Distribution of Rare Elements in Distillation Processing of Polymetallic Matte
Abstract
:1. Introduction
2. Equilibrium Distribution of Some Rare Metals under Vacuum Distillation of Mattes
2.1. Iron Chalcogenides
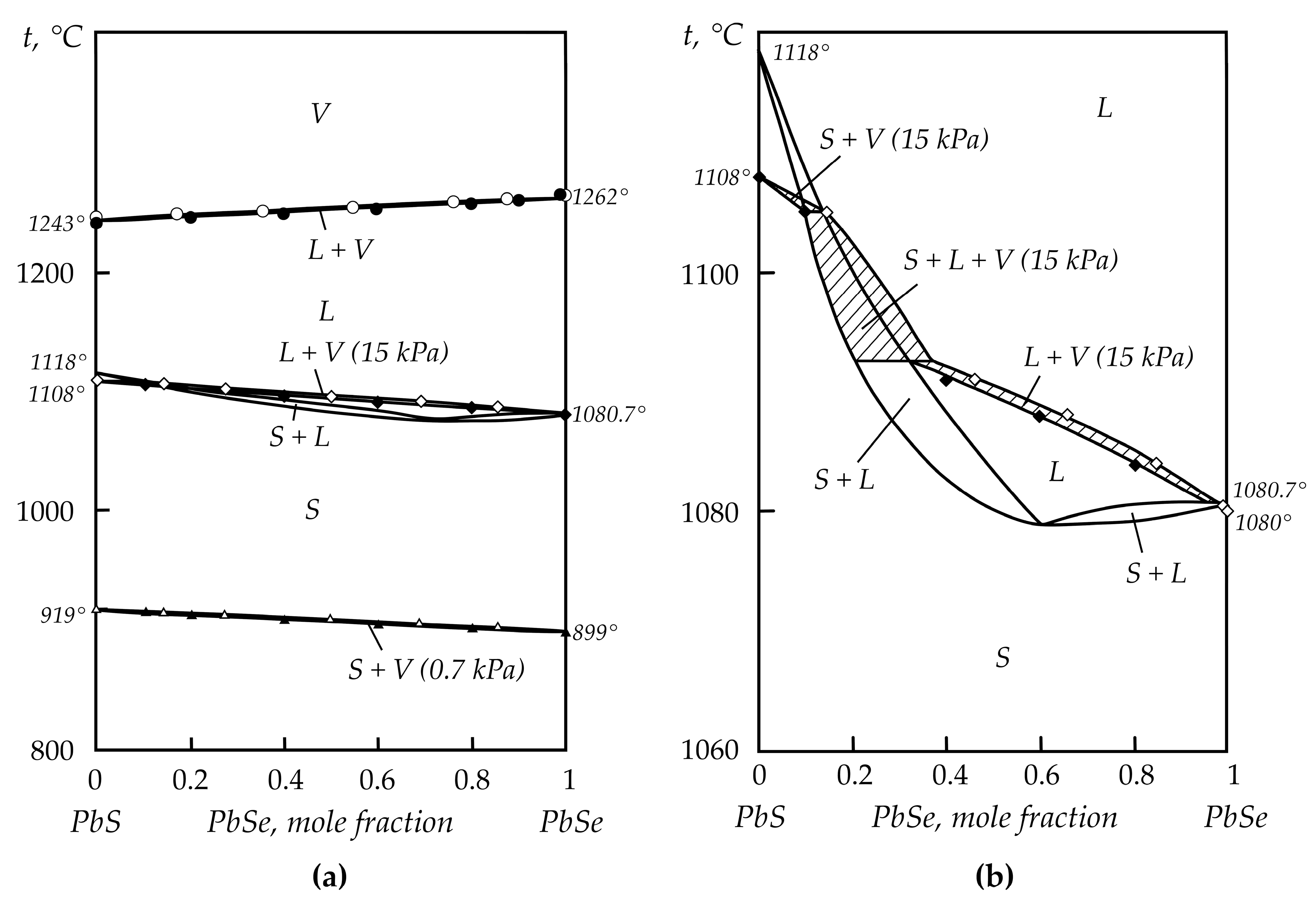
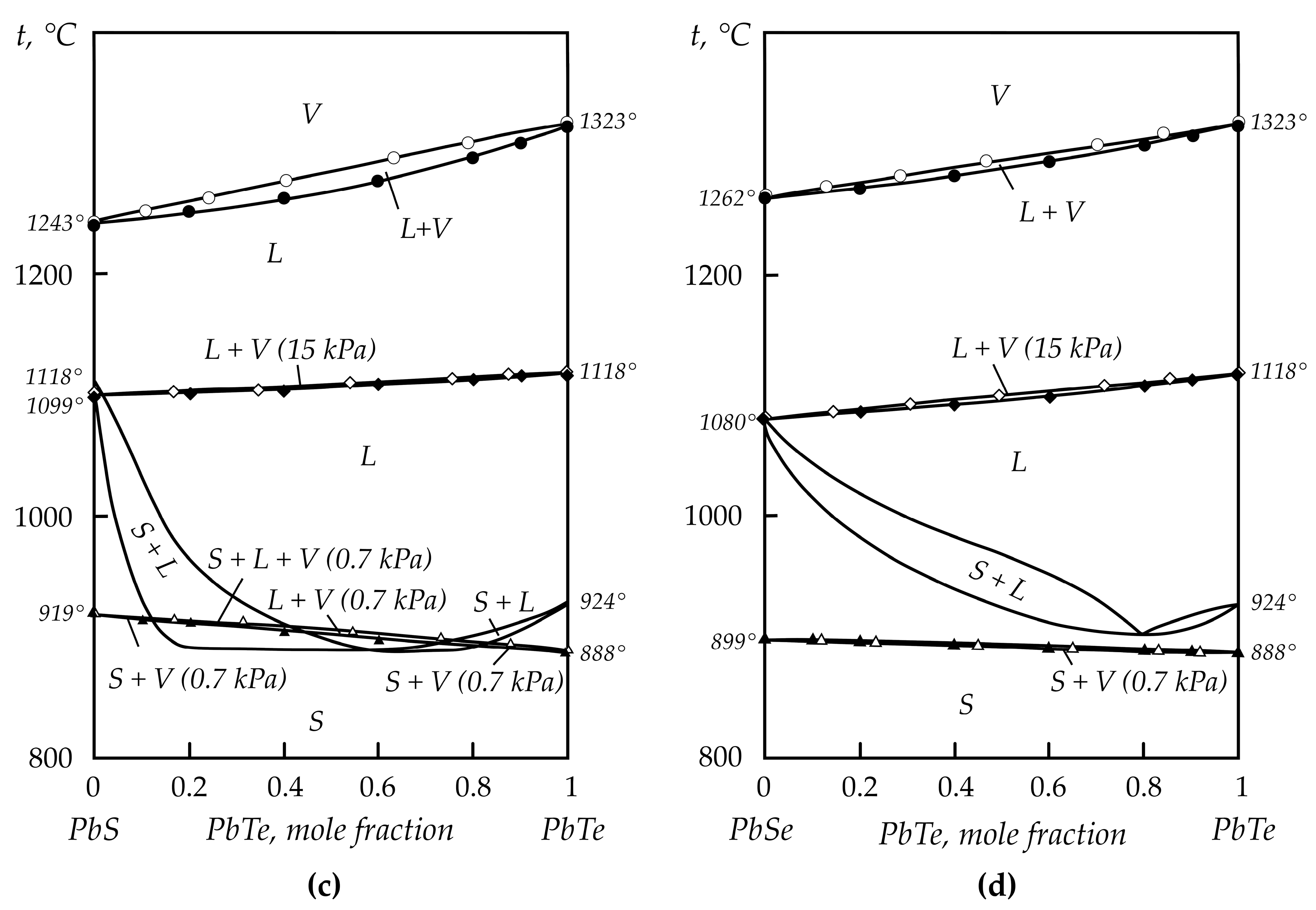
2.2. Lead Chalcogenides
2.3. Antimony Chalcogenides
2.4. Dual Systems of Antimony Trisulfide with Copper, Iron, and Lead Sulfides
3. Distribution of Rare and Semi-Metals during Vacuum-Thermal Processing
3.1. Materials
- Mattes from the settling tanks of shaft furnaces from the lead production, which are sent to reflective smelting to reduce the content of lead;
- Mattes from reduction shaft smelting of intermediate products and waste of the lead plant;
- Reflection smelting matte of the copper smelter;
- Electromelting matte of antimony smelter.
