Chelating Extractants for Metals
Abstract
:1. Introduction
2. Extractants Containing Amide Groups
2.1. Actinides
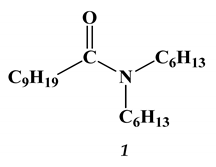
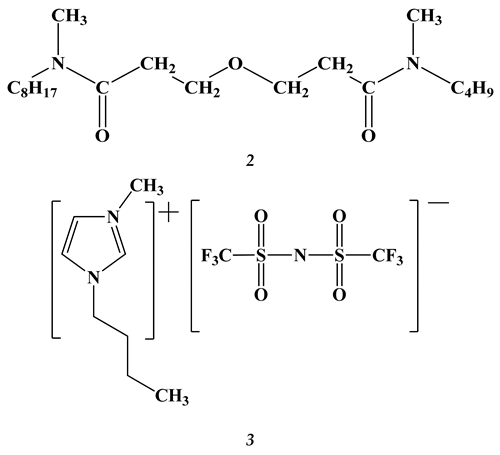

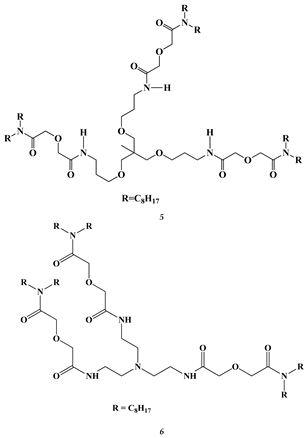

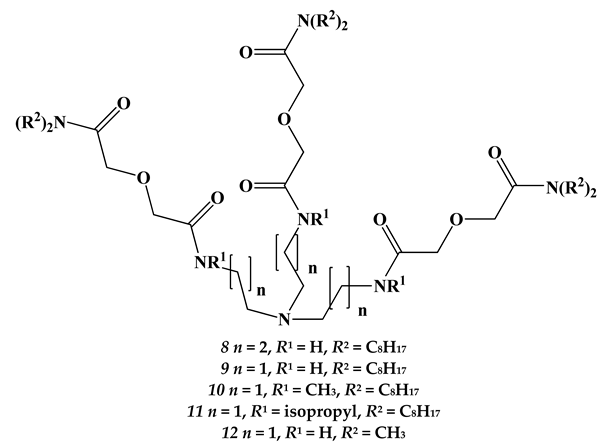
2.2. Lanthanides

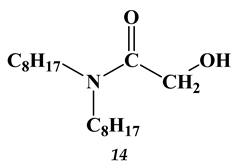
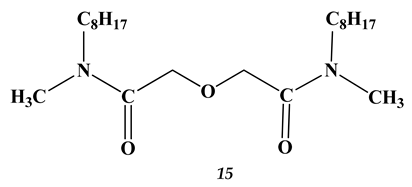
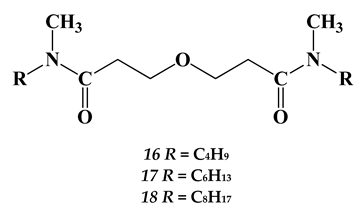
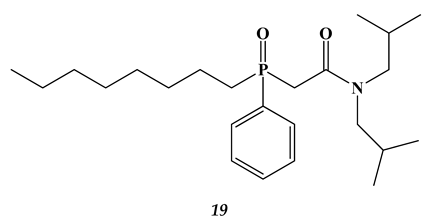
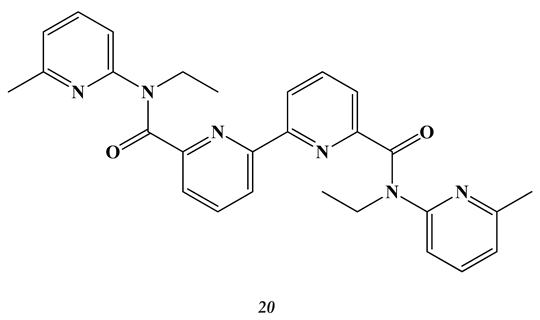
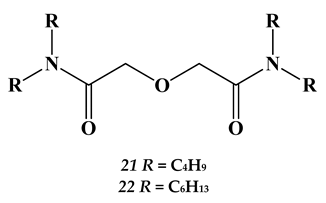
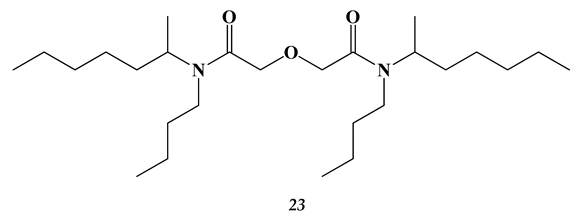
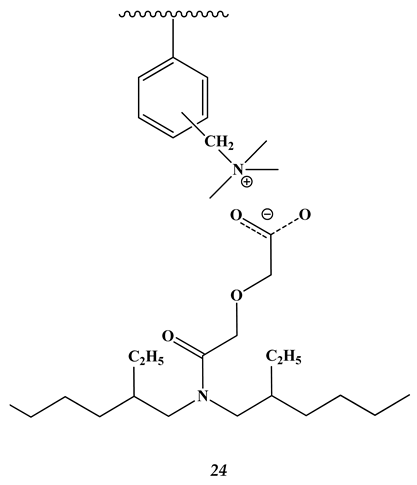

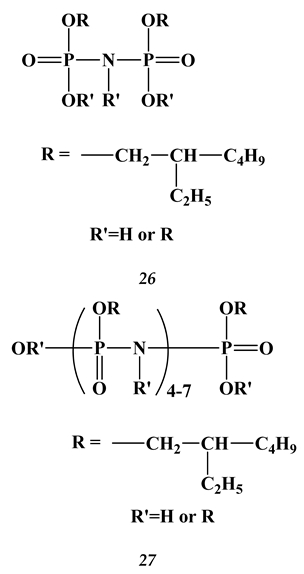
2.3. Heavy and Noble Metals

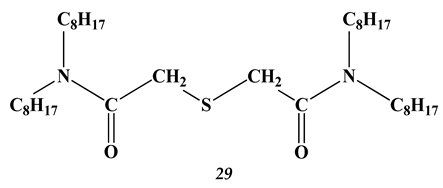

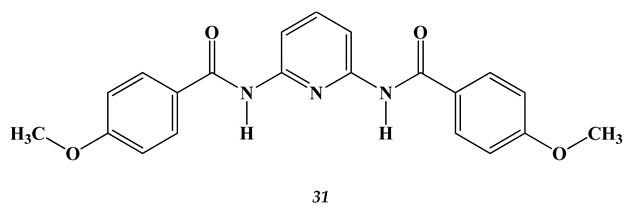

3. Amino Acids
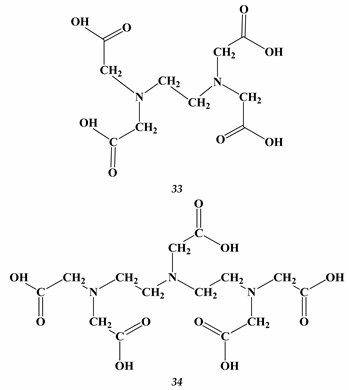
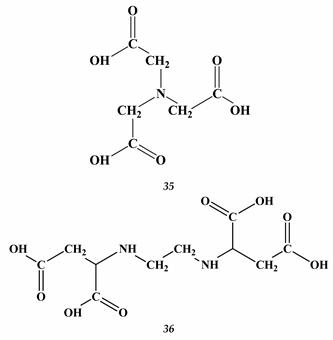
Heavy Metals

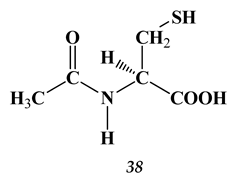
4. Shiff Bases (Azomethines) and Oximes
4.1. Actinides and Lanthanides

