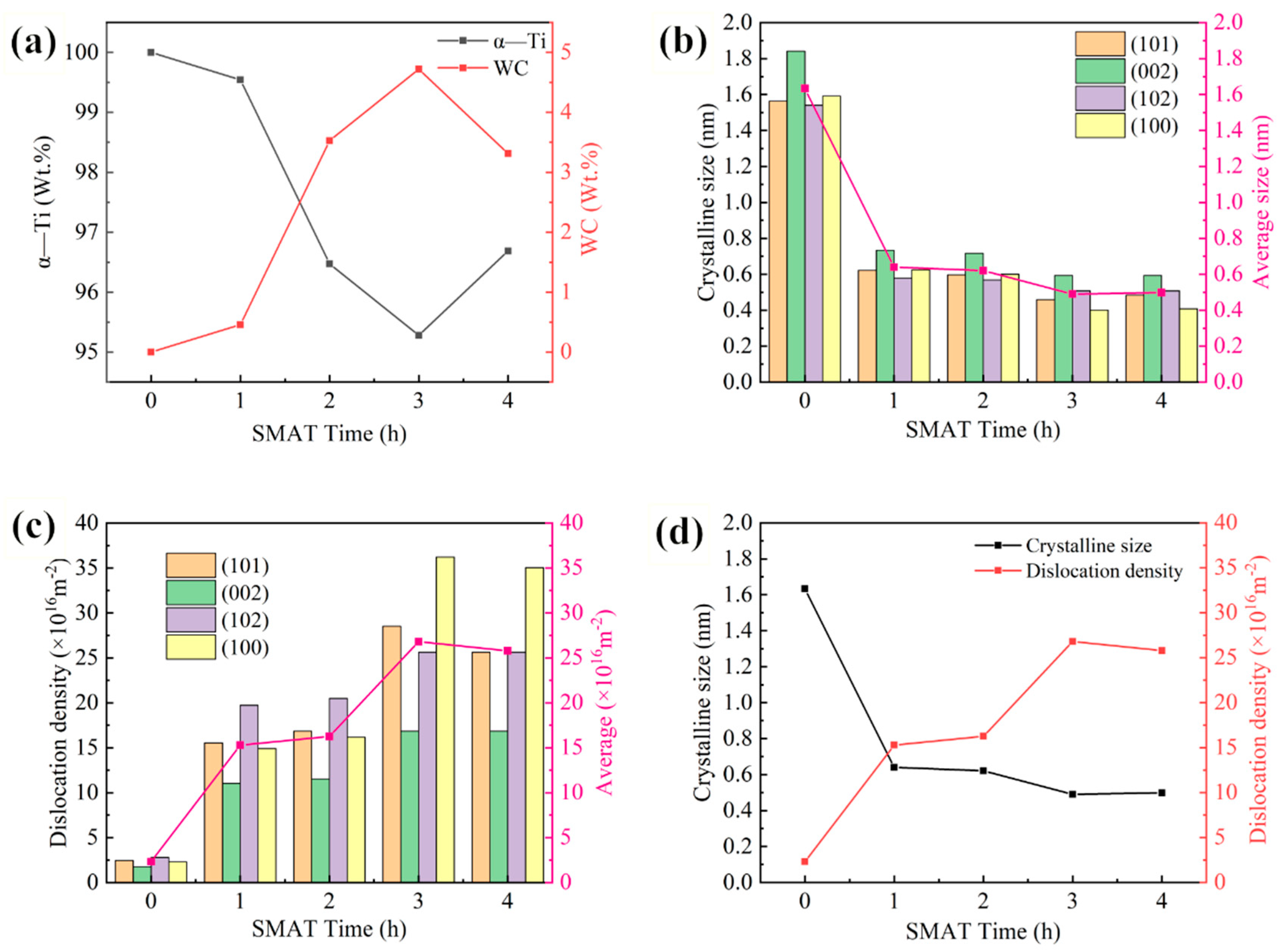
3.1. XRD Analysis
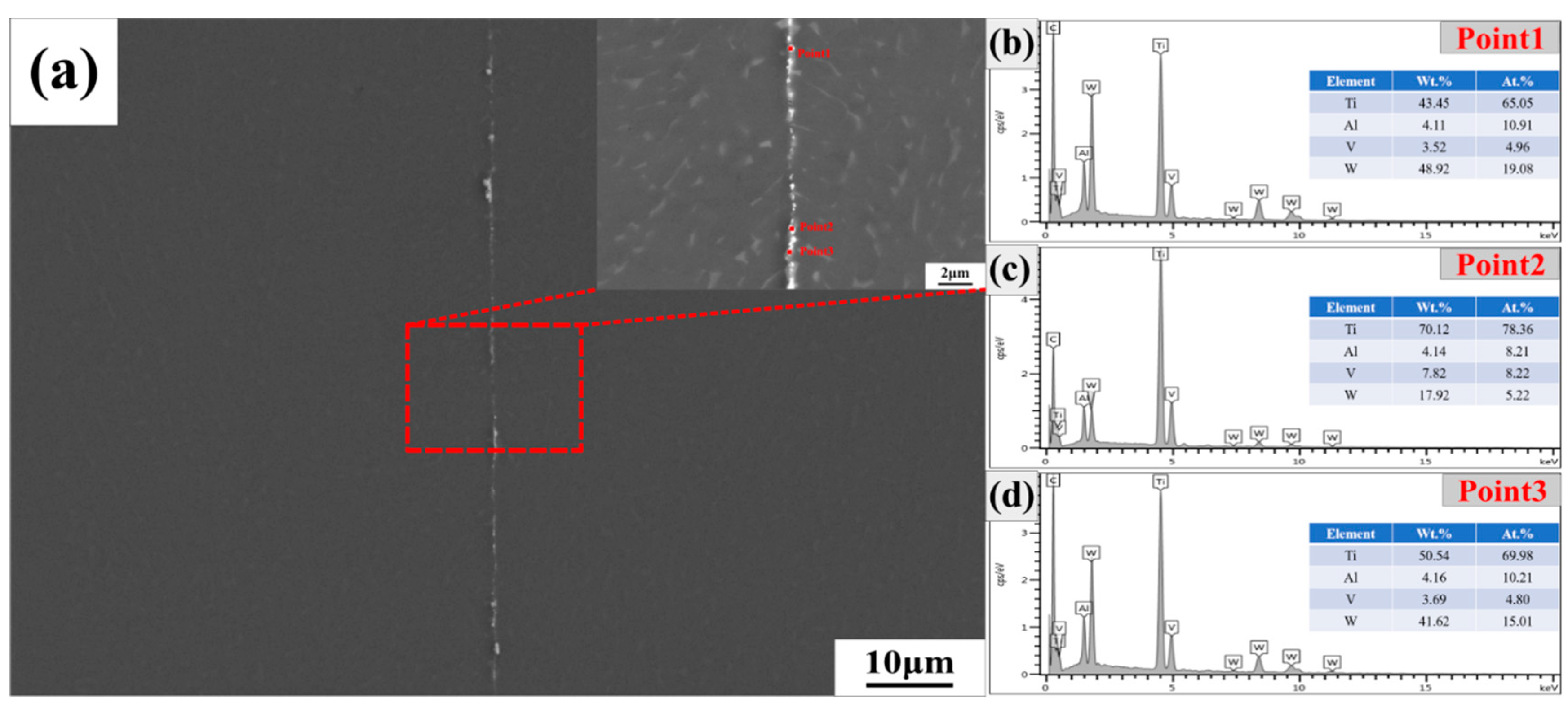
3.2. Microstructure Analysis of Diffusion Bond
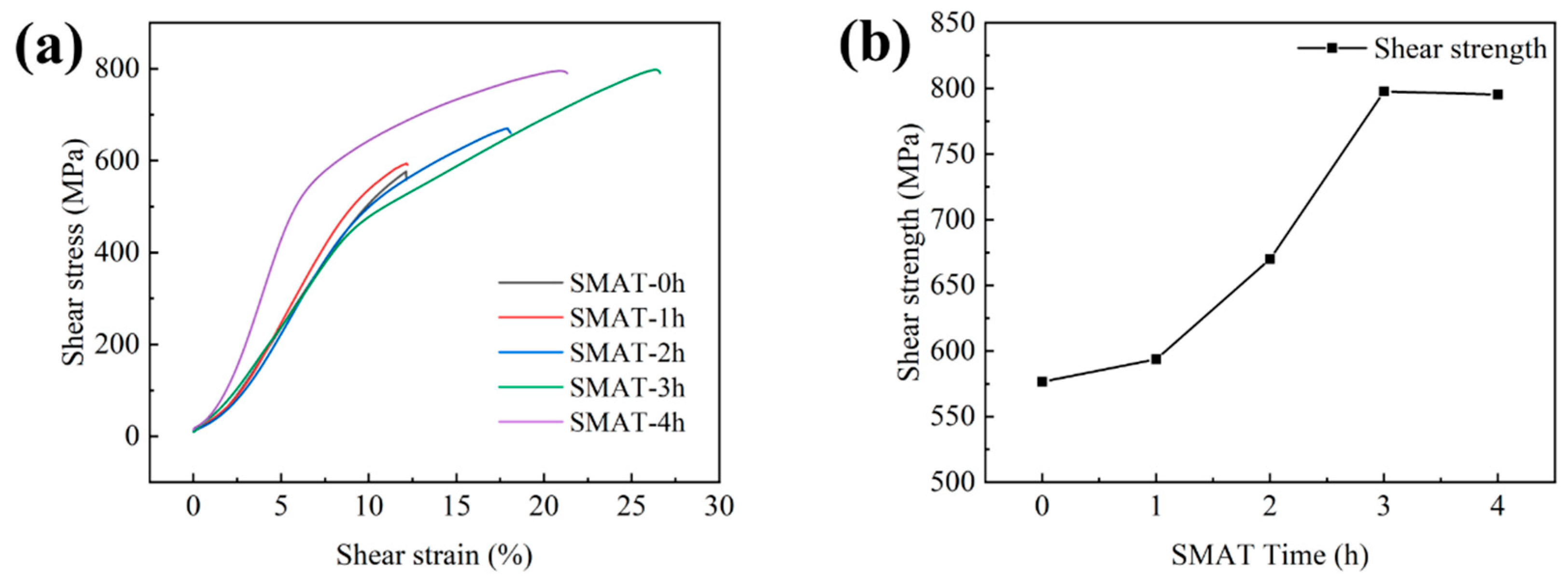
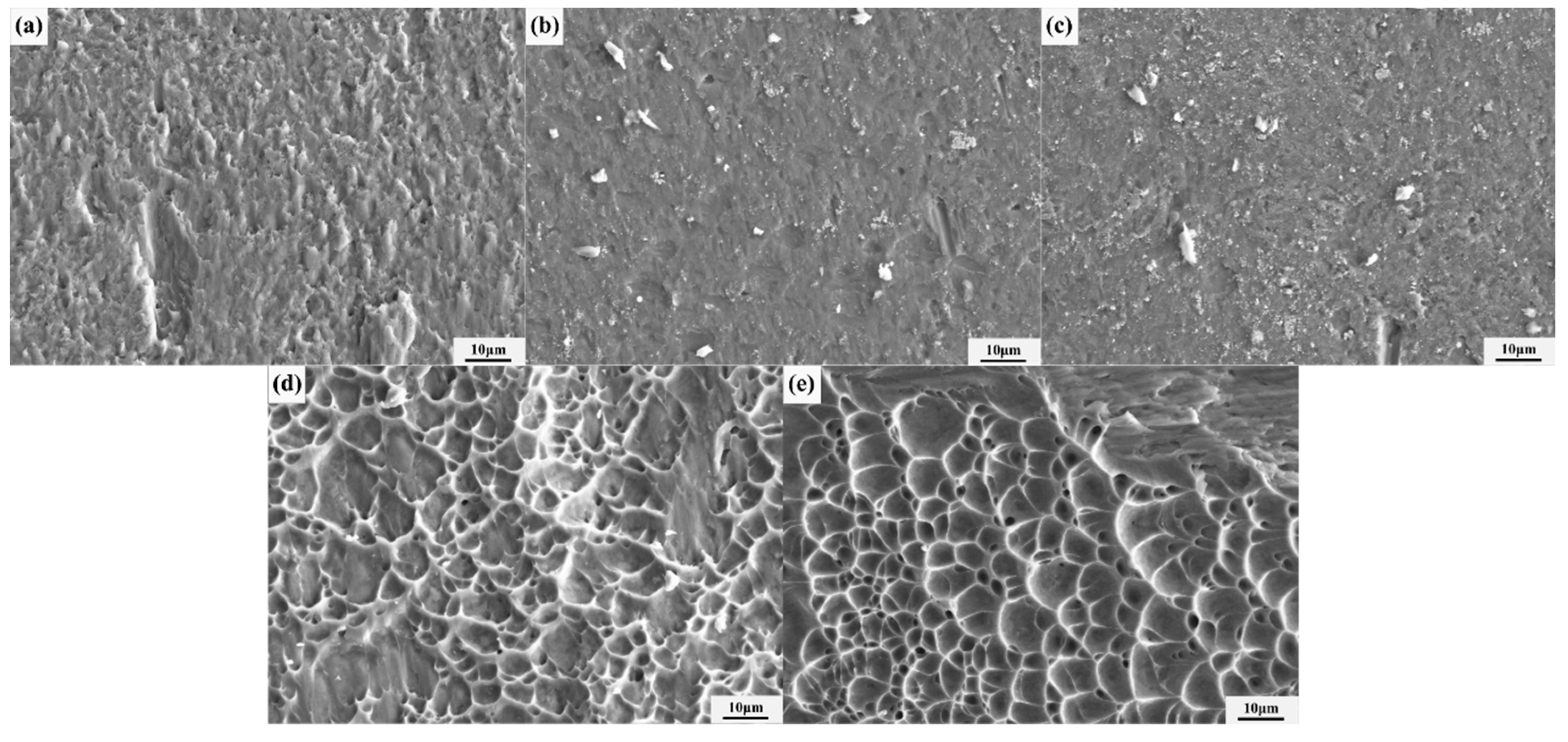
3.3. Analysis of Mechanical Properties of Diffusion Bonds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, D.; Williams, J.C. Perspectives on titanium science and technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Wyatt, Z.; Ankem, S. The effect of metastability on room temperature deformation behavior of β and α + β titanium alloys. J. Mater. Sci. 2010, 45, 5022–5031. [Google Scholar] [CrossRef]
- Shen, J.; Sun, Y.; Ning, Y.; Yu, H.; Yao, Z.; Hu, L. Superplasticity induced by the competitive DRX between BCC beta and HCP alpha in Ti-4Al-3V-2Mo-2Fe alloy. Mater. Charact. 2019, 153, 304–317. [Google Scholar] [CrossRef]
- Liu, J. Properties and Applications of Titanium Alloys. Nonferrous Met. Process. 2002, 4, 1–9. [Google Scholar]
- Kashaev, N.; Ventzke, V.; Fomichev, V.; Fomin, F.; Riekehr, S. Effect of Nd:YAG laser beam welding on weld morphology and mechanical properties of Ti–6Al–4V butt joints and T-joints. Opt. Lasers Eng. 2016, 86, 172–180. [Google Scholar] [CrossRef]
- Mironov, S.; Ozerov, M.; Kalinenko, A.; Stepanov, N.; Plekhov, O.; Sikhamov, R.; Ventzke, V.; Kashaev, N.; Salishchev, G.; Semiatin, L.; et al. On the relationship between microstructure and residual stress in laser-shock-peened Ti-6Al-4V. J. Alloys Compd. 2021, 163383. [Google Scholar] [CrossRef]