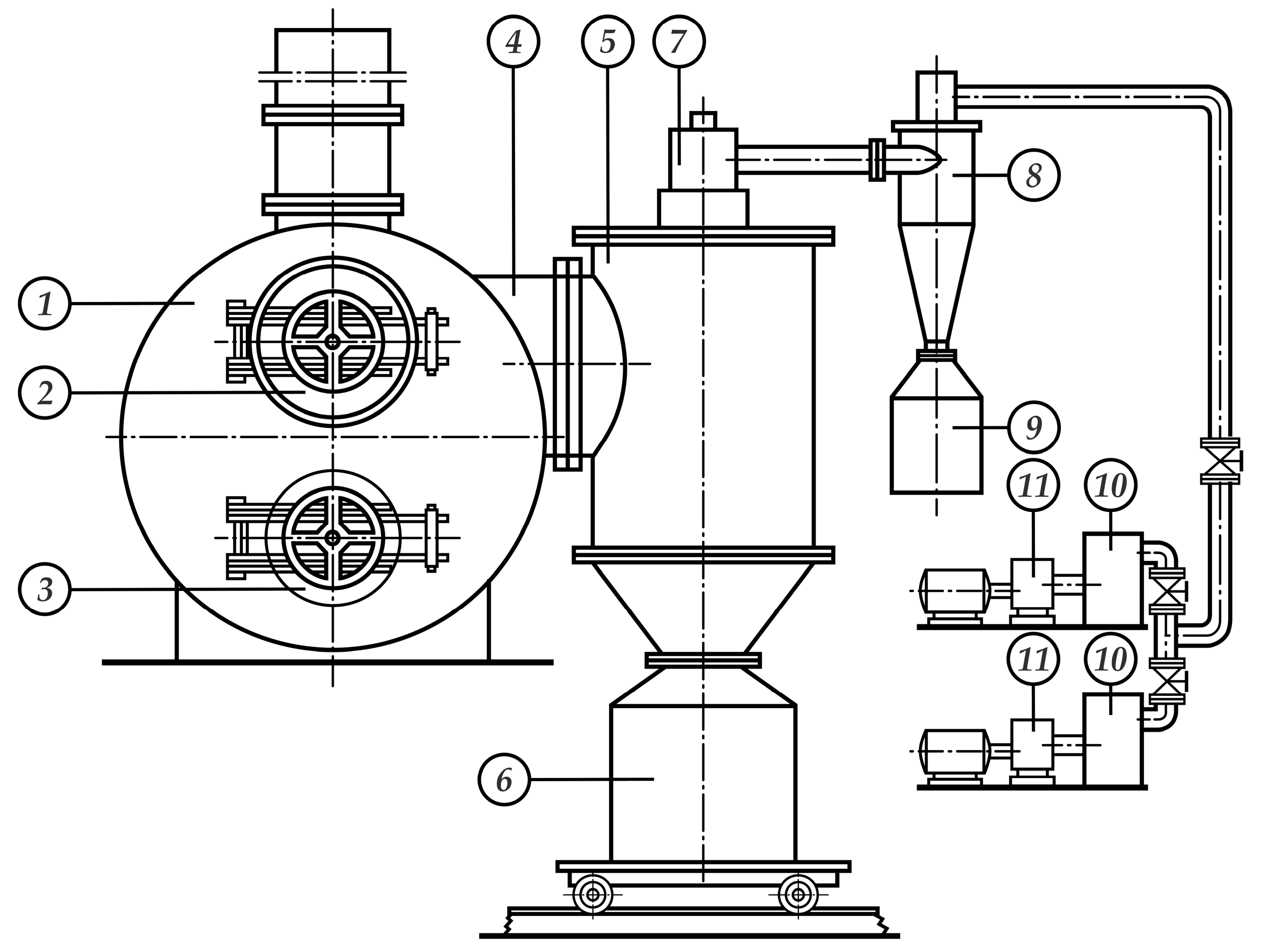
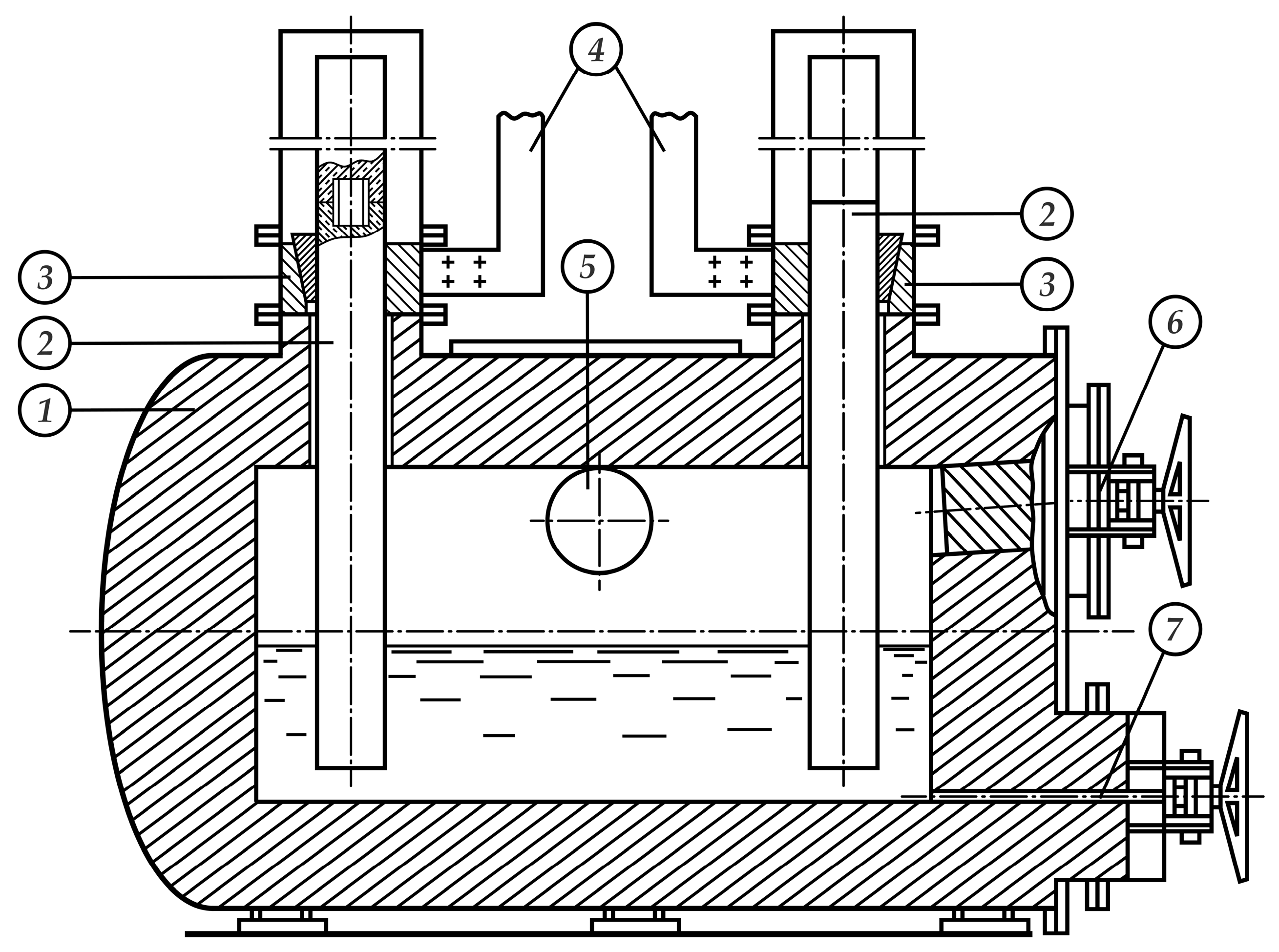
3.2. Technological Equipment for Distillation Processing of Polymetallic Matte
3.3. Operating Procedure
3.4. Technological Test Results for Matte Processing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Issakova, R.A.; Nesterov, V.N.; Chelokhsaev, L.S. Fundamentals of Polymetallic Raw Materials Vacuum Pyro-Selection; Nauka: Alma-Ata, Kazakhstan, 1976; 255p. [Google Scholar]
- Neustroev, V.I.; Karimov, K.A.; Naboichenko, S.S.; Kovyazin, A.A. Autoclave leaching of arsenic from copper concentrate and matte. Metallurgist 2015, 59, 177–179. [Google Scholar] [CrossRef]
- Dosmukhamedov, N.; Zholdasbay, E. The solubility of Cu, Pb, As, Sb of copper-lead matte in the slag. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2020, 312, 31–40. [Google Scholar] [CrossRef]
- Argyn, A.; Zoldasbay, E.; Dosmukhamedov, N. Improving the quality of converting products by the joint smelting of high-sulfur copper concentrate with copper-lead matte. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2023, 328, 50–58. [Google Scholar] [CrossRef]
- Dyussebekova, M.A.; Kenzhaliyev, B.K.; Kvyatkovskiy, S.A.; Sit’ko, E.A.; Nurkhadianto, D. The main reasons for increased copper losses with slags from vanyukov furnace. Metallugija 2021, 60, 309–312. [Google Scholar]
- Wright, S.; Sun, S.Y.; Jahanshahi, S. Rate of removal of lead from copper matte by submerged gas injection. Miner. Process. Extr. Metall. 2023, 13, 99–109. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Kvyatkovskiy, S.A.; Dyussebekova, M.A.; Semenova, A.S.; Nurhadiyanto, D. Analysis of existing technologies for depletion of dump slags of autogenous melting. Kompleks. Ispolz. Mineral. Syra Comp. Use Min. Res. 2022, 323, 23–29. [Google Scholar] [CrossRef]