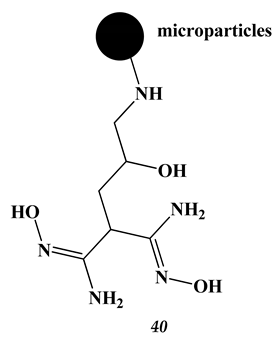
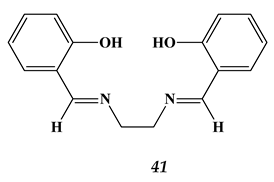
4.2. Heavy Metals
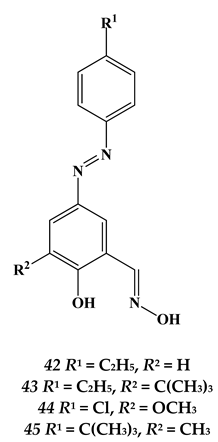
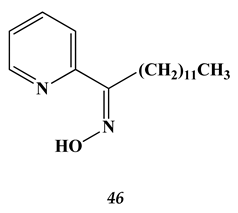
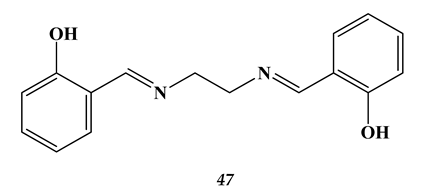
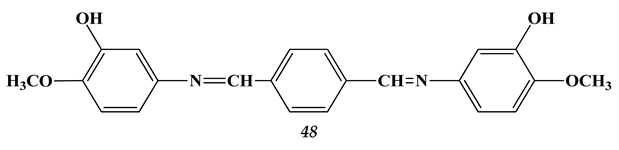
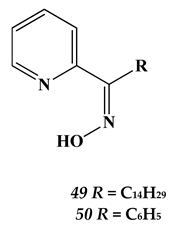
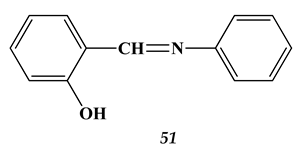
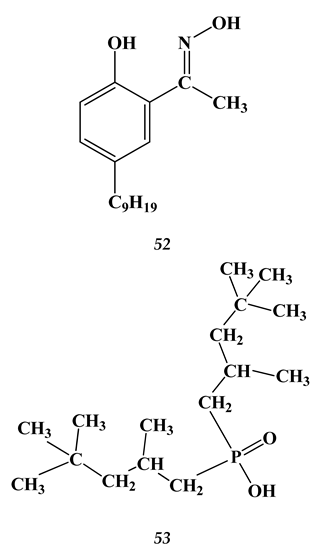
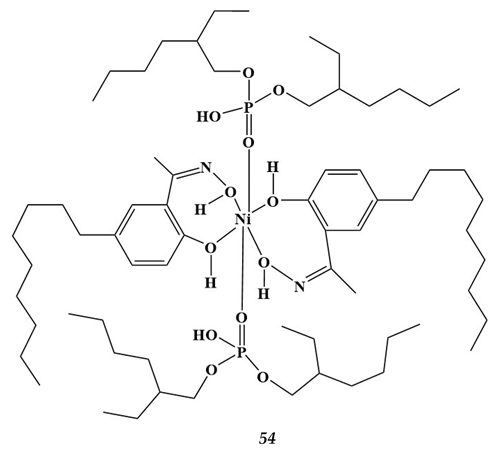

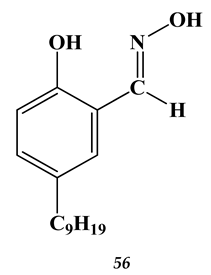
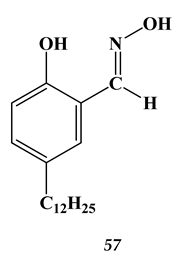
4.3. Noble Metals
5. Crown Ethers
5.1. Lithium
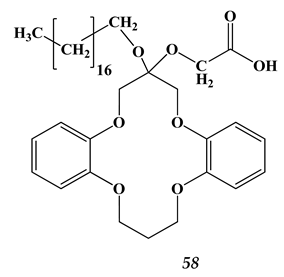
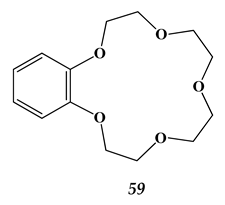
5.2. Heavy Metals and Strontium
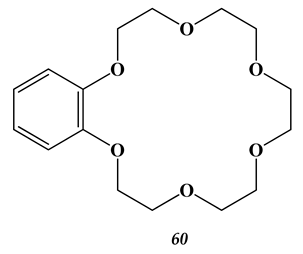
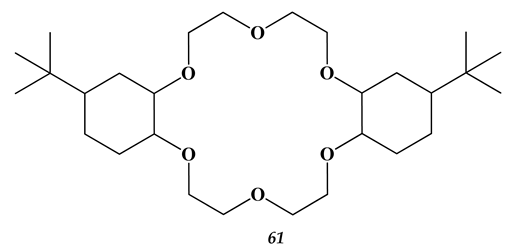
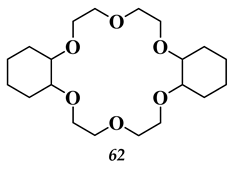
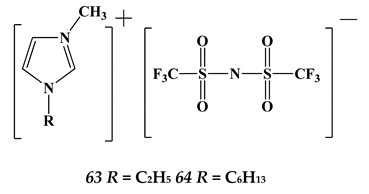
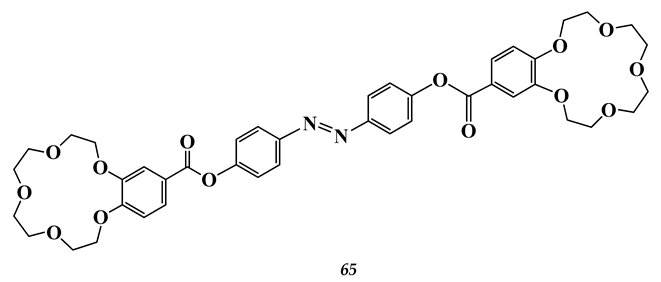
5.3. Elements of 7th Group of the Periodic Table of Elements
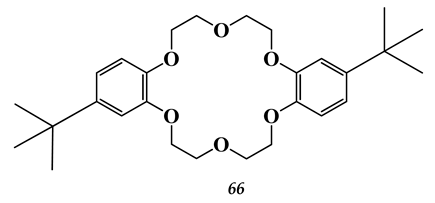
5.4. Noble Metals
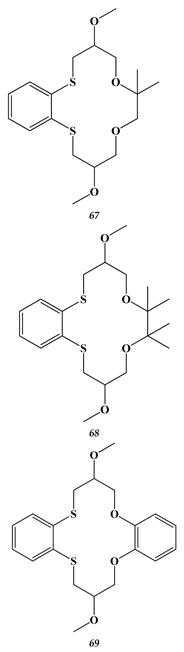
6. Calixarenes
6.1. Lanthanides and Actinides
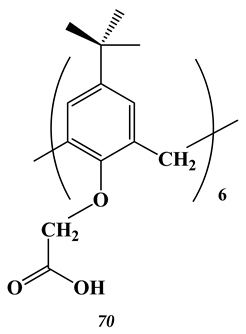
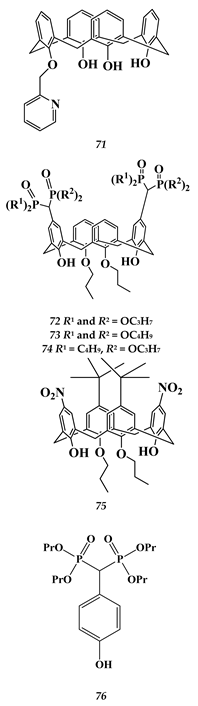
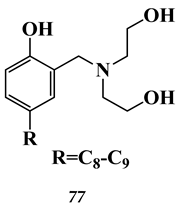
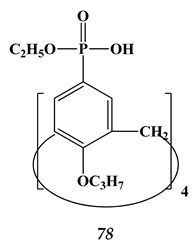
6.2. Noble Metals
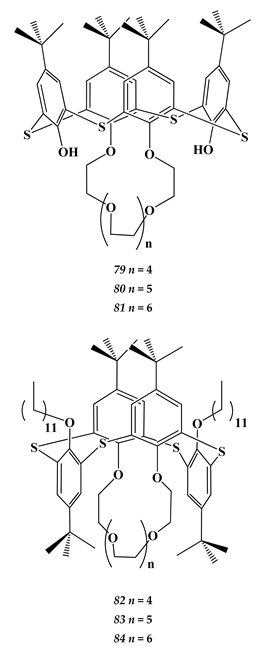

