Effect of Ce on the Microstructure and Corrosion Resistance of Al-5Mg-3Zn-1Cu Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
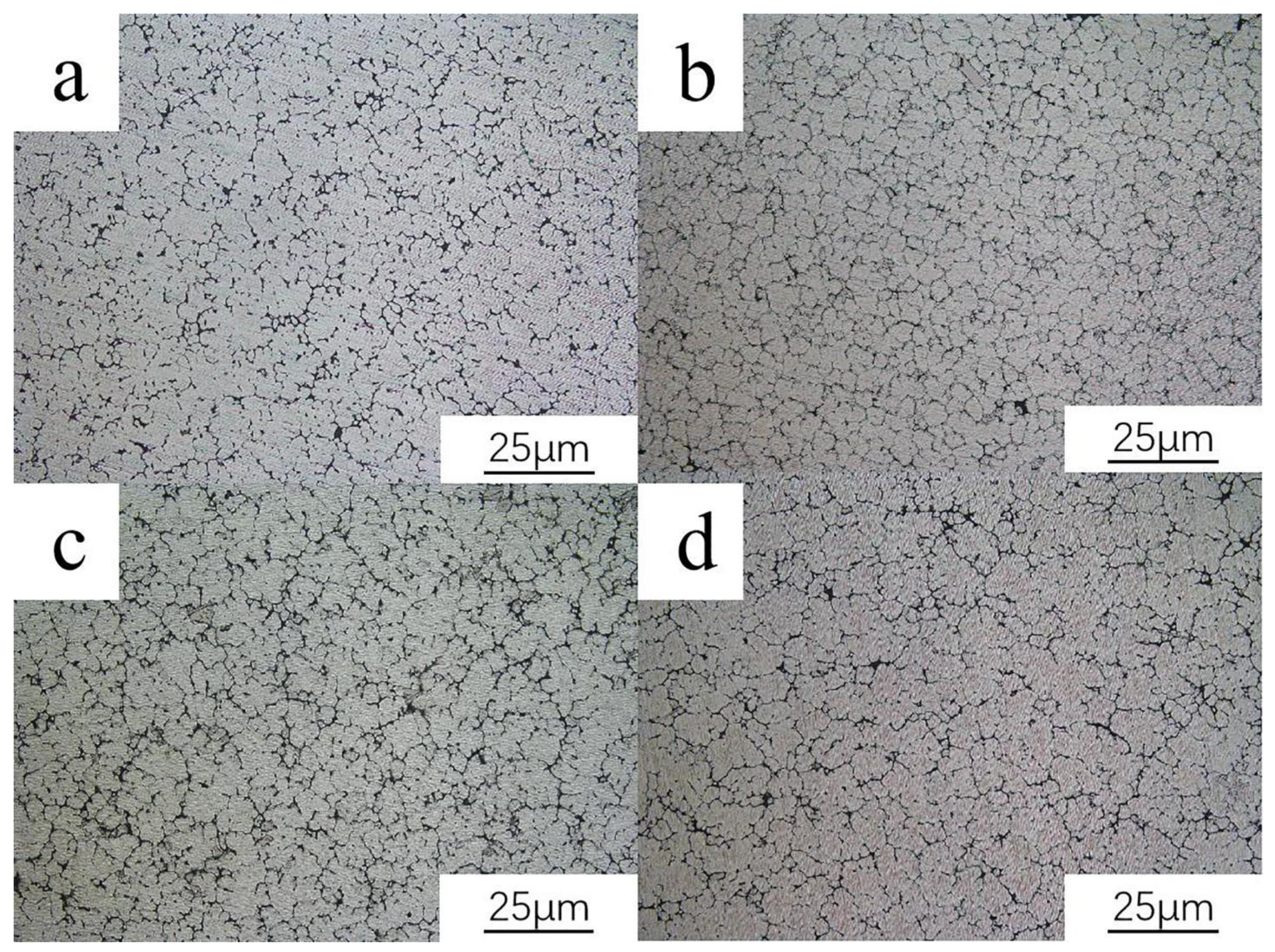
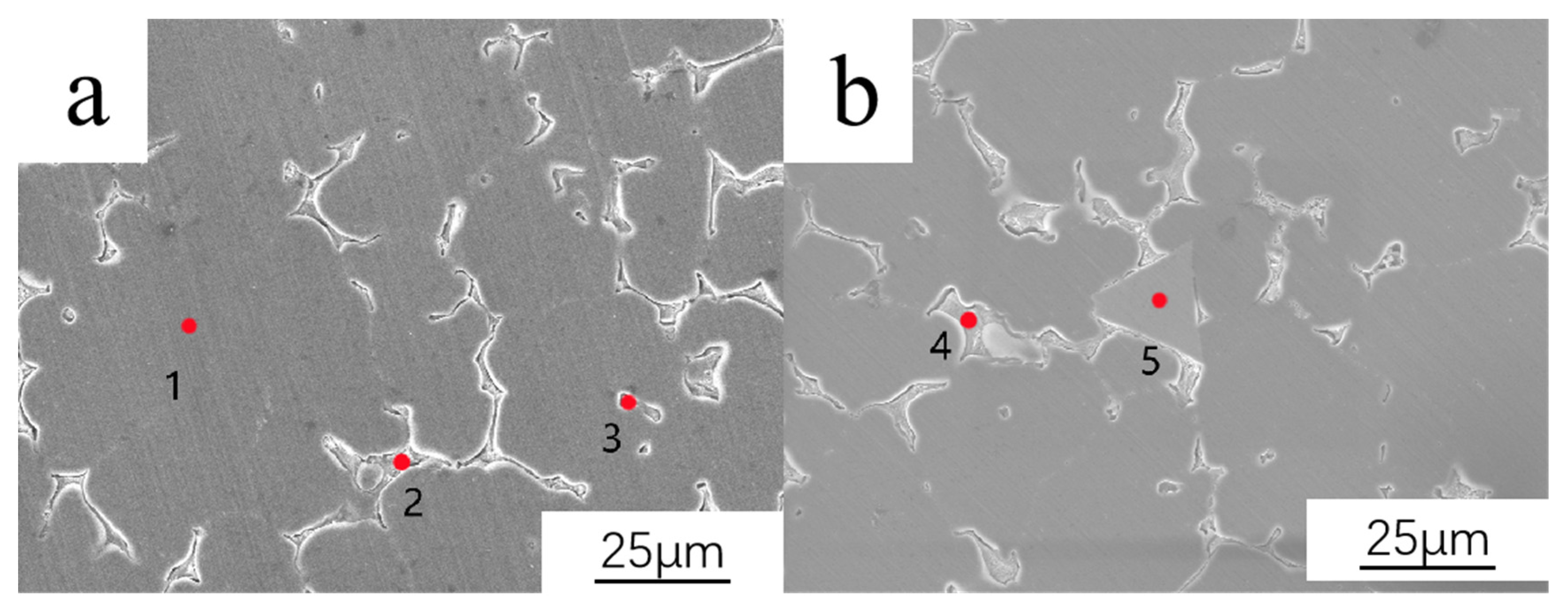
3.1. Microstructure of As-Cast Alloy