- Minic, D.; Strbac, N.; Mihajlovic, I.; Zivkovic, Z. Thermal analysis and kinetics of the copper-lead matte roasting process. J. Therm. Anal. Calorim. 2005, 82, 383–388. [Google Scholar] [CrossRef]
- Sineva, S.; Shevchenko, M.; Shishin, D.; Hidayat, T.; Chen, J.; Hayes, P.C.; Jak, E. Phase equilibria and minor element distributions in complex copper/slag/matte systems. JOM 2020, 72, 3401–3409. [Google Scholar] [CrossRef]
- Volodin, V.N.; Trebukhov, S.A.; Kenzhaliyev, B.K.; Nitsenko, A.V.; Burabaeva, N.M. Melt–Vapor Phase Diagram of the Te–S System. Rus. J. Phys. Chem. A 2018, 92, 407–410. [Google Scholar] [CrossRef]
- Sohn, H.S.; Fukunaka, Y.; Oishi, T.; Sohn, H.Y.; Asaki, Z. Kinetics of As, Sb, Bi and Pb volatilization from industrial copper matte during Ar+O2 bubbling. Metall. Mater. Trans. B 2004, 35, 651–661. [Google Scholar] [CrossRef]
- Dosmukhamedov, N.K.; Zholdasbay, E.E.; Argyn, A.A.; Kurmanseitov, M.B. Behavior of Zn, Pb, As compounds during copper-zinc concentrate and matte melting in converters. Non-Ferr. Metal. 2020, 49, 11–18. [Google Scholar] [CrossRef]
- Volodin, V.N.; Issakova, R.A. Distillation Separation Processes of Sulfide and Metallic Melts: Theory and Technology; Tengri Ltd.: Karaganda, Kazakhstan, 2015; 260p. [Google Scholar]
- Maekawa, T.; Yokokawa, T.; Niwa, K. Enthalpies of mixing in the liquid state IV. Bi + Se and Sb + Se. J. Chem. Thermodyn. 1972, 4, 873–878. [Google Scholar] [CrossRef]
- Predel, B.; Piehl, J.; Pool, M.J. BeitragzurKenntnis der thermodynamischenEigenschaftenflüssiger Thallium-Selen-, Wismut-Selen- und Antimon- Selen-Legierungen. Int. J. Mater. Res. 1975, 66, 388–395. [Google Scholar] [CrossRef]
- Predel, B.; Gerdes, F.; Gerling, U. Berücksichtigung der Assoziation in der Dampfphase bei Aktivitätbestimmungen und Revision der Aktivitäten flüssiger Legierungen der Systeme Selen-Thallium, Selen-Wismut und Selen-Antimon. Int. J. Mater. Res. 1979, 70, 109–112. [Google Scholar] [CrossRef]