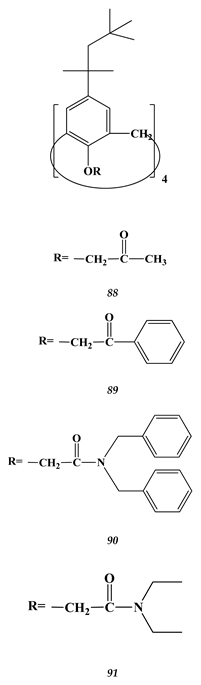
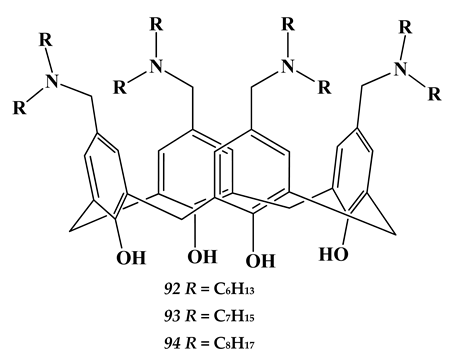
6.3. Heavy Metals

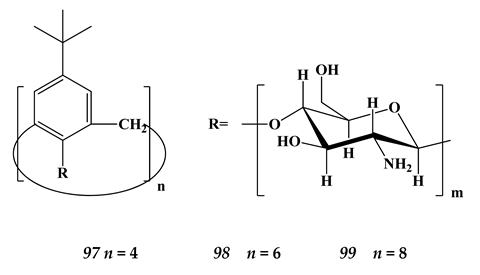
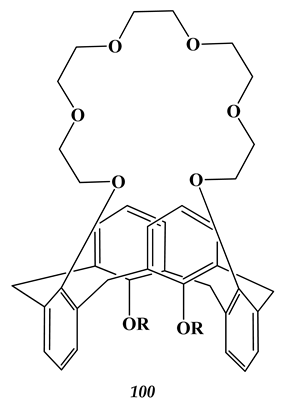
6.4. Strontium
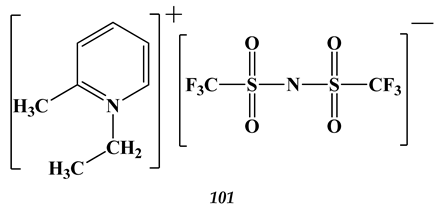
(n = 2)
(n = 2)
7. Phenanthroline Derivatives
7.1. Actinides and Lanthanides
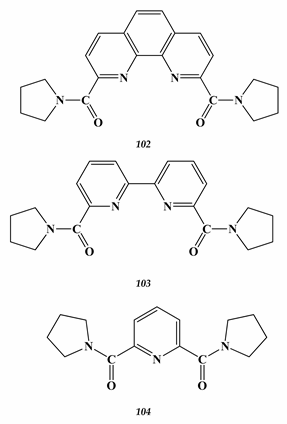

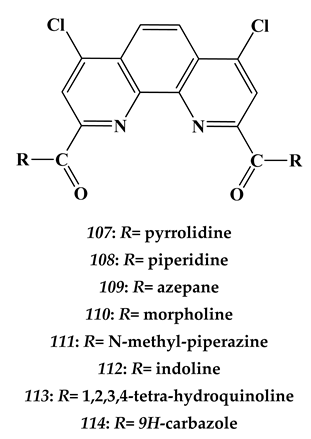
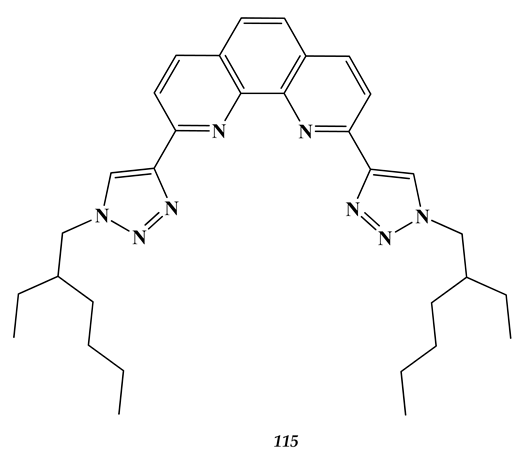
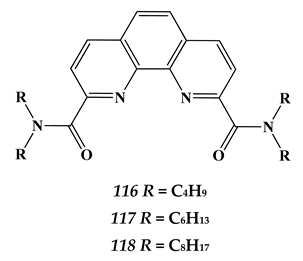
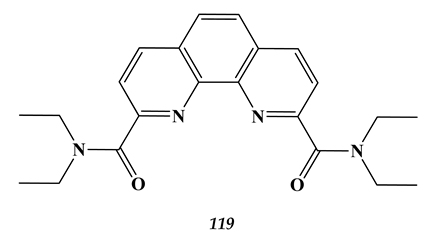

7.2. Palladium
8. Other Chelating Extractants
8.1. Lanthanides and Actinides
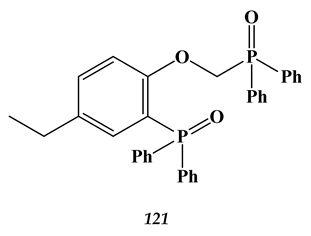
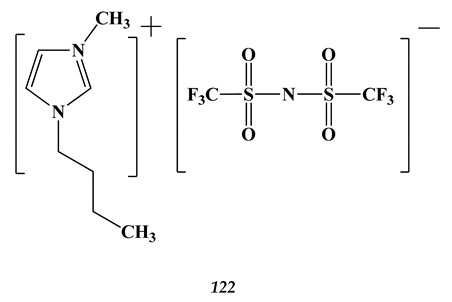
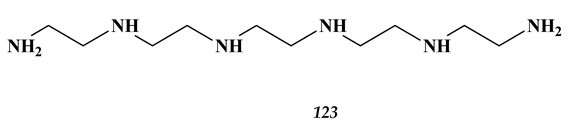
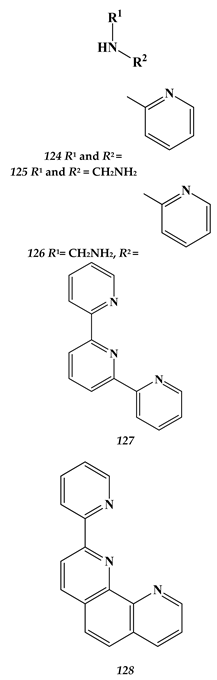

