Magnetocaloric Effect in CoFe-Electroplated Ni50Mn33In16Cr1 Alloy
Abstract
:1. Introduction
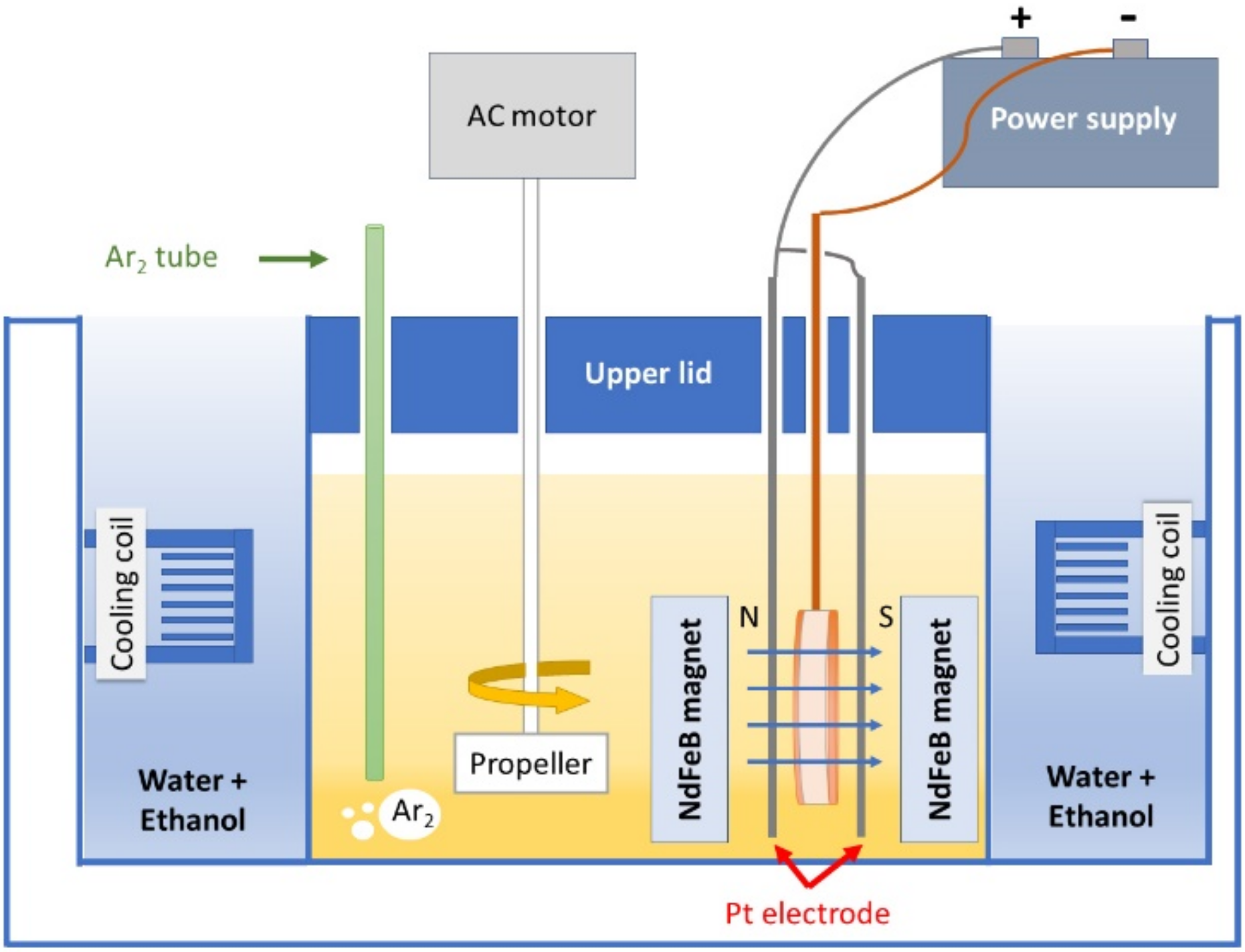
2. Material and Methods
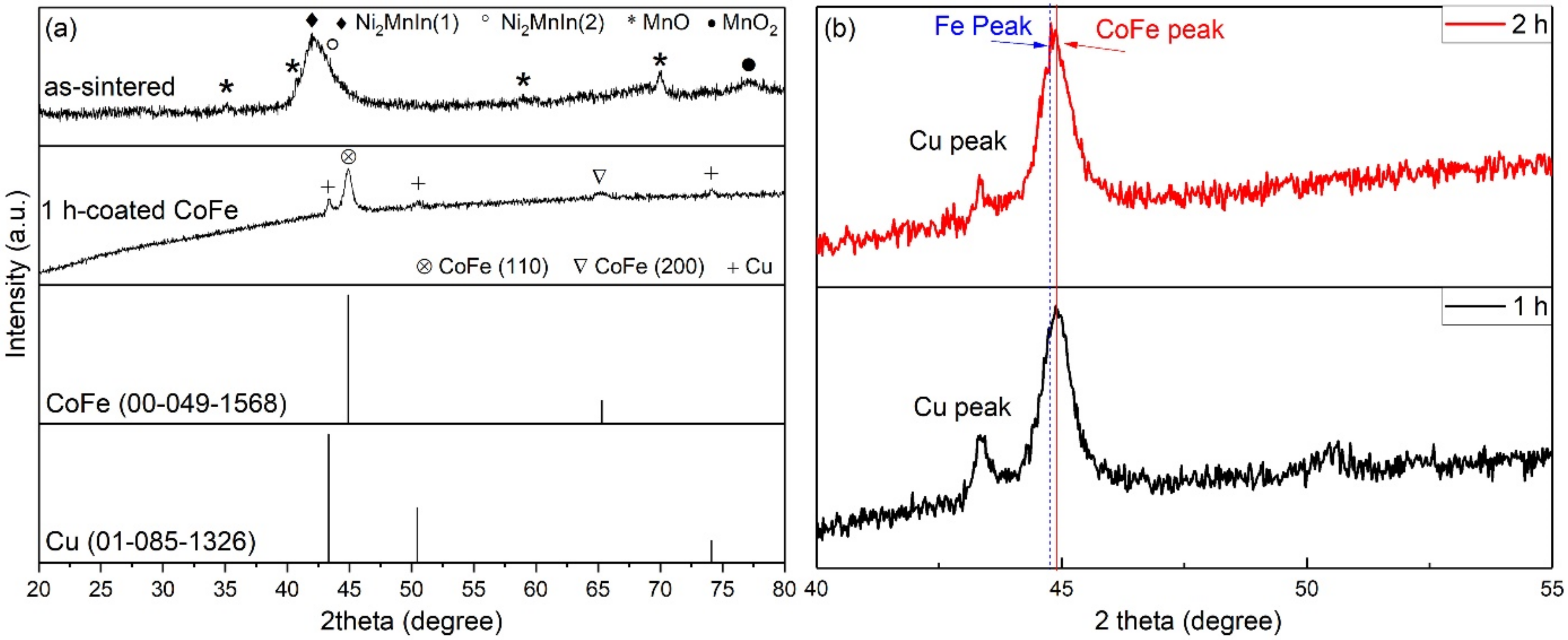
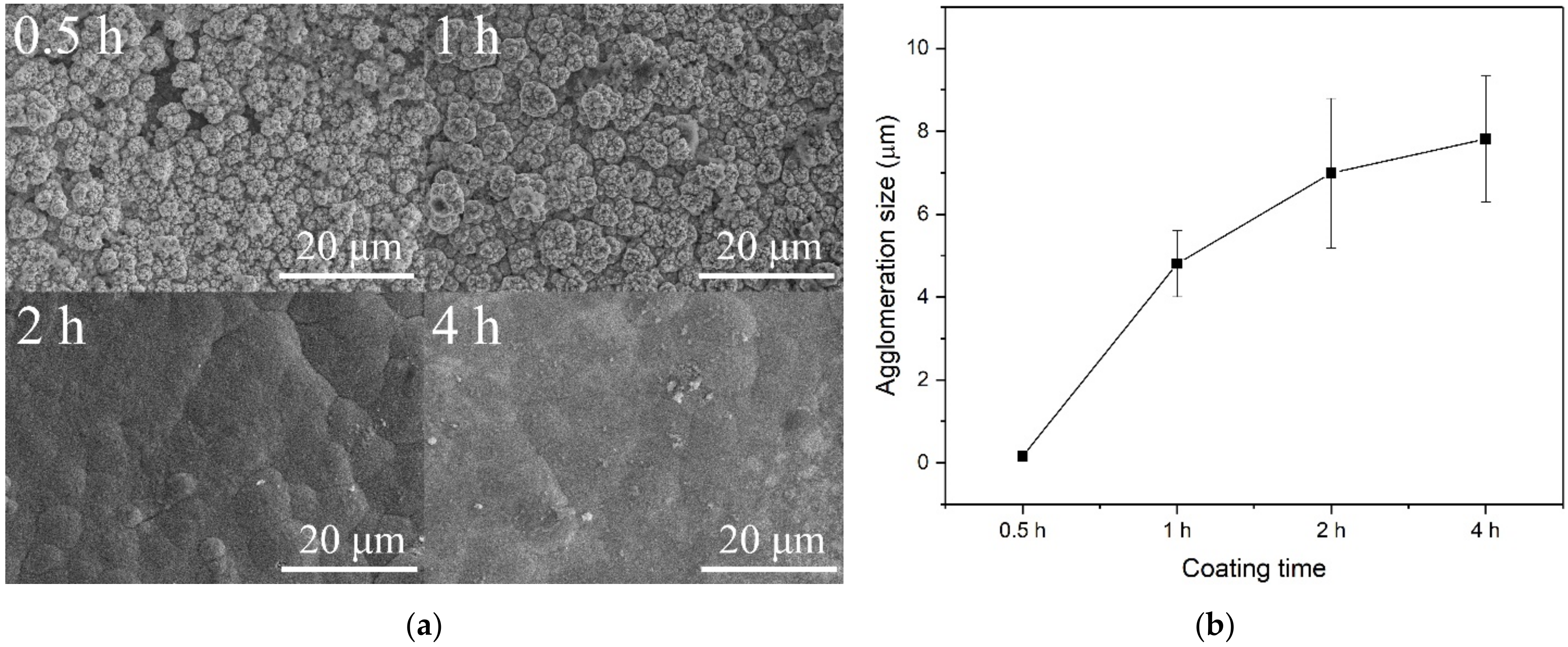
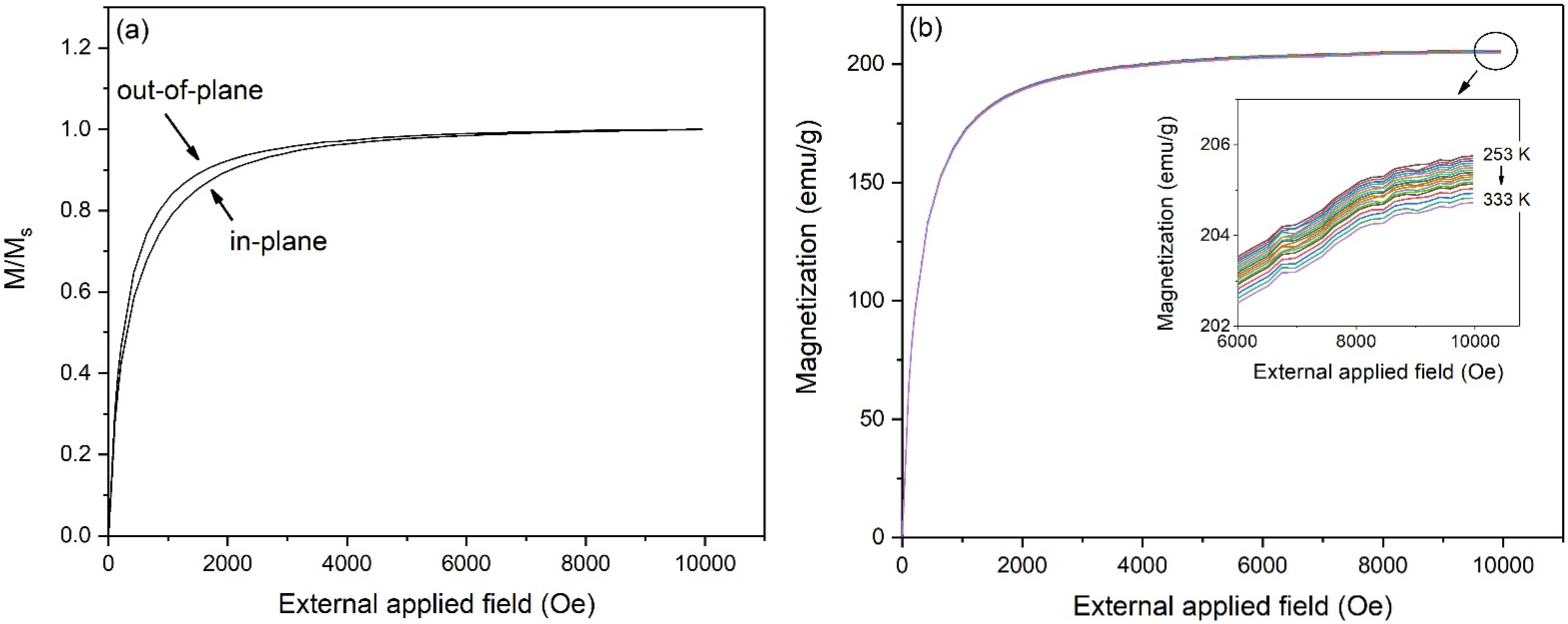
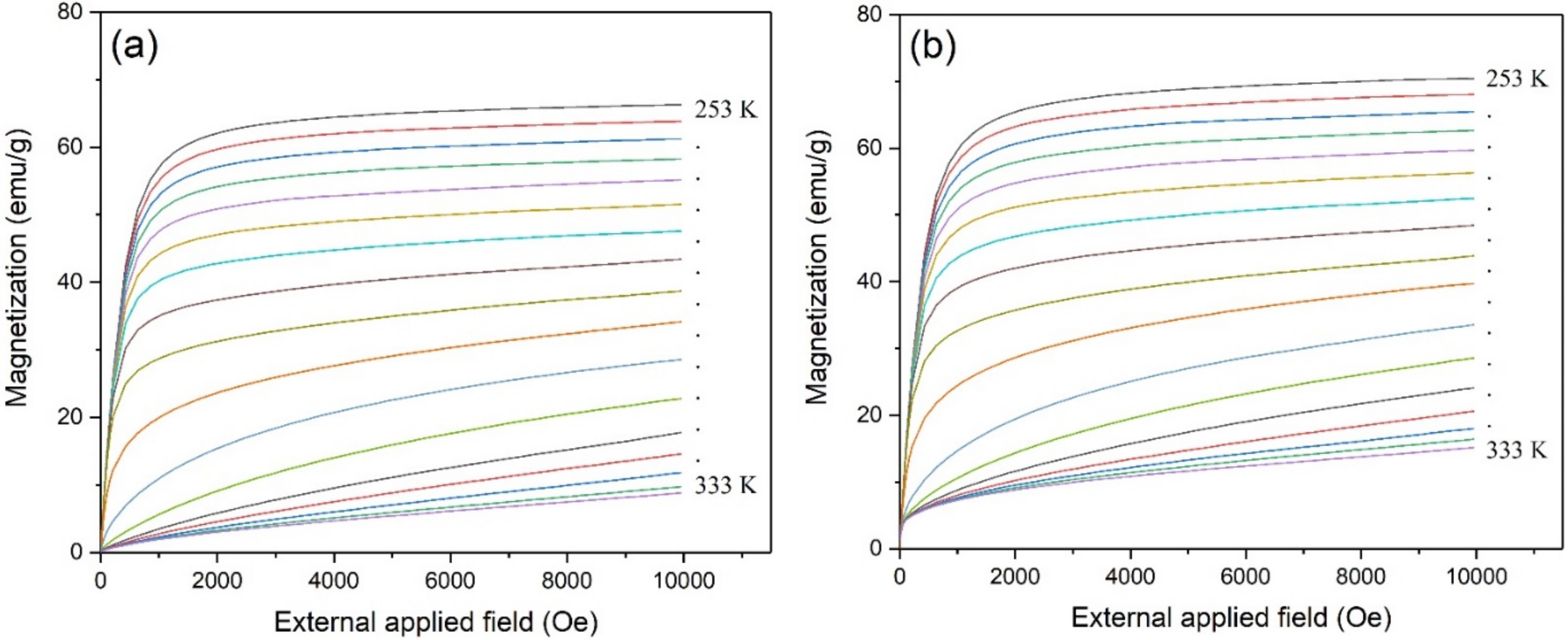
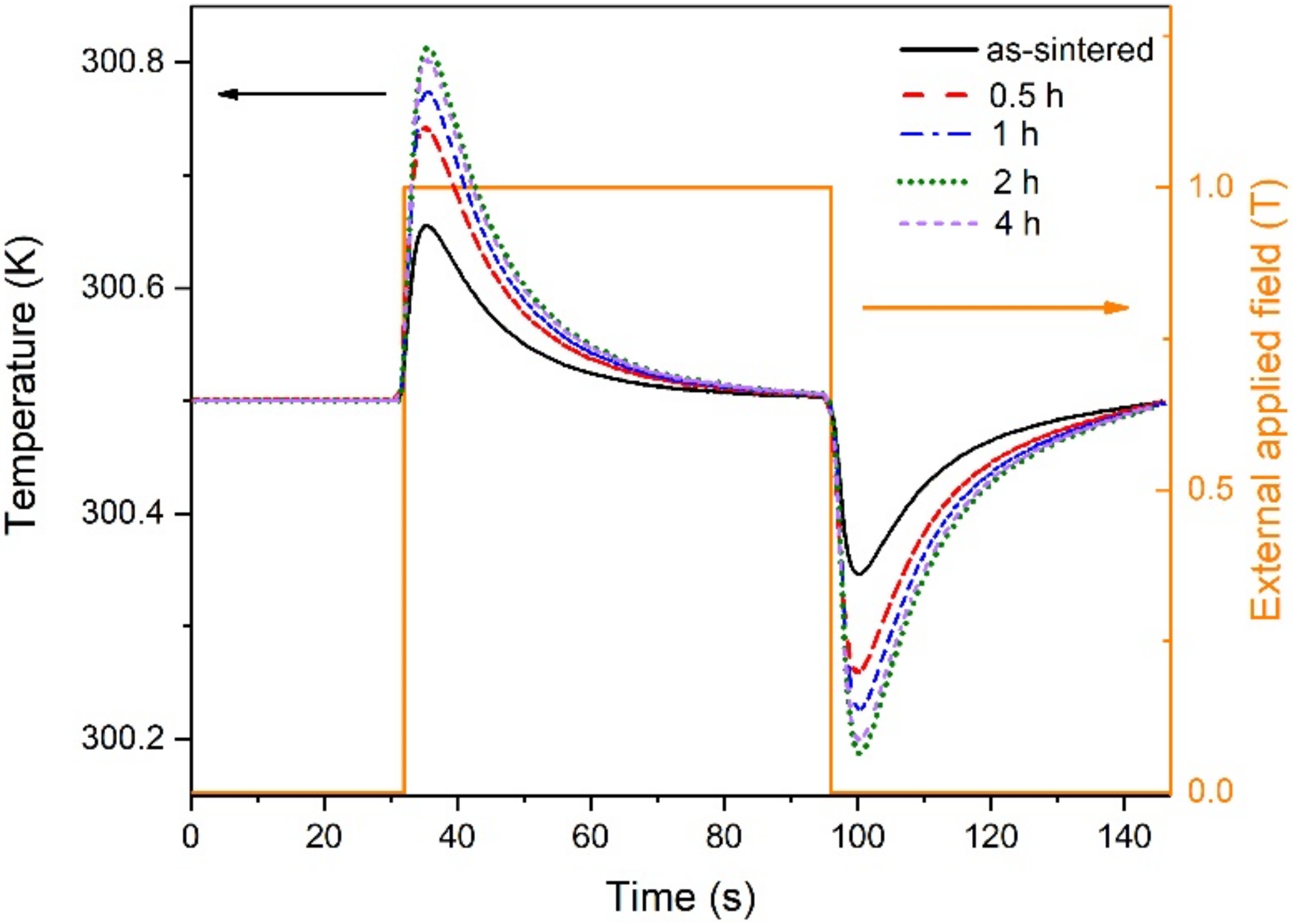
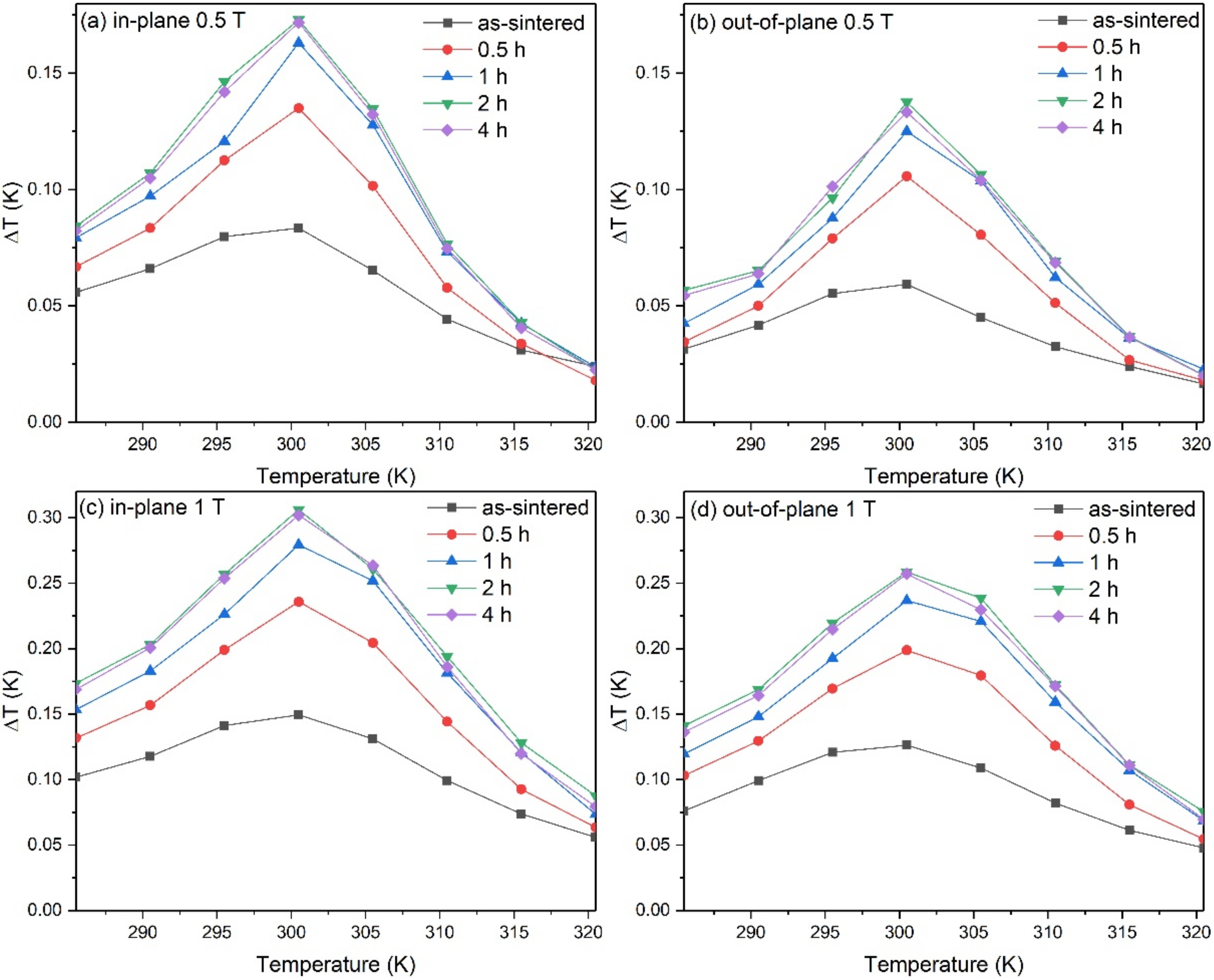
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warburg, E. Magnetische Untersuchungen. Ueber einige Wirkungen der Cöercitivkraft. Ann. Der Phys. 1881, 249, 141–164. [Google Scholar] [CrossRef] [Green Version]
- Pecharsky, V.K.; Gschneidner, K.A., Jr.; Pecharsky, A.O.; Tishin, A.M. Thermodynamics of the magnetocaloric effect. Phys. Rev. B 2001, 64, 144406. [Google Scholar] [CrossRef]
- Herrero, A.; Oleaga, A.; Manfrinetti, P.; Provino, A.; Salazar, A. Critical behavior of the ferromagnetic transition in GdSc(Si,Ge) intermetallic compounds. Intermetallics 2018, 101, 64–71. [Google Scholar] [CrossRef]