- Sticchi, M.; Schnubel, D.; Kashaev, N.; Huber, N. Review of Residual Stress Modification Techniques for Extending the Fatigue Life of Metallic Aircraft Components. Appl. Mech. Rev. 2014, 67, 010801. [Google Scholar] [CrossRef]
- Du, Z.; Wang, C.; Liu, Q.; Wang, S.; Liu, Y.; Wang, G. The superplastic forming/diffusion bonding of TA7 titanium alloy for manufacturing hollow structure with stiffeners. J. Manuf. Process. 2021, 73, 385–394. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Gu, Y.; Li, D.; Fang, H. Investigation on electrically-assisted diffusion bonding of Ti 2 AlNb alloy sheet by microstructural observation, mechanical tests and heat treatment. Mater. Des. 2018, 157, 351–361. [Google Scholar] [CrossRef]
- Du, Z.; Ma, S.; Han, G.; Wei, X.; Han, J.; Zhang, K. The parameter optimization and mechanical property of the honeycomb structure for Ti2AlNb based alloy. J. Manuf. Process. 2021, 65, 206–213. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Mosleh, A.O.; Kotov, A.D.; Kwame, J.S.; Pourcelot, T.; Golovin, I.S.; Portnoy, V.K. Superplastic deformation behavior and microstructure evolution of near-α Ti-Al-Mn alloy. Mater. Sci. Eng. A 2017, 708, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Zhang, K.; Wang, G. Superplastic forming and diffusion bonding for honeycomb structure of Ti–6Al–4V alloy. J. Mater. Process. Technol. 2007, 183, 450–454. [Google Scholar] [CrossRef]