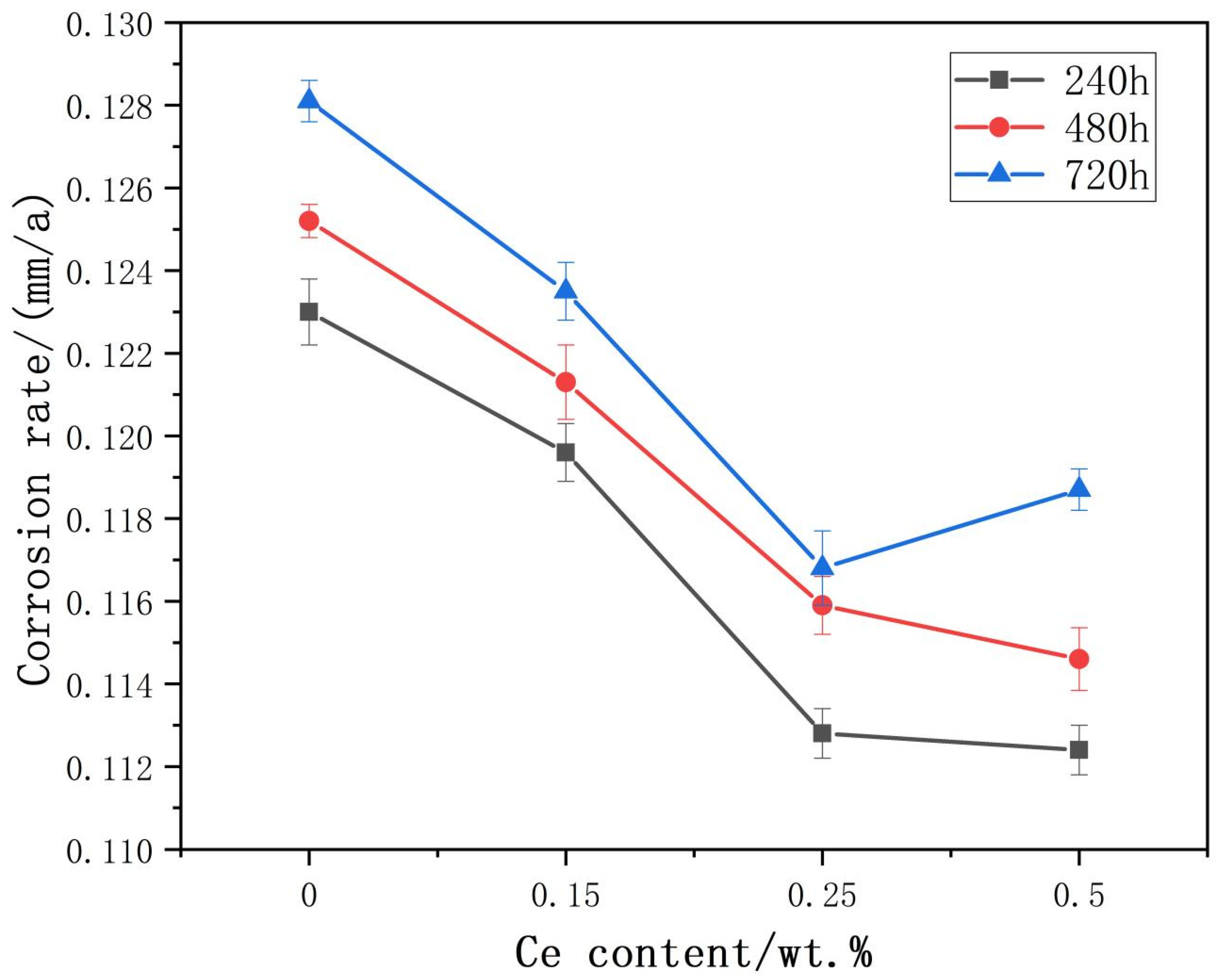
3.2. Results and Analysis of Corrosion Resistance
4. Conclusions
- After adding Ce element to the alloy, the main microstructure of the alloy was α-Al, T-AlMgZnCu, Al2Cu, and a bulk rich Ce phase.
- The addition of Ce can effectively refine the microstructure of the alloy. When the addition of Ce is 0.15 wt.%, the refinement effect is the most obvious, and the precipitated second phase is the most uniform.