- Matsumoto, K.T.; Hiraoka, K. Magnetocaloric effect in Gd-based ferromagnet GdZn2. J. Magn. Magn. Mater. 2017, 423, 318–320. [Google Scholar] [CrossRef]
- Xue, L.; Li, J.; Yang, W.; Yuan, C.; Shen, B. Effect of Fe substitution on magnetocaloric effects and glass-forming ability in Gd-based metallic glasses. Intermetallics 2018, 93, 67–71. [Google Scholar] [CrossRef]
- Vuong, V.-H.; Do-Thi, K.-A.; Nguyen, D.-T.; Nguyen, Q.-H.; Hoang, N.-N. Low field magnetocaloric effect in bulk and ribbon alloy La(Fe 0.88 Si 0.12 ) 13. Phys. B Condens. Matter 2018, 532, 115–118. [Google Scholar] [CrossRef]
- Funk, A.; Freudenberger, J.; Waske, A.; Krautz, M. Getting magnetocaloric materials into good shape: Cold-working of La(Fe, Co, Si)13 by powder-in-tube-processing. Mater. Today Energy 2018, 9, 223–228. [Google Scholar] [CrossRef]
- Liu, J.; He, C.; Zhang, M.; Yan, A. A systematic study of the microstructure, phase formation and magnetocaloric properties in off-stoichiometric La-Fe-Si alloys. Acta Mater. 2016, 118, 44–53. [Google Scholar] [CrossRef]