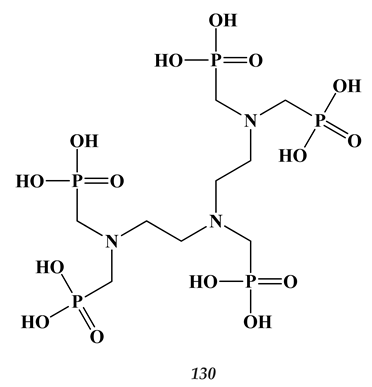
8.2. Heavy Metals
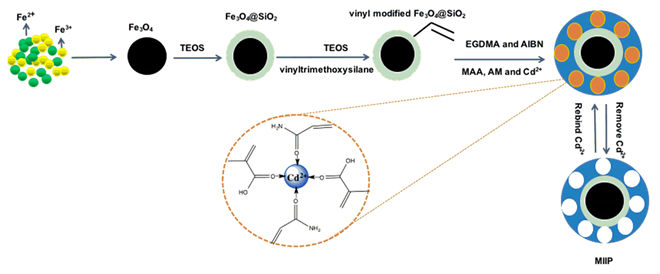
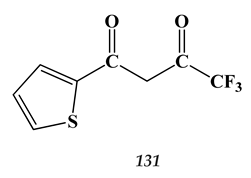
8.3. Platinum-Group Metals
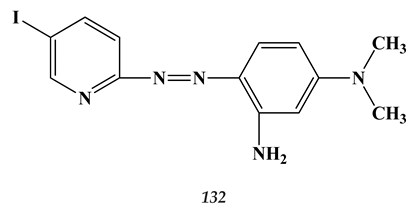