- The results of weight loss experiments show that Ce can effectively reduce the corrosion rate of Al-5Mg-3Zn-1Cu alloy, and also increase the corrosion potential of Al-5Mg-3Zn-1Cu and reduce the local corrosion sensitivity of the alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.J.; Zhang, W.Z.; Marthinsen, K. Precipitation crystallography of plate-shaped Al 6(Mn, Fe) dispersoids in AA5182 alloy. Acta Mater. 2012, 60, 5963–5974. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, P.; Sui, Y.; Jiang, Y.; Zhou, R. Influence of Zr Content on Microstructure and Mechanical Properties of As-cast Al-Zn-Mg-Cu Alloy. J. Alloy. Compd. 2021, 867, 158920. [Google Scholar] [CrossRef]
- Barabi, A.; Zarei-Hanzaki, A.; Abedi, H.; Anoushe, A.; Cho, J.H. The correlation of macrostructure, microstructure, and texture with room temperature mechanical properties of a twinning-induced plasticity automotive steel after friction stir spot welding/processing. Steel Res. Int. 2018, 89, 1800245. [Google Scholar] [CrossRef] [Green Version]
- Prach, O.; Trudonoshyn, O.; Randelzhofer, P.; Körner, C.; Durst, K. Effect of Zr, Cr and Sc on the Al–Mg–Si–Mn high-pressure die casting alloys. Mater. Sci. Eng. A 2019, 759, 603–612. [Google Scholar] [CrossRef]
- Shin, S.S.; Lim, K.M.; Park, I.M. Characteristics and microstructure of newly designed Al–Zn-based alloys for the die-casting process. J. Alloy. Compd. 2016, 671, 517–526. [Google Scholar] [CrossRef]
- Tang, H.P.; Wang, Q.D.; Lei, C.; Ye, B.; Wang, K.; Jiang, H.Y.; Zhang, X.-F.; Lin, Z.; Zhang, J.-B. Effect of cooling rate on microstructure and mechanical properties of an Al-5.0Mg-3.0Zn-1.0Cu cast alloy. J. Alloy. Compd. 2019, 801, 596–608. [Google Scholar] [CrossRef]
- Pourbahari, B.; Emamy, M. Effects of La intermetallics on the structure and tensile properties of thin section gravity die-cast A357 Al alloy. Mater. Des. 2016, 94, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Stemper, L.; Tunes, M.A.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Giant hardening response in AlMgZn(Cu) alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Ji, S.; Watson, D.; Fan, Z.; White, M. Development of a super ductile diecast Al–Mg–Si alloy. Mater. Sci. Eng. A 2012, 556, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.P.; Wang, Q.D.; Luo, C.; Lei, C.; Liu, T.-W.; Li, Z.-Y.; Jiang, H.-P.; Ding, W.-J.; Fang, J.; Zhang, J.-W. Effects of aging treatment on the precipitation behaviors and mechanical properties of Al-5.0Mg-3.0Zn-1.0Cu cast alloys. J. Alloy. Compd. 2020, 842, 155707. [Google Scholar] [CrossRef]
- Liu, T.-W.; Wang, Q.-D.; Tang, H.-P.; Li, Z.-Y.; Lei, C.; Ebrahimi, M.; Jiang, H.-Y.; Wen-Jiang, D.I.N.G. Microstructure and mechanical properties of squeeze-cast Al−5.0Mg−3.0Zn−1.0Cu alloys in solution-treated and aged conditions. Trans. Nonferrous Met. Soc. China 2020, 30, 2326–2338. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Zhuang, L.Z.; Zhang, J.S. Correlations between stress corrosion cracking, grain boundary precipitates and Zn content of Al–Mg–Zn alloys. J. Alloys Compd. 2016, 655, 178–187. [Google Scholar] [CrossRef]
- Carroll, M.C.; Gouma, P.I.; Mills, M.J.; Daehn, G.S.; Dunbar, B.R. Effects of Zn additions on the grain boundary precipitation and corrosion of Al-5083. Scr. Mater. 2000, 42, 335–340. [Google Scholar] [CrossRef]