- Lee, A.; Kim, S.; Kim, Y.; Lee, M. The effect of refractory (Zr, Hf) elements on the magnetocaloric property of Mn-based alloys. Appl. Surf. Sci. 2019, 478, 1004–1008. [Google Scholar] [CrossRef]
- Govor, G.; Mitsiuk, V.; Nikitin, S.; Pankratov, N.; Smarzhevskaya, A. Magnetostructural phase transitions and magnetocaloric effect in Mn(As,P) compounds and their composites. J. Alloys Compd. 2019, 801, 428–437. [Google Scholar] [CrossRef]
- Ren, Q.; Hutchison, W.; Wang, J.; Studer, A.; Campbell, S. First-order magneto-structural transition and magnetocaloric effect in Mn(Co0.96Fe0.04)Ge. J. Alloys Compd. 2017, 693, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Cavazzini, G.; Cugini, F.; Gruner, M.; Bennati, C.; Righi, L.; Fabbrici, S.; Albertini, F.; Solzi, M. Tuning the magnetic and magnetocaloric properties of austenitic Ni-Mn-(In,Sn) Heuslers. Scr. Mater. 2019, 170, 48–51. [Google Scholar] [CrossRef]
- Sepehri-Amin, H.; Taubel, A.; Ohkubo, T.; Skokov, K.; Gutfleisch, O.; Hono, K. Microstructural origin of hysteresis in Ni-Mn-In based magnetocaloric compounds. Acta Mater. 2018, 147, 342–349. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Qian, M.; Yuan, B.; Geng, L. Effect of partial metamagnetic and magnetic transition coupling on the magnetocaloric effect of Ni-Mn-Sn-Fe alloy. Intermetallics 2019, 105, 124–129. [Google Scholar] [CrossRef]
- Lekkla, P.; Jantaratana, P. Near room-temperature magnetocaloric effect of liquid phase sintered Ni50Mn34-xIn16Crx alloys. Solid State Commun. 2022, 342, 114628. [Google Scholar] [CrossRef]
- Laks, D.B.; Van de Walle, C.G.; Neumark, G.F.; Blöchl, P.E.; Pantelides, S.T. Native defects and self-compensation in ZnSe. Phys. Rev. B 1992, 45, 10965–10978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leriche, A.; Hampshire, S.; Cambier, F. Control of the Microstructure in Ceramics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 349–366. [Google Scholar] [CrossRef]
