The Microstructure Formation in Slag Solidification at Continuous Casting Mold
Abstract
:1. Introduction
2. Materials and Methods
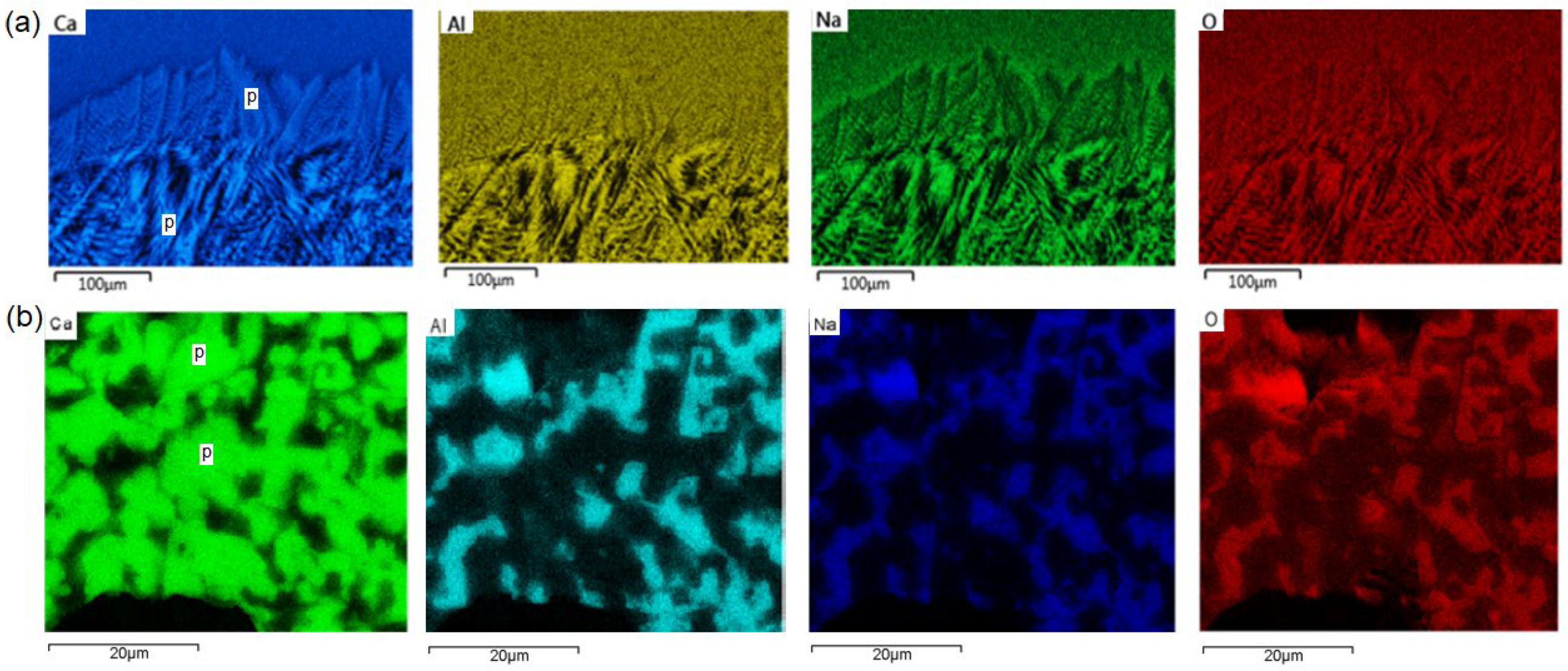
3. Results
4. Theoretical Consideration and Discussion
4.1. Microstructure Formation Sequences
4.2. Formation of Sub-Bands
4.3. Grain Size and Surface Instability
5. Conclusions
- The microstructure contains a crystalline layer and an amorphous layer. The crystalline layer contains three bands, with each band containing two sub-bands of blocky and dendritic morphologies, respectively;
- The crystalline bands are formed directly from solidification instead of from the crystallization of the amorphous state. This clarifies a major puzzle in slag solidification. The result was achieved according to the analysis of crystal morphology and growth direction;
- The solute segregation in liquid slag during cuspidine crystal growth causes a considerable surge of viscosity. This is one of the major reasons for porosity formation and glass formation. The segregation of Al can cause an increase in both the melting temperature and viscosity of liquid slag. This increases constitutional undercooling. The retained liquid can hence be frozen into an amorphous state;
- The segregation-induced increase of viscosity reduces diffusivity, which contributes to the block-to-dendrite transformation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, K.C.; Fox, A.B. The role of mould fluxes in continuous casting-so simple yet so complex. ISIJ Int. 2003, 43, 1479–1486. [Google Scholar] [CrossRef]
- Shu, Q.F.; Li, Q.Q.; Medeiros, S.L.S.; Klug, J.L. Development of non-reactive f-free mold fluxes for high aluminum steels: Non-isothermal crystallization kinetics for devitrification. Metall. Mater. Trans. B 2020, 51, 1169–1180. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Huang, S.H.; Wen, G.G.; Jiang, W.B.; Tang, P. Computational insight into the thermal conductivity of CaO-SiO2-Al2O3-MgO-Na2O melts. Metall. Mater. Trans. B 2020, 51, 2391–2399. [Google Scholar] [CrossRef]
- Hooli, P. Study on the Layers in the Film Originating from the Casting Powder between Steel Shell and Mould and Associated Phenomena in Continuous Casting of STAINLESS steel. Ph.D. Thesis, Helsinki University of Technology, Helsinki, Finland, 2007. [Google Scholar]
- Wang, W.L.; Yu, J.; Zhou, L.J.; Wu, Z.Y.; Li, H. Optimization of mold flux for the continuous casting of Cr-contained steels. Metall. Mater. Trans. B 2018, 49, 1580–1587. [Google Scholar] [CrossRef]
- Yang, C.L.; Wen, G.H.; Tang, P.; Xi, C.C.; Sun, Q.H. Quantification of crystalline fraction of solid slag film using X-ray powder diffraction. Powder Diffr. 2016, 31, 40–51. [Google Scholar] [CrossRef]