- Tan, Z.; Bai, L.; Bai, B.; Zhao, B.; Li, Z.; Hou, H. Fabrication of lattice truss structures by novel super-plastic forming and diffusion bonding process in a titanium alloy. Mater. Des. 2016, 92, 724–730. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Ma, P.; Xiong, J.; Zhang, F. Study on microstructure and impact toughness of TC4 titanium alloy diffusion bonding joint. Vacuum 2018, 152, 272–277. [Google Scholar] [CrossRef]
- de Salazar, J.G.; Ureña, A.; Carrión, J. Charpy impact test of Ti-6Al-4V joints diffusion welded at low temperature. Scr. Mater. 1996, 35, 479–484. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.; Carreño, F.; Ruano, O.; Sarkeeva, A.; Kruglov, A.; Lutfullin, R. Influence of interfacial defects on the impact toughness of solid state diffusion bonded Ti–6Al–4V alloy based multilayer composites. Mater. Sci. Eng. A 2013, 563, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Tuppen, S.J.; Bache, M.R.; Voice, W.E. Structural integrity of diffusion bonds in Ti–6Al–4V processed via low cost route. Mater. Sci. Technol. 2006, 22, 1423–1430. [Google Scholar] [CrossRef]
- Li, H.; Li, M.-Q.; Yu, W.-X.; Liu, H.-B. Significance and interaction of bonding parameters with bonding ratio in press bonding of TC4 alloy. Rare Met. 2014, 35, 235–241. [Google Scholar] [CrossRef]
- Olugbade, T.; Lu, J. Literature review on the mechanical properties of materials after surface mechanical attrition treatment (SMAT). Nano Mater. Sci. 2020, 2, 3–31. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, J.; Wang, L.; Xu, T.; Xue, Q. Surface nanocrystallization by surface mechanical attrition treatment and its effect on structure and properties of plasma nitrided AISI 321 stainless steel. Acta Mater. 2006, 54, 5599–5605. [Google Scholar] [CrossRef]
- Chang, H.-W.; Kelly, P.; Shi, Y.-N.; Zhang, M.-X. Thermal stability of nanocrystallized surface produced by surface mechanical attrition treatment in aluminum alloys. Surf. Coatings Technol. 2012, 206, 3970–3980. [Google Scholar] [CrossRef]
- Sun, Y.; Bailey, R. Improvement in tribocorrosion behavior of 304 stainless steel by surface mechanical attrition treatment. Surf. Coatings Technol. 2014, 253, 284–291. [Google Scholar] [CrossRef]
- Sun, J.; Yao, Q.; Zhang, Y.; Du, X.; Wu, Y.; Tong, W. Simultaneously improving surface mechanical properties and in vitro biocompatibility of pure titanium via surface mechanical attrition treatment combined with low-temperature plasma nitriding. Surf. Coatings Technol. 2017, 309, 382–389. [Google Scholar] [CrossRef]