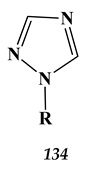
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Xu, Y.; Zhang, C.; Rong, H.; Zeng, G. New trends in removing heavy metals from wastewater. Biotechnology 2016, 100, 6509–6518. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2018, 17, 145–155. [Google Scholar] [CrossRef]
- Davidson, C.M. Methods for the Determination of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils. Environmental Pollution; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 22, pp. 97–140. [Google Scholar] [CrossRef]
- Soltani, F.; Darabi, H.; Aram, R.; Ghadiri, M. Leaching and solvent extraction purification of zinc from Mehdiabad complex oxide ore. Sci. Rep. 2021, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Kim, H.; Srivastava, R.R. Hydrometallurgical Recycling of Rare Earth Metal–Cerium from Bio-processed Residual Waste of Exhausted Automobile Catalysts. JOM 2021, 73, 19–26. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhou, L.P.; Yan, L.L.; Dong, Y.M.; Bai, Z.L.; Sun, X.Q.; Diwu, J.; Wang, S.; Bunzli, J.C.; Sun, Q.F. A supramolecular lanthanide separation approach based on multivalent cooperative enhancement of metal ion selectivity. Nat. Commun. 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudaev, P.A.; Kolpinskaya, N.A.; Chistyakov, E.M. Organophosphorous extractants for metals. Hydrometallurgy 2021, 201, 105558. [Google Scholar] [CrossRef]
- Hirayama, N. Chelate extraction of Metals into Ionic Liquids. Solvent Ext. Res. Dev. 2011, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zalloum, H.M.; Mubarak, M.S. Antioxidant Polymers: Metal Chelating Agents. In Antioxidant Polymers: Synthesis, Properties, and Applications; Cirilo, G., Iemma, F., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; pp. 87–114. [Google Scholar] [CrossRef]
- Memon, S.Q.; Memon, N.; Mallah, A.; Soomro, R.; Khuhawar, M.Y. Schiff Bases as Chelating Reagents for Metal Ions Analysis. Curr. Anal. Chem. 2014, 10, 393–417. [Google Scholar] [CrossRef]
- Laskar, M.A.; Siddiqui, S.; Islam, A. Reflection of the physiochemical characteristics of 1-(2-pyridylazo)-2-naphthol on the preconcentration of trace heavy metals. Crit. Rev. Anal. Chem. 2016, 46, 413–423. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Q.; Hou, X.; Fang, T. Supercritical Fluid Extraction of Metal Chelate: A Review. Crit. Rev. Anal. Chem. 2016, 47, 99–118. [Google Scholar] [CrossRef]
- Mahanty, B.; Kanekar, A.S.; Ansari, S.A.; Bhattacharyya, A.; Mohapatra, P.K. Separation of neptunium from actinides by monoamides: A solvent extraction study. Radiochim. Acta 2018, 107, 369–376. [Google Scholar] [CrossRef]
- Ren, P.; Li, Y.; Wang, Z.; Geng, Y.; Yu, T.; Hua, R. Extraction and separation of thorium(IV) and uranium(VI) with 4-oxaheptanediamide into ionic liquid system from aqueous solution. Chem. Pap. 2020, 74, 2049–2057. [Google Scholar] [CrossRef]
- Kumar, S.S.; Rao, A.; Yadav, K.K.; Lenka, R.K.; Singh, D.K.; Tomar, B.S. Selective removal of Am(III) and Pu(IV) from analytical waste solutions of quality control operations using extractant encapsulated polymeric beads. J. Radioanal. Nucl. Chem. 2020, 324, 375–384. [Google Scholar] [CrossRef]
- Gujar, R.B.; Mohapatra, P.; Verboom, W. Extraction of Np4+ and Pu4+ from nitric acid feeds using three types of tripodal diglycolamide ligands. Sep. Purif. Technol. 2020, 247, 116986. [Google Scholar] [CrossRef]
- Ansari, S.A.; Mohapatra, P.K.; Verma, P.K.; Leoncini, A.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Verboom, W. Highly Efficient Extraction of Trivalent f-Cations Using Several N -Pivot Tripodal Diglycolamide Ligands in an Ionic Liquid: The Role of Ligand Structure on Metal Ion Complexation. Eur. J. Inorg. Chem. 2020, 2, 191–199. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Boltoeva, M. Solvent extracvation of intra-lanthanides using a mixture of TBP and TODGA in ionic liquid. Hydrometallurgy 2020, 195, 105367. [Google Scholar] [CrossRef]
- Prathibha, T.; Swami, K.R.; Sriram, S.; Venkatesan, K.A. Interference of Zr(IV) during the extraction of trivalent Nd(III) from the aqueous waste generated from metallic fuel reprocessing. Radiochim. Acta 2020, 108, 543–554. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Wei, Z.; Zhou, Y.; Zhang, M.; Hou, H.; Tian, G.; He, H. Extraction behavior and third phase formation of neodymium (III) from nitric acid medium in N, N′-dimethyl-N, N′-dioctyl-3-oxadiglcolamide. J. Radional. Nucl. Chem. 2018, 318, 2087–2096. [Google Scholar] [CrossRef]
- Ravi, J.; Venkatesan, K.A.; Antony, M.P.; Srinivasan, T.G.; Vasudeva Rao, P.R. Tuning the diglycolamides for modifier-free minor actinide partitioning. J. Radioanal. Nucl. Chem. 2013, 295, 1283–1292. [Google Scholar] [CrossRef]
- Venkatesan, K.A.; Antony, M.P.; Srinivasan, T.G.; Vasudeva Rao, P.R. New unsymmetrical digycolamide ligands for trivalent actinide separation. Radiochim. Acta 2014, 102, 609–617. [Google Scholar] [CrossRef]
- Swami, K.R.; Venkatesan, K.A.; Selvan, B.R. Studies on the aggregation behaviour of radiolytically degraded tetra (2-ethyhexyl) diglycolamide in n-dodecane medium during the extraction of trivalent metal ions. J. Radioanal. Nucl. Chem. 2020, 325, 283–291. [Google Scholar] [CrossRef]
- Swami, K.R.; Venkatesan, K.A. Unraveling the role of phase modifiers in the extraction of Nd(III) from nitric acid medium in tetra-bis(2-ethylhexyl)diglycolamide in n-dodecane containing long chain aliphatic alcohols. J. Mol. Liq. 2019, 296, 111741. [Google Scholar] [CrossRef]
- Niu, Y.-N.; Ren, P.; Zhang, F.; Yan, Z.-Y. Solvent extraction of Eu3+ with 4-oxaheptanediamide into ionic liquid system. Sep. Sci. Technol. 2018, 53, 2750–2755. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Wang, X. Studies on the extraction of actinides, europium and technetium by diamide derivcatives. In Proceeding of the International Conference Global 2003, New Orleans, LA, USA, 16–20 September 2003; Volume 2, pp. 1915–1919. [Google Scholar]
- Parvathy, N.; Swami, K.R.; Prathibha, T.; Venkatesan, K.A. Antagonism in the aggregation behaviour of N,N,N′,N′-tetraoctyldiglycolamide in n-dodecane upon adding N, N-dioctylhydroxyacetamide during trivalent metal extraction. J. Mol. Liq. 2020, 317, 113940. [Google Scholar] [CrossRef]
- Atanassova, M. Investigation of synergism and selectivity using mixture of two neutral extractants in IL media for lanthanoids extraction. Sep. Purif. Technol. 2016, 169, 253–261. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ivanov, A.V.; Kharcheva, A.V.; Sumyanova, T.B.; Surkova, U.V.; Matveev, P.I.; Patsaeva, S.V. Effect of Heterocyclic Ring on Ln III Coordination, Luminescence and Extraction of Diamides of 2,2′-Bipyridyl-6,6′-Dicarboxylic Acid. Molecules 2020, 25, 62. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Hong, W.; Zhou, Y.; Pu, B.; Gong, A.; Xu, T.; Yang, Q.; Li, F.; Qiu, L.; Zhang, W.; et al. Extraction and back-extraction behaviors of 14 rare earth elements from sulfuric acid medium by TODGA. J. Rare Earth 2018, 36, 642–647. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Song, L.; Wang, X.; Xiao, Q.; Xu, H.; Feng, Q.; Ding, S. Extraction and complexation of trivalent rare earth elements with tetraalkyl diglycolamides. Inorg. Chim. Acta 2020, 513, 119928. [Google Scholar] [CrossRef]
- Sun, G.J.; Yang, J.H.; Yang, H.X.; Sun, G.X.; Cui, Y. Extraction study of rare earth elements with N,N′-dibutyl–N,N′-di (1-methylheptyl)-diglycolamide from hydrochloric acid. Nucl. Sci. Tech. 2016, 27, 75. [Google Scholar] [CrossRef]
- Case, M.E.; Fox, R.V.; Baek, D.L.; Mincher, B.J.; Wai, C.M. Extraction behavior of selected rare earth metals from acidic chloride media using tetrabutyl diglycolamide. Solvent Extr. Ion Exch. 2017, 35, 496–506. [Google Scholar] [CrossRef]