- Sullivan, C.L.; Prusaczyk, L.E.; Carlson, K.D. Molecules in the equilibrium vaporization of antimony sulfide and selenide. J. Chem. Phys. 1970, 53, 1289–1290. [Google Scholar] [CrossRef]
- Bagdavadze, J.; Chagelishvili, R.; Gagnidze, N.; Kandelaki, A.; Rasmadze, R.; Tsikaridze, Z. Study of high-temperature process for obtaining antimony sulfide and metallic Sb. Bull. Georg. Natl. Acad. Sci. 2013, 7, 75–79. [Google Scholar]
- Shi, C.; Li, N.; Zhang, W. Thermodynamic assessment of the Sb–S and In–S binary systems. Intern. J. Mater. Res. 2021, 112, 373–381. [Google Scholar] [CrossRef]
- Aliev, F.R.; Orujlu, E.N.; Babanly, D.M. Thermodynamic properties of the Sb2Te3 compound. Azerb. Chem. J. 2021, 4, 53–59. [Google Scholar] [CrossRef]
- Ilyasheva, N.A. Study of Cu2S–Sb2S3 system at 320–400 °C. Inorg. Mater. 1973, 9, 1677–1679. [Google Scholar]
- Ibragimov, Y.T.; Shendyapin, A.S.; Nesterov, V.N.; Khobdabergenov, R.Z. Vapor pressure in the Sb2S3–Cu2S system. Proc. Inst. Metall. Ore Benef. Acad. Sci. KazSSR 1977, 52, 61–65. [Google Scholar]
- Lee, Y.H.; Itagaki, K. Thermodynamic study of liquid Sb–S and Sb2S3–FeS systems by the use of a drop-calorimeter. Trans. Jpn. Inst. Met. 1986, 27, 987–995. [Google Scholar] [CrossRef]
- Dong, Z.; Li, L.; Xiong, H.; Liu, G.; Wang, Y.; Zhou, Z.; Xu, B.; Yang, B. Application of modified molecular interaction volume model for phase equilibrium of PbS–Sb2S3 system in vacuum distillation. Vacuum 2022, 20, 111067. [Google Scholar] [CrossRef]
- Rametani, H.; Yamauchi, C.; Murao, K.; Hayashida, M. A fundamental study on the vacuum treatment of molten matte and white metal. Trans. Jpn. Inst. Met. 1973, 14, 218–223. [Google Scholar] [CrossRef]
- Allaire, A.; Harris, R. Vacuum distillation of copper matte to remove lead, arsenic, bismuth, and antimony. Metall. Mater. Trans. B 1989, 20, 793–804. [Google Scholar] [CrossRef]