- Malyshev, A.; Petrova, A.; Sokolovskiy, A.; Surzhikov, A. Effect of fast cooling on defects level, microstructure and magnetic properties of LiTiZn ferrite ceramics. Mater. Chem. Phys. 2019, 227, 219–223. [Google Scholar] [CrossRef]
- Wada, H.; Asano, T. Effect of heat treatment on giant magnetocaloric properties of Mn1+As1−Sb. J. Magn. Magn. Mater. 2005, 290–291, 703–705. [Google Scholar] [CrossRef]
- Taskaev, S.; Buchelnikov, V.; Pellenen, A.P.; Kuz’Min, M.D.; Skokov, K.; Karpenkov, D.; Bataev, D.S.; Gutfleisch, O. Influence of thermal treatment on magnetocaloric properties of Gd cold rolled ribbons. J. Appl. Phys. 2013, 113, 17A933. [Google Scholar] [CrossRef]
- Poddar, P.; Srinath, S.; Gass, J.; Prasad, B.L.V.; Srikanth, H. Magnetic Transition and Large Magnetocaloric Effect Associated with Surface Spin Disorder in Co and CocoreAgshell Nanoparticles. J. Phys. Chem. C 2007, 111, 14060–14066. [Google Scholar] [CrossRef]
- Srinath, S.; Poddar, P.; Das, R.; Sidhaye, D.; Prasad, B.L.V.; Gass, J.; Srikanth, H. Large Magnetocaloric Effect, Moment, and Coercivity Enhancement after Coating Ni Nanoparticles with Ag. ChemPhysChem 2014, 15, 1619–1623. [Google Scholar] [CrossRef]