- Xu, D.; Shah, Z.; Sun, G.; Peng, X.; Cui, Y. Recovery of rare earths from phosphate ores through supported liquid membrane using N,N,N′,N′-tetraoctyl diglycol amide. Miner. Eng. 2019, 139, 105861. [Google Scholar] [CrossRef]
- Cui, H.; Feng, X.; Shi, J.; Liu, W.; Yan, N.; Rao, G.; Wang, W. A facile process for enhanced rare earth elements separation from dilute solutions using N,N-di (2-ethylhexyl)-diglycolamide grafted polymer resin. Sep. Purif. Technol. 2020, 234, 116096. [Google Scholar] [CrossRef]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Gergoric, M.; Barrier, A.; Retegan, T. Recovery of rare-earth elements from neodymium magnet waste using glycolic, maleic, and ascorbic acids followed by solvent extraction. J. Sustain. Metall. 2018, 5, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Tunsu, C.; Petranikova, M. Perspectives for the recovery of critical elements from future energy-efficient refrigeration materials. J. Clean. Prod. 2018, 197, 232–242. [Google Scholar] [CrossRef]
- Bredov, N.S.; Gorlov, M.V.; Esin, A.S.; Bykovskaya, A.A.; Kireev, V.V.; Sinegribova, O.A.; Ryabochenko, M.D. Linear 2-Ethylhexyl Imidophosphoric Esters as Effective Rare-Earth Element Extractants. Appl. Sci. 2020, 10, 1229. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.P.; Martins, M.E.; Ortet, O. Palladium (II) recovery from hydrochloric acid solutions by N,N′- dimethyl-N,N′-dibutylthiodiglycolamide. Metals 2015, 5, 2303–2315. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Morita, K.; Saeki, M.; Hisamatsu, S.; Yoshizuka, K. Precious metal extraction by N,N,N′,N′-tetraoctyl-thiodiglycolamide and its comparison with N,N,N′,N′-tetraoctyl-diglycolamide and methylimino-N,N′- dioctylacetamide. Hydrometallurgy 2017, 169, 576–584. [Google Scholar] [CrossRef]
- Bożejewicz, D.; Witt, K.; Kaczorowska, M.A.; Urbaniak, W.; Ośmiałowski, B. The Application of 2,6-Bis (4-Methoxybenzoyl)-Diaminopyridine in Solvent Extraction and Polymer Membrane Separation for the Recovery of Au (III), Ag (I), Pd (II) and Pt (II) Ions from Aqueous Solutions. Int. J. Mol. Sci. 2021, 22, 9123. [Google Scholar] [CrossRef]
- Bożejewicz, D.; Witt, K.; Kaczorowska, M.A.; Ośmiałowski, B. The copper (II) ions solvent extraction with a new compound: 2,6-bis (4-methoxybenzoyl)-diaminopyridine. Processes 2019, 7, 954. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab. J. Chem. 2020, 13, 5160. [Google Scholar] [CrossRef]
- Dolev, N.; Katz, Z.; Ludmer, Z.; Ullmann, A.; Brauner, N.; Goikhman, R. Natural amino acids as potential chelators for soil remediation. Environ. Res. 2020, 183, 109140. [Google Scholar] [CrossRef]
- Nair, N.M.; Varghese, G.K. Optimization of parameters for the extraction of Pb from lateritic soil using EDTA. SN Appl. Sci. 2020, 2, 1344. [Google Scholar] [CrossRef]
- Ayyanar, A.; Thatikonda, S. Enhanced Electrokinetic Removal of Heavy Metals from a Contaminated Lake Sediment for Ecological Risk Reduction. Soil Sediment Contam. 2021, 30, 12–34. [Google Scholar] [CrossRef]
- Sun, T.; Beiyuan, J.; Gielen, G.; Mao, X.; Song, Z.; Xu, S.; Ok, Y.S.; Rinklebe, J.; Liu, D.; Hou, D.; et al. Optimizing extraction procedures for better removal of potentially toxic elements during EDTA-assisted soil washing. J. Soils Sediments 2020, 20, 3417–3426. [Google Scholar] [CrossRef]
- Alpaslan, O.; Yaras, A.; Arslanoglu, H. A Kinetic Model for Chelating Extraction of Metals from Spent Hydrodesulphurization Catalyst by Complexing Agent. Trans. Indian Inst. Met. 2020, 73, 1925–1937. [Google Scholar] [CrossRef]
- Sharma, S.; Gautam, A.; Gautam, S. A Greener Approach to Extract Copper from Fertilizer Industry Spent Catalyst. Arab. J. Sci. Eng. 2020, 45, 7529–7538. [Google Scholar] [CrossRef]
- Orr, R.; Hocking, R.K.; Pattison, A.; Nelson, P.N. Extraction of metals from mildly acidic tropical soils: Interactions between chelating ligand, pH and soil type. Chemosphere 2020, 248, 126060. [Google Scholar] [CrossRef]
- Hughes, D.L.; Afsar, A.; Laventine, D.M.; Shaw, E.J.; Harwood, L.M.; Hodson, M.E. Metal removal from soil leachates using DTPA-functionalised maghemite nanoparticles, a potential soil washing technology. Chemosphere 2018, 209, 480–488. [Google Scholar] [CrossRef]
- Kobylinska, N.; Kostenko, L.; Khainakov, S.; Garcia-Granda, S. Advanced core-shell EDTA-functionalized magnetite nanoparticles for rapid and efficient magnetic solid phase extraction of heavy metals from water samples prior to the multi-element determination by ICP-OES. Microchim. Acta 2020, 187, 289. [Google Scholar] [CrossRef]
- Liang, F.; Guo, Z.H.; Men, S.H.; Xiao, X.Y.; Peng, C.; Wu, L.H.; Christie, P. Extraction of Cd and Pb from contaminated-paddy soil with EDTA, DTPA, citric acid and FeCl3 and effects on soil fertility. J. Cent. S. Univ. 2019, 26, 2987. [Google Scholar] [CrossRef]
- Jani, Y.; Hogland, W. Chemical extraction of trace elements from hazardous fine fraction at an old glasswork dump. Chemosphere 2018, 195, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jeon, E.-K.; Kitae, B. Role of reducing agent in extraction of arsenic and heavy metals from soils by use of EDTA. Chemosphere 2016, 152, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Golmaei, M.; Kinnarinen, T.; Jernström, E.; Häkkinen, A. Extraction of hazardous metals from green liquor dregs by ethylenediaminetetraacetic acid. J. Environ. Manag. 2018, 212, 219–227. [Google Scholar] [CrossRef]
- Beiyuan, J.; Tsang, D.C.W.; Valix, M.; Baek, K.; Ok, Y.S.; Zhang, W.; Bolan, N.S.; Rinklebe, J.; Li, X.-D. Combined application of EDDS and EDTA for removal of potentially toxic elements under multiple soil washing schemes. Chemosphere 2018, 205, 178–187. [Google Scholar] [CrossRef]
- Shukla, M.; Baksi, B.; Mohanty, S.P.; Mahanty, B.; Mansi, A.; Rene, E.R.; Behera, S.K. Remediation of chromium contaminated soil by soil washing using EDTA and N-acetyl-L-cysteine as the chelating agents. Prog. Org. Coat. 2022, 165, 106704. [Google Scholar] [CrossRef]
- Al Zoubi, W. Solvent extraction of metal ions by use of Schiff bases. J. Coord. Chem. 2013, 66, 2264. [Google Scholar] [CrossRef]
- Hawkins, C.A.; Bustillos, C.G.; May, I.; Copping, R.; Nilsson, M. Water-soluble Schiff base-actinyl complexes and their effect on the solvent extraction of f-elements. Dalton Trans. 2016, 45, 15415–15426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, M.F.; Roux, J.C.; Guibal, E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Shiri-Yekta, Z. Removal of Th(IV) ion from wastewater using a proper Schiff base impregnated onto Amberlite XAD-4. Particul. Sci. Technol. 2020, 38, 495–504. [Google Scholar] [CrossRef]