- Nitsenko, A.; Linnik, X.; Volodin, V.; Tuleutay, F.; Burabaeva, N.; Trebukhov, S.; Ruzakhunova, G. Phase transformation and tellurium recovery from technical copper telluride by oxidative-distillate roasting at 0.67 kPa. Metals 2022, 12, 1774. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, D.; Xiong, H.; Wang, C.; Ma, B.; Wei, L.; Chen, Y.; Huang, K. A vacuum distillation process for separation of antimony trisulfide and lead sulfide from jamesinite. Vacuum 2021, 188, 110172. [Google Scholar] [CrossRef]
- Nitsenko, A.; Volodin, V.; Linnik, X.; Burabaeva, N.; Tuleutai, F. Behavior of copper chalcogenides during vacuum-thermal processing. Metalurgija 2023, 62, 125–128. [Google Scholar]
- Richardson, F.D.; Jeffes, H.E. The thermodynamics of substances of interest in iron and steel making. J. Iron Steel Inst. 1952, 171, 165–168. [Google Scholar]
- Placente, V.; Scardala, P.; Fontana, D. Decomposition pressure and standard enthalpies of sublimation and formation of iron monoselenide. J. Alloys Compd. 1992, 189, 263–267. [Google Scholar] [CrossRef]
- Ipser, H.; Komarek, K.L. Thermodynamic properties of iron-tellurium alloys. Monatshefte Für Chem./Chem. Mon. 1974, 105, 1344–1361. [Google Scholar] [CrossRef]
- Yelagina, Y.I.; Abricosov, N.K. Study of the PbTe–PbSe system. Rep. Acad. Sci. USSR 1956, 111, 353–354. [Google Scholar]
- Simpson, D.R. The binary System PbS–PbSe. Econ. Geol. 1964, 59, 150–153. [Google Scholar] [CrossRef]
- Sokolov, V.V.; Dolgikh, V.A.; Pashinkin, A.S.; Novoselova, A.V. Saturated steam pressure in the PbTe–PbSe system. Inorg. Mat. 1969, 5, 279–282. [Google Scholar]


| Matte | Composition and Approximate Content of Minerals, Mass. % |
|---|---|
| Lead concentrate I | FeS2—40, PbS—40, ZnS—10, silicates—10, spinel grains |
| Lead concentrate II | PbS—50, ZnS—10, FeS2, CuFeS, silicates, Cu2S |