- Chotibhawaris, T.; Tachai, L.; Jantaratana, P.; Boonyongmaneerat, Y. Influence of the Electrodeposited Co-Fe Alloys’ Characteristics on their Magnetic Properties. In Advanced Materials Research; Trans Tech Publications: Bäch, Switzerland, 2014; Volume 1025–1026, pp. 709–716. [Google Scholar] [CrossRef]
- Ispas, A. Electrochemical Phase Formation of Ni and Ni-Fe alloys in a Magnetic Field. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 2007. [Google Scholar]
- Du, J.; Li, G.; Wang, Q.; Ma, Y.; Cao, Y.; He, J. Microstructural evolution and magnetic properties of nanocrystalline Fe films prepared in a high magnetic field. Vacuum 2015, 121, 88–95. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Miękoś, E.; Zieliński, M.; Jaksender, M.; Szczukocki, D.; Czarny, K.; Krawczyk, B. Influence of constant magnetic field on electrodeposition of metals, alloys, conductive polymers, and organic reactions. J. Solid State Electrochem. 2018, 22, 1629–1647. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tang, X.; Wei, R.; Zhu, S.; Yang, J.; Song, W.; Dai, J.; Zhu, X.; Sun, Y. Microstructure refinement and magnetization improvement in CoFe thin films by high magnetic field annealing. J. Alloys Compd. 2017, 729, 730–734. [Google Scholar] [CrossRef]
- Chen, Y.; Jen, S.; Yao, Y.; Wu, J.; Hwang, G.; Tsai, T.; Chang, Y.; Sun, A. Magnetic, structural and electrical properties of ordered and disordered Co50Fe50 films. J. Magn. Magn. Mater. 2006, 304, e71–e74. [Google Scholar] [CrossRef]
- Jakobsson, A.; Şaşıoğlu, E.; Mavropoulos, P.; Ležaić, M.; Sanyal, B.; Bihlmayer, G.; Blügel, S. Tuning the Curie temperature of FeCo compounds by tetragonal distortion. Appl. Phys. Lett. 2013, 103, 102404. [Google Scholar] [CrossRef] [Green Version]
- Prozorov, R.; Kogan, V.G. Effective Demagnetizing Factors of Diamagnetic Samples of Various Shapes. Phys. Rev. Appl. 2018, 10, 014030. [Google Scholar] [CrossRef] [Green Version]