- Karabörk, M.; Kirpik, H.; Sayin, K.; Köse, M. New diazo-containing phenolic oximes: Structural characterization, computational studies, and solvent extraction of Cu(II), Ni(II), and Zn(II) ions. Turk. J. Chem. 2019, 43, 197–212. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Krupa, M.; Wojciechowska, A.; Wojciechowska, I.; Olszanowski, A. Equilibrium studies of cobalt (II) extraction with 2-pyridineketoxime from mixed sulphate/chloride solution. J. Radioanal. Nucl. Chem. 2016, 307, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Witt, K.; Bożejewicz, D.; Kaczorowska, M.A. N,N’-Bis (salicylidene) ethylenediamine (Salen) as an Active Compound for the Recovery of Ni (II), Cu (II), and Zn (II) Ions from Aqueous Solutions. Membranes 2020, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Al Zoubi, W.; Kandil, F.; Chebani, M.K. Solvent extraction of chromium and copper using Schiff base derived from terephthaldialdehyde and 5-amino-2-methoxy-phenol. Arab. J. Chem. 2016, 9, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Mazarakioti, E.C.; Beobide, A.S.; Angelidou, V.; Efthymiou, C.G.; Terzis, A.; Psycharis, V.; Voyiatzis, G.A.; Perlepes, S.P. Modeling the Solvent Extraction of Cadmium (II) from Aqueous Chloride Solutions by 2-pyridyl Ketoximes: A Coordination Chemistry Approach. Molecules 2019, 24, 2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almi, S.; Bouzgou, M.; Adjal, F.; Barkat, D. Methyl-isobutyl ketone and 1-octanol as synergistic agents and phase modifiers in solvent extraction of cobalt (II) by the N-(2-hydroxybenzylidene) aniline from sulfate medium. Inorg. Nano-Met. Chem. 2020, 50, 8–15. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, W.; Yang, L.; Huang, S.; Xu, Z.; Ji, Z.; Li, Y.; Hu, Y. Separation and recovery of heavy metals from concentrated smelting wastewater by synergistic solvent extraction using a mixture of 2-hydroxy-5- nonylacetophenone oxime and bis (2,4,4-trimethylpentyl)-phosphinic acid. Solvent Extr. Ion Exch. 2018, 36, 175–190. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, L.; Huang, S.; Xu, Z.; Li, Y.; Wang, W. Synergistic solvent extraction of nickel by 2-hydroxy-5-nonylacetophenone oxime mixed with neodecanoic acid and bis (2-ethylhexyl) phosphoric acid: Stoichiometry and structure investigation. Miner. Eng. 2019, 132, 284–292. [Google Scholar] [CrossRef]
- Reis, M.T.A.; Ismael, M.R.C.; Wojciechowska, A.; Wojciechowska, I.; Aksamitowski, P.; Wieszczycka, K.; Carvalho, J.M. Zinc (II) recovery using pyridine oxime-ether–novel carrier in pseudo-emulsion hollow fiber strip dispersion system. Sep. Purif. Technol. 2019, 223, 168–177. [Google Scholar] [CrossRef]
- Xiao, B.Q.; Jiang, F.; Peng, J.H.; Ju, S.H.; Zhang, L.H.; Yin, S.H.; Zhang, L.B.; Xu, L.; Tian, S.H. Optimization study of operation parameters for extracting Cu2+ from sulfuric solution containing Co2+. Arab. J. Sci. Eng. 2018, 43, 2145. [Google Scholar] [CrossRef]
- Jiang, F.; Yin, S.; Zhang, L.; Peng, J.; Ju, S.; Miller, J.D.; Wang, X. Solvent extraction of Cu (II) from sulfate solutions containing Zn (II) and Fe (III) using an interdigital micromixer. Hydrometallurgy 2018, 177, 116–122. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Huang, J.; Zheng, Q.; Liu, H. Extraction of vanadium (V) from a vanadium-bearing shale leachate through bifunctional coordination in Mextral 984H extraction system. Sep. Purif. Technol. 2022, 288, 120452. [Google Scholar] [CrossRef]
- Witt, K.; Kaczorowska, M.A.; Bożejewicz, D.; Urbaniak, W. Efficient Recovery of Noble Metal Ions (Pd2+, Ag+, Pt2+, and Au3+) from Aqueous Solutions Using N,N’-Bis (salicylidene) ethylenediamine (Salen) as an Extractant (Classic Solvent Extraction) and Carrier (Polymer Membranes). Membranes 2021, 11, 863. [Google Scholar] [CrossRef]
- Elizalde, M.P.; del Sol Rúa, M.; Menoyo, B. Palladium Extraction from Nitric Acid Solutions by LIX 84 and LIX 860-I. Solvent Extr. Ion Exc. 2019, 37, 411–421. [Google Scholar] [CrossRef]
- Torrejos, R.E.; Nisola, G.M.; Song, H.S.; Han, J.W.; Lawagon, C.P.; Seo, J.G.; Koo, S.; Kim, H.; Chung, W.J. Liquid-liquid extraction of lithium using lipophilic dibenzo-14-crown-4 ether carboxylic acid in hydrophobic room temperature ionic liquid. Hydrometallurgy 2016, 164, 362–371. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, M.; Yao, Y.; Wang, H.; Tong, B.; Zhao, Z. A novel approach for the selective extraction of Li+ from the leaching solution of spent lithium-ion batteries using benzo-15-crown-5-ether as extractant. Sep. Purif. Technol. 2020, 237, 116325. [Google Scholar] [CrossRef]
- Kudo, Y.; Nakamori, T.; Numako, C. Pb (II) Extraction with Benzo-18-Crown-6 Ether into Benzene under the Co-Presence of Cd (II) Nitrate in Water. Inorganics 2018, 6, 77. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Ye, G.; Wang, S.; Duan, W.; Wang, J.; Chen, J. Solvent extraction of strontium from nitric acid medium by di-tert-butyl cyclohexano-18-crown-6 in n-octanol: Extraction behavior and flowsheet demonstration. Solvent Extr. Ion. Exc. 2013, 31, 731–742. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, Y.; Zhou, Y.; Jiao, C.; Zhang, M.; Hou, H.; Liu, W. The extraction of Sr2+ with dicyclohexano-18-crown-6 in conventional organic solvent and ionic liquid diluents. J. Serb. Chem. Soc. 2020, 85, 909–922. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wang, X.; Zhao, S.; He, P.; Luo, T.; Jiang, J.; Cai, J.; Xu, H. A New Photoresponsive Bis (Crown Ether) for Extraction of Metal Ions. Chem. Sel. 2019, 4, 10316–10319. [Google Scholar] [CrossRef]
- Favre-Réguillon, A.; Draye, M.; Cote, G.; Czerwinsky, K.R. Insights in uranium extraction from spent nuclear fuels using dicyclohexano-18-crown-6–Fate of rhenium as technetium homolog. Sep. Purif. Technol. 2018, 209, 338–342. [Google Scholar] [CrossRef]
- Popova, N.N.; Tananaev, I.G.; Rovnyi, S.I.; Myasoedov, B.F. Technetium: Behaviour during processing of irradiated fuel and in environmental objects. Rus. Chem. Rev. 2003, 72, 115–137. [Google Scholar] [CrossRef]
- Sharma, J.N.; Sinharoy, P.; Kharwandikar, B.; Thorat, V.S.; Tessy, V.; Kaushik, C.P. Process for separation of technetium from alkaline low level waste using di-tert-butyldibenzo-18-crown-6+ isodecyl alcohol/n-dodecane solvent. Sep. Purif. Technol. 2018, 207, 416–419. [Google Scholar] [CrossRef]
- Kudo, Y.; Ikeda, S.; Morioka, S.; Tomokata, S. Silver (I) extraction with benzo-18-crown-6 ether from water into 1, 2-dichloroethane: Analyses on ionic strength of the phases and their equilibrium potentials. Inorganics 2017, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Torrejos, R.E.C.; Nisola, G.M.; Min, S.H.; Han, J.W.; Lee, S.P.; Chung, W.J. Highly selective extraction of palladium from spent automotive catalyst acid leachate using novel alkylated dioxa-dithiacrown ether derivatives. J. Ind. Eng. Chem. 2020, 89, 428–435. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, D.; He, S.; Feng, J.; Tesfay, A.R.; Liu, C.; Yang, Z.; Shi, L.; Li, J. Reactive extraction of europium (III) and neodymium (III) by carboxylic acid modified calixarene derivatives: Equilibrium, thermodynamics and kinetics. Sep. Purif. Technol. 2017, 188, 250–259. [Google Scholar] [CrossRef]
- Smirnov, I.V.; Stepanova, E.S.; Drapailo, A.B.; Kalchenko, V.I. Extraction of americium and europium with functionalized calixarenes from alkaline solutions. Radiochemistry 2016, 58, 42–51. [Google Scholar] [CrossRef]
- Lu, Y.; Liao, W. Extraction and separation of trivalent rare earth metal ions from nitrate medium by p- phosphonic acid calix[4]arene. Hydrometallurgy 2016, 165, 300–305. [Google Scholar] [CrossRef]
- Muravev, A.; Yakupov, A.; Gerasimova, T.; Nugmanov, R.; Trushina, E.; Babaeva, O.; Nizameeva, G.; Syakaev, V.; Katsyuba, S.; Selektor, S.; et al. Switching Ion Binding Selectivity of Thiacalix [4] arene Monocrowns at Liquid–Liquid and 2D-Confined Interfaces. Int. J. Mol. Sci. 2021, 22, 3535. [Google Scholar] [CrossRef]
- Kim, J.Y.; Morisada, S.; Kawakita, H.; Ohto, K. Comparison of interfacial behavior and silver extraction kinetics with various types calix[4]arene derivatives at heterogeneous liquid-liquid interfaces. J. Chromatogr. A 2018, 1558, 107–114. [Google Scholar] [CrossRef]