- Kashaev, N.; Ventzke, V.; Horstmann, M.; Chupakhin, S.; Riekehr, S.; Falck, R.; Maawad, E.; Staron, P.; Schell, N.; Huber, N. Effects of laser shock peening on the microstructure and fatigue crack propagation behavior of thin AA2024 specimens. Int. J. Fatigue 2017, 98, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Prokhorov, A.; Vshivkov, A.; Plekhov, O.; Kashaev, N.; Fomin, F.; Ozerov, M.; Zherebtsov, S. The Effect of LSP on the Structure Evolution and Self-Heating of ARMCO Iron under Cyclic Loading. Metals 2021, 11, 1198. [Google Scholar] [CrossRef]
- Han, J.; Sheng, G.; Zhou, X.; Sun, J. Diffusion Bonding of Titanium Alloy and Stainless Steel with Surface Nanocrystallization. Rare Met. Mater. Eng. 2010, 39, 42–45. [Google Scholar]
- Li, C.; Si, X.; Bian, S.; Dong, Z.; Huang, Y.; Qi, J.; Feng, J.; Cao, J. Diffusion bonding of Ti and Zr at ultra-low temperature via surface nano-crystallization treatment. Mater. Sci. Eng. A 2020, 785, 139413. [Google Scholar] [CrossRef]
- Han, J.; Sheng, G.M.; Zhou, X.L. Diffusion Bonding of Surface Self-nanocrystallized Ti–4Al–2V and 0Cr18Ni9Ti by Means of High Energy Shot Peening. ISIJ Int. 2008, 48, 1238–1245. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, G.; Han, Z.; Lu, K. Grain size effect on wear resistance of a nanostructured AISI52100 steel. Scr. Mater. 2008, 58, 445–448. [Google Scholar] [CrossRef]