- Yan, X.; Tan, P.; Sui, Y.; Jiang, Y.; Wang, Q. Effects of different holding pressures on the microstructure and mechanical properties of Al-5.9Zn-2.2Mg-1.8Cu alloy. J. Alloys Compd. 2022, 894, 162367. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.H.; Fang, H.C.; Qi, X.W.; Gang, L.I.U. Effect of Yb addition on strength and fracture toughness of Al-Zn-Mg-Cu-Zr aluminum alloy. Trans. Nonferrous Met. Soc. China 2008, 18, 1037–1042. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, J.; Jiang, H.; Zhang, L.; Zhao, J.; He, J. Effect of micro-alloying element La on corrosion behavior of Al-Mg-Si alloys. Corros. Sci. 2021, 179, 109113. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Zhang, X.H.; Chen, H.L. Effect of cerium on intergranular corrosion and exfoliation corrosion behavior of 7249 aluminum alloy. Trans. Mater. Heat Treat. 2014, 35, 74–78. [Google Scholar]
- Hu, G.; Zhu, C.; Xu, D.; Dong, P.; Chen, K. Effect of cerium on microstructure, mechanical properties and corrosion properties of Al-Zn-Mg alloy. J. Rare Earths 2021, 39, 208–216. [Google Scholar] [CrossRef]
- Ly, R.; Karayan, A.I.; Hartwig, K.T.; Castaneda, H. Insights into the electrochemical response of a partially recrystallized Al-Mg-Si alloy and its relationship to corrosion events. Electrochim. Acta 2019, 308, 35–44. [Google Scholar] [CrossRef]
- Orłowska, M.; Ura-Bińczyk, E.; Olejnik, L.; Lewandowska, M. The effect of grain size and grain boundary misorientation on the corrosion resistance of commercially pure aluminium. Corros. Sci. 2019, 148, 57–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, F.; Mao, J.; Niu, G. The difference of La and Ce as additives of electrical conductivity aluminum alloys. Mater. Charact. 2019, 158, 109963. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Wang, R.; Peng, C.; Liu, L. Influence of cerium on microstructures and electrochemical properties of Al-Mg-Sn-Hg anode materials for seawater battery. J. Rare Earths 2015, 33, 1010–1016. [Google Scholar] [CrossRef]




| Parameter Name | Unit | Parameter Value |
|---|---|---|
| Rated power | Kw | 5 |
| Rated voltage | Volt | 220 |
| Rated temperature | °C | 1000 |
| Connection method of heating element | / | Parallel connection |
| Heating time | Min | ≤50 |
| Loss of power | Kw | ≤1.2 |
| Furnace size | Mm | Φ200 × h250 |
| Weight | Kg | 80 |
| Alloys | Mg | Zn | Cu | Ce | Al |
|---|---|---|---|---|---|
| Al-5Mg-3Zn-1Cu | 5.21 | 2.94 | 0.94 | \ | Bal. |
| Al-5Mg-3Zn-1Cu-0.15Ce | 4.94 | 2.91 | 1.05 | 0.12 | Bal. |
| Al-5Mg-3Zn-1Cu-0.25Ce | 4.92 | 3.12 | 0.98 | 0.27 | Bal. |
| Al-5Mg-3Zn-1Cu-0.50Ce | 5.18 | 3.06 | 0.96 | 0.53 | Bal. |
| Element | Al | Mg | Zn | Cu | Ce | Phase |
|---|---|---|---|---|---|---|
| 1 | 97.00 | 2.61 | 0.17 | 0.22 | \ | ɑ-Al |
| 2 | 79.91 | 10.08 | 3.89 | 4.89 | \ | T-AlMgZnCu |
| 3 | 78.76 | 0.04 | 3.25 | 17.89 | 0.06 | Al2Cu |
| 4 | 82.25 | 9.45 | 2.95 | 5.22 | 0.13 | T-AlMgZnCu |
| 5 | 91.24 | 4.23 | 1.22 | 0.04 | 3.27 | AlMgZnCe |
| Alloys | Ecorr(V) | Icorr(10−6 A) | Rp(Ω) |
|---|---|---|---|
| Al-5Mg-3Zn-1Cu | −1.253 | 8.334 | 3596 |
| Al-5Mg-3Zn-1Cu-0.15Ce | −1.193 | 5.850 | 4413 |
| Al-5Mg-3Zn-1Cu-0.25Ce | −1.231 | 7.253 | 4193 |
| Al-5Mg-3Zn-1Cu-0.50Ce | −1.234 | 7.769 | 4136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Sui, Y.; Jiang, Y.; Wang, Q. Effect of Ce on the Microstructure and Corrosion Resistance of Al-5Mg-3Zn-1Cu Alloy. Metals 2022, 12, 371. https://doi.org/10.3390/met12030371
Zhang X, Sui Y, Jiang Y, Wang Q. Effect of Ce on the Microstructure and Corrosion Resistance of Al-5Mg-3Zn-1Cu Alloy. Metals. 2022; 12(3):371. https://doi.org/10.3390/met12030371
Chicago/Turabian StyleZhang, Xingwen, Yudong Sui, Yehua Jiang, and Qudong Wang. 2022. "Effect of Ce on the Microstructure and Corrosion Resistance of Al-5Mg-3Zn-1Cu Alloy" Metals 12, no. 3: 371. https://doi.org/10.3390/met12030371