- Watanabe, T.; Hashimoto, H.; Hayashi, M.; Nagata, K. Effect of alkali oxides on crystallization in CaO-SiO2-CaF2 glasses. ISIJ Int. 2008, 48, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Susa, M.; Kubota, S.; Hayashi, M.; Mills, K.C. Thermal conductivity and structure of alkali silicate melts containing fluorides. Ironmak. Steelmak. 2001, 28, 390–395. [Google Scholar] [CrossRef]
- Wang, Z.J.; Sohn, I. Experimental determination of phase equilibria in the CaO-BaO-SiO2-12 mol pct. Al2O3-13 mol pct. MgO system at 1573 K and 1623 K. J. Am. Ceram. Soc. 2019, 102, 5632–5644. [Google Scholar] [CrossRef]
- Hayashi, M.; Watanabe, T.; Nakada, H.; Nagata, K. Effect of Na2O on crystallization of mould fluxes for continuous casting of steel. ISIJ Int. 2006, 46, 1805–1809. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, A.; Sorimachi, K.; Yamauchi, T. Effect of solidus temperature and crystalline phase of mould flux on heat transfer in continuous casting mould. Ironmak. Steelmak. 2002, 29, 203–207. [Google Scholar] [CrossRef]
- Susa, M.; Kushimoto, A.; Toyota, H.; Hayashi, M.; Endo, R.; Kobayashi, Y. Effects of both crystallisation and iron oxides on the radiative heat transfer in mould fluxes. ISIJ Int. 2009, 49, 1722–1729. [Google Scholar] [CrossRef]
- Huang, X.C.; Duan, Y.R.; Liu, Z.Q.; Li, B.K.; Wang, F. Role of electrode rotation on improvement of metal pool profile in electroslag remelting process. Metals 2021, 11, 1675. [Google Scholar] [CrossRef]
- Persson, E.S.; Brorson, S.; Mitchell, A.; Jonsson, P.G. Impact of solidification on inclusion morphology in ESR and PESR remelted martensitic stainless steel ingots. Metals 2021, 11, 408. [Google Scholar] [CrossRef]
- Shibata, H.; Emi, T.; Waseda, Y.; Kondo, K.; Ohta, H.; Nakajima, K. Thermal diffusivities of continuous casting mold fluxes for steel in the glassy and crystalline states. J. Iron Steel Inst. Jpn. 1996, 82, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Seo, M.D.; Shi, C.B.; Cho, J.W.; Kim, S.H. Control of crystal morphology for mold flux during high-aluminum AHSS continuous casting process. Metall. Mater. Trans. 2016, 47B, 2211–2221. [Google Scholar] [CrossRef] [Green Version]
- Saburi, S.; Kawahara, A.; Henmi, C.; Kusachi, I.; Kihara, K. The refinement of the crystal structure of cuspidine. Mineral. Mag. 1977, 8, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Vulić, P.; Kahlenberg, V.; Konzett, J. On the existence of a Na-deficient monoclinic trinepheline with composition Na7.85Al7.85Si8.15O32. Am. Mineral. 2008, 93, 1072–1079. [Google Scholar] [CrossRef]