- Chamgordani, S.A.; Miresmaeili, R.; Aliofkhazraei, M. Improvement in tribological behavior of commercial pure titanium (CP-Ti) by surface mechanical attrition treatment (SMAT). Tribol. Int. 2018, 119, 744–752. [Google Scholar] [CrossRef]
- Rajabi, M.; Miresmaeili, R.; Aliofkhazraei, M. Hardness and wear behavior of surface mechanical attrition treated titanium. Mater. Res. Express 2019, 6, 065003. [Google Scholar] [CrossRef]

| Element | Al | V | Fe | C | N | H | O | Ti |
|---|---|---|---|---|---|---|---|---|
| Atom fraction | 5.5~6.8 | 3.5~4.5 | ≤0.50 | ≤0.10 | ≤0.05 | ≤0.015 | ≤0.20 | Bal. |
| (101) | SMAT-0 h | SMAT-1 h | SMAT-2 h | SMAT-3 h | SMAT-4 h |
|---|---|---|---|---|---|
| TTH | 40.6123 | 40.4855 | 40.4791 | 40.4402 | 40.4292 |
| FWHM | 0.0946 | 0.2377 | 0.2475 | 0.3221 | 0.3053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, G.; Liu, Y.; Zhan, L.; Diao, H.; Wang, Y. Diffusion Bonding of Ti6Al4V at Low Temperature via SMAT. Metals 2022, 12, 94. https://doi.org/10.3390/met12010094
Chen Y, Wang G, Liu Y, Zhan L, Diao H, Wang Y. Diffusion Bonding of Ti6Al4V at Low Temperature via SMAT. Metals. 2022; 12(1):94. https://doi.org/10.3390/met12010094
Chicago/Turabian StyleChen, Yuqing, Guofeng Wang, Yongkang Liu, Liqiang Zhan, He Diao, and Yuelin Wang. 2022. "Diffusion Bonding of Ti6Al4V at Low Temperature via SMAT" Metals 12, no. 1: 94. https://doi.org/10.3390/met12010094