- Yamada, M.; Kaneta, Y.; Gandhi, M.R.; Kunda, U.; Shibayama, A. Calix [4] arene-Based Amino Extractants Containing n-Alkyl Moieties for Separation of Pd (II) and Pt (IV) from Leach Liquors of Automotive Catalysts. Metals 2018, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Kaneta, Y.; Gandhi, M.R.; Kunda, U.M.R.; Shibayama, A. Recovery of Pd (II) and Pt (IV) from leach liquors of automotive catalysts with calixarene-based di-n-alkylamino extractants in saturated hydrocarbon diluents. Hydrometallurgy 2019, 184, 103. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Sathuluri, R.R.; Iwasaki, W.; Morisada, S.; Kawakita, H.; Ohto, K.; Miyazaki, M.; Jimina. Microfluidic reactor for Pb (II) ion extraction and removal with an amide derivative of calix [4] arene supported by spectroscopic studies. Microchem. J. 2018, 142, 377–384. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Z. Extraction of heavy metals, dichromate anions and rare metals by new calixarene-chitosan polymers. J. Inorg. Organomet. Polym. 2018, 28, 962–967. [Google Scholar] [CrossRef]
- Pathak, S.; Jayabun, S.; Boda, A.; Musharaf Ali, S.; Sengupta, A. Experimental and theoretical insight into the extraction mechanism, kinetics, thermodynamics, complexation and radiolytic stability of novel calix crown ether in ionic liquid with Sr2+. J. Mol. Liq. 2020, 316, 113864. [Google Scholar] [CrossRef]
- Meng, R.; Xu, L.; Yang, X.; Sun, M.; Xu, C.; Borisova, N.E.; Zhang, X.; Lei, L.; Xiao, C. Influence of a N-Heterocyclic Core on the Binding Capability of N,O-Hybrid Diamide Ligands toward Trivalent Lanthanides and Actinides. Inorg. Chem. 2021, 60, 8754. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Ye, G.; Xu, C.; Chen, J.; Zhang, X.; Lei, L.; Xiao, C. Unraveling the complexation mechanism of actinide (iii) and lanthanide (iii) with a new tetradentate phenanthroline-derived phosphonate ligand. Inorg. Chem. Front. 2020, 7, 1726. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Li, Y.; Wei, P.; Sun, T.; Xiao, C.; Cheng, J.; Xu, C. Selective Separation and Complexation of Trivalent Actinide and Lanthanide by a Tetradentate Soft–Hard Donor Ligand: Solvent Extraction, Spectroscopy, and DFT Calculations. Inorg. Chem. 2019, 58, 4420. [Google Scholar] [CrossRef] [PubMed]
- Lemport, P.S.; Matveev, P.I.; Yatsenko, A.V.; Evsiunina, M.V.; Petrov, V.S.; Tarasevich, B.N.; Roznyatovsky, V.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Zhokhov, S.S.; et al. The impact of alicyclic substituents on the extraction ability of new family of 1,10-phenanthroline-2,9-diamides. RSC Adv. 2020, 10, 26022. [Google Scholar] [CrossRef]
- Zsabka, P.; Opsomer, T.; Van Hecke, K.; Dehaen, W.; Wilden, A.; Modolo, G.; Verwerft, M.; Cardinaels, T. Solvent extraction studies for the separation of trivalent actinides from lanthanides with a triazole-functionalized 1,10-phenanthroline extractant. Solvent Extr. Ion Exch. 2020, 38, 719. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, L.; Chai, Z.; Shi, W. Towards understanding the correlation between UO22+ extraction and substitute groups in 2,9-diamide-1, 10-phenanthroline. Sci. China Chem. 2018, 61, 1285. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, L.; Chai, Z.; Shi, W. A new solvent system containing N,N’-diethyl-N,N’-ditolyl-2,9-diamide-1,10-phenanthroline in 1-(trifluoromethyl)-3-nitrobenzene for highly selective UO22+ extraction. Sep. Purif. Technol. 2016, 168, 232. [Google Scholar] [CrossRef]
- Xiao, Q.; Song, L.; Wang, X.; Xu, H.; He, L.; Li, Q.; Ding, S. Highly Efficient Extraction of Palladium (II) in Nitric Acid Solution by a Phenanthroline-Derived Diamide Ligand. Sep. Purif. Technol. 2021, 280, 119805. [Google Scholar] [CrossRef]
- Kovalenko, O.; Baulin, V.; Baulin, D.; Tsivadze, A. Solvent-Impregnated Resins Based on the Mixture of (2-Diphenylphosphoryl)-4-ethylphenoxy) methyl) diphenylphosphine Oxide and Ionic Liquid for Nd (III) Recovery from Nitric Acid Media. Molecules 2021, 26, 2440. [Google Scholar] [CrossRef]
- Cristiani, C.; Bellotto, M.; Dotelli, G.; Latorrata, S.; Ramis, G.; Gallo Stampino, P.; Zubiani, E.M.L.; Finocchio, E. Rare Earths (La, Y, and Nd) Adsorption Behaviour towards Mineral Clays and Organoclays: Monoionic and Trionic Solutions. Minerals 2021, 11, 30. [Google Scholar] [CrossRef]
- Jeong, K.; Jeong, H.J.; Woo, S.M.; Bae, S. Prediction of binding stability of Pu (IV) and PuO2 (VI) by nitrogen tridentate ligands in aqueous solution. Int. J. Mol. Sci. 2020, 21, 2791. [Google Scholar] [CrossRef]
- Ahmad, H.; BinSharfan, I.I.; Khan, R.A.; Alsalme, A. 3D Nanoarchitecture of Polyaniline-MoS2 Hybrid Material for Hg (II) Adsorption Properties. Polymers 2020, 12, 2731. [Google Scholar] [CrossRef]
- Ahmad, H.; Alharbi, W.; BinSharfan, I.I.; Khan, R.A.; Alsalme, A. Aminophosphonic Acid Functionalized Cellulose Nanofibers for Efficient Extraction of Trace Metal Ions. Polymers 2020, 12, 12370. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, M.; Wu, Q.; Xu, Z.; Tian, X. Synthesis and application of novel magnetic ion-imprinted polymers for selective solid phase extraction of cadmium (II). Polymers 2017, 9, 360. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, M.; Mizuna, K.; Sazawa, K.; Okazaki, T.; Kuramitz, H.; Taguchi, S.; Hata, N. Organic Ion-Associate Phase Microextraction/Back-Microextraction for Preconcentration: Determination of Nickel in Environmental Water Using 2-Thenoyltrifluoroacetone via GF-AAS. Appl. Chem. 2021, 1, 130–141. [Google Scholar] [CrossRef]
- Han, Q.; Huo, Y.; Wu, J.; He, Y.; Yang, X.; Yang, L. Determination of ultra-trace rhodium in water samples by graphite furnace atomic absorption spectrometry after cloud point extraction using 2-(5-iodo-2-pyridylazo)-5-dimethylaminoaniline as a chelating agent. Molecules 2017, 22, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. New Polymer Inclusion Membranes in the Separation of Palladium, Zinc and Nickel Ions from Aqueous Solutions. Polymers 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
| Ligand | UO22+ | Cs (I) | Sr (II) |
|---|---|---|---|
| Compound 5 | 435 | 87,000 | 2200 |
| Compound 6 | 837 | 50,000 | 3100 |
| Compound 7 | 7300 | 70,000 | 10,000 |
| Extractant | Am (III) | U (VI) | Np (IV) | Pu (IV) | Sr (II) |
|---|---|---|---|---|---|
| 8 | 59.9 | 0.04 (1500) | 3.11 (19.2) | 3.86 (15.5) | 0.06 (1000) |
| 9 | 9.51 | 0.01 (951) | 1.47 (6.47) | 1.73 (5.50) | 0.04 (238) |
| 10 | 0.96 | 0.02 (48) | 1.01 (0.95) | 1.04 (0.92) | 0.02 (48) |
| 11 | 0.13 | 0.01 (13) | 0.66 (0.20) | 0.71 (0.18) | 0.01 (13) |
| 12 | 0.31 | 0.01 (31) | 0.79 (0.39) | 0.84 (0.37) | 0.02 (16) |
| Hardly Extracted Metals | Extractable Metals | Well-Extractable Metals | |||
|---|---|---|---|---|---|
| Metal | D | Metal | D | Metal | D |
| W | 0.080 | Pu | 8.260 | Pd | 470 |
| Ca | 0.045 | Re | 1.990 | Ag | 240 |
| Co | 0.035 | Bi | 1.870 | Hg | 110 |
| Ni | 0.027 | U | 1.610 | Au | 65 |
| Cu | 0.026 | Zr | 1.430 | ||
| Fe | 0.016 | Sb | 0.810 | ||
| Zn | 0.024 | Tc | 0.761 | ||
| Cd | 0.017 | Ta | 0.740 | ||
| Nd | 0.015 | Ir | 0.710 | ||
| Mg | 0.010 | Ru | 0.500 | ||
| Pt | 0.005 | Mo | 0.270 | ||
| V | 0.003 | Pb | 0.120 | ||
| Ions | Concentration (mg L−1) | Recovery (%) | |||
|---|---|---|---|---|---|
| Cu2+ | Cd2+ | Pb2+ | Cr3+ | ||
| Ca2+ | 5000 | 95 | 92 | 96 | 99 |
| Na+ | 1000 | 95 | 98 | 99 | 98 |
| Fe3+ | 500 | 89 | 87 | 95 | 91 |
| Mg2+ | 500 | 84 | 90 | 94 | 87 |
| Co2+ | 300 | 82 | 89 | 93 | 85 |
| NO3− | 5000 | 95 | 98 | 99 | 98 |
| SO42− | 5000 | 95 | 92 | 98 | 99 |
| PO43− | 5000 | 99 | 98 | 99 | 97 |
| F− | 1000 | 96 | 96 | 97 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudaev, P.; Chistyakov, E. Chelating Extractants for Metals. Metals 2022, 12, 1275. https://doi.org/10.3390/met12081275
Yudaev P, Chistyakov E. Chelating Extractants for Metals. Metals. 2022; 12(8):1275. https://doi.org/10.3390/met12081275
Chicago/Turabian StyleYudaev, Pavel, and Evgeniy Chistyakov. 2022. "Chelating Extractants for Metals" Metals 12, no. 8: 1275. https://doi.org/10.3390/met12081275