- Castillejos E, A.H.; Tania, M.; Flores, F. Characterization of roughness, porosity and thermal resistances of continuous casting mold slag layers devitrified and crystallized in laboratory. Metall. Mater. Trans. B 2019, 50, 2436–2453. [Google Scholar] [CrossRef]
- Arrhenius, S.A. Über die dissociationswärme und den einfluss der temperatur auf den dissociationsgrad der elektrolyte. Z. Phys. Chem. 1889, 4, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.S.; Zhou, B.L. Effect of electric current pulses on grain size in castings. Int. J. Non-Equilib. Proc. 1998, 11, 77–86. [Google Scholar]
- Hooli, P.O. Mould flux film between mould and steel shell. Ironmak. Steelmak. 2002, 29, 293–296. [Google Scholar] [CrossRef]
- Qin, R.S.; Wallach, E.R.; Thomson, R.C. A phase-field model for the solidification of multicomponent and multiphase alloys. J. Crys. Growth 2005, 279, 163–169. [Google Scholar] [CrossRef]
- Wang, X.J.; Jin, H.B.; Zhu, L.G.; Xu, Y.; Liu, R.; Piao, Z.L.; Qu, S. Effect of CaF2 on the viscosity and microstructure of CaO-SiO2-Al2O3 based continuous casting mold flux. Metals 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.T.; Gao, Y.H. Influence of Al2O3, CaO/SiO2, and B2O3 on viscous behavior of high alumina and medium titania blast furnace slag. J. Chem. 2017, 2017, 6895928. [Google Scholar] [CrossRef] [Green Version]
- Witten, T.A.; Sander, L.M. Diffusion-limited aggregation, a kinetic critical phenomenon Phys. Rev. Lett. 1981, 47, 1400–1403. [Google Scholar] [CrossRef]
- Liu, H.; Qin, Y.L.; Yang, Y.H.; Zhang, Q.Y.; Deng, N.Y. Influence of Al2O3 content on the melting and fluidity of blast furnace type slag with low TiO2 content. J. Chem. 2018, 2018, 9502304. [Google Scholar] [CrossRef]
- Mullins, W.W.; Sekerka, R.F. Stability of a planar interface during solidification of a dilute binary alloy. J. Appl. Phys. 1964, 35, 444. [Google Scholar] [CrossRef]
- Qin, R.S. Suppression of the surface roughness and fluctuation frequency by electric method. Mater. Today Commun. 2021, 28, 102512. [Google Scholar] [CrossRef]





| SiO2 | CaO | Na2O | F | Al2O3 | MgO | C |
|---|---|---|---|---|---|---|
| 38.44 | 35.74 | 12.9 | 9.4 | 2.5 | <1 | balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhagurkar, A.G.; Qin, R. The Microstructure Formation in Slag Solidification at Continuous Casting Mold. Metals 2022, 12, 617. https://doi.org/10.3390/met12040617
Bhagurkar AG, Qin R. The Microstructure Formation in Slag Solidification at Continuous Casting Mold. Metals. 2022; 12(4):617. https://doi.org/10.3390/met12040617
Chicago/Turabian StyleBhagurkar, Ashutosh G., and Rongshan Qin. 2022. "The Microstructure Formation in Slag Solidification at Continuous Casting Mold" Metals 12, no. 4: 617. https://doi.org/10.3390/met12040617