- Omelyanchik, A.; Varvaro, G.; Maltoni, P.; Rodionova, V.; Murillo, J.-P.M.; Locardi, F.; Ferretti, M.; Sangregorio, C.; Canepa, F.; Chernavsky, P.; et al. High-Moment FeCo Magnetic Nanoparticles Obtained by Topochemical H2 Reduction of Co-Ferrites. Appl. Sci. 2022, 12, 1899. [Google Scholar] [CrossRef]
- Yuan, J.; Li, C.-F.; Liu, Z.-Q.; Wu, D.; Cao, L. Synthesis of variously shaped magnetic FeCo nanoparticles and the growth mechanism of FeCo nanocubes. CrystEngComm 2017, 19, 6506–6515. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekkla, P.; Jantaratana, P.; Chotibhawaris, T. Magnetocaloric Effect in CoFe-Electroplated Ni50Mn33In16Cr1 Alloy. Metals 2022, 12, 2137. https://doi.org/10.3390/met12122137
Lekkla P, Jantaratana P, Chotibhawaris T. Magnetocaloric Effect in CoFe-Electroplated Ni50Mn33In16Cr1 Alloy. Metals. 2022; 12(12):2137. https://doi.org/10.3390/met12122137
Chicago/Turabian StyleLekkla, Peerapat, Pongsakorn Jantaratana, and Thanakrit Chotibhawaris. 2022. "Magnetocaloric Effect in CoFe-Electroplated Ni50Mn33In16Cr1 Alloy" Metals 12, no. 12: 2137. https://doi.org/10.3390/met12122137