| Lead concentrate III | PbS—60, FeS2—20, ZnS—5, silicates—10, grains of chalcopyrite, bornite, chalcocite |
| Shared concentrate I | Galena, bornite, chalcopyrite |
| Shared concentrate II | PbS, ZnS, FeS2, carbonates |
| Shared concentrate III | ZnS—40, PbS—30, FeS2—10, Cu2S—5 |
| Copper–lead concentrate I | PbS—30, Cu2S—10, bornite —10, silicates—40, CuS, chalcopyrite—5, Cu2S—CuS solid solution |
| Copper–lead concentrate II | Cu2S—25, bornite—30, ZnS—3, chalcopyrite, Fe2O3 |
| Matte | Element Content, wt. % | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Fe | Sb | As | Cd | Bi | In | Ge | |
| Reflection smelting of the lead plant | 55.00 | 4.50 | 0.19 | 2.03 | 0.48 | 1 × 10−2 | (1–3) × 10−2 | 1 × 10−3 |
| Reduction smelting of the lead plant | 39.37 | 14.43 | 0.17 | 0.67 | – | 1 × 10−3 | – | – |
| Copper plant | 38.00 | 14.32 | 0.30 | 0.65 | 0.30 | – | – | – |
| Antimony production | – | 47.50 | 5.21 | 0.12 | – | 6 × 10−3 | – | – |
| Products | Mass | Sb | As | Cd | ||||
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||||
| Matte | 3600 | 100 | 0.19 | 100 | 2.03 | 100 | 0.48 | 100 |
| Received: | ||||||||
| Distillation residue | 2680 | 74.44 | 0.15 | 58.77 | 0.24 | 8.80 | 6 × 10−3 | 0.93 |
| Condensation | 870 | 24.17 | 0.32 | 40.70 | 5.60 | 66.67 | 1.90 | 95.66 |
| Cyclone dust and losses | 50 | 1.39 | - | 0.53 | - | 24.53 | - | 3.41 |
| Products | Mass | Bi | In | Ge | ||||
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||||
| Matte | 3600 | 100 | 0.19 | 100 | 2.03 | 100 | 0.48 | 100 |
| Received: | ||||||||
| Distillation residue | 2680 | 74.44 | 1 × 10−3 | 7.44 | 4 × 10−4 | 0.99 | 1 × 10−4 | 7.44 |
| Condensation | 870 | 24.17 | 3 × 10−2 | 72.50 | 0.11 | 88.61 | 3 × 10−3 | 72.50 |
| Cyclone dust and losses | 50 | 1.39 | - | 20.06 | - | 10.40 | - | 20.06 |
| Products | Mass | Sb | As | |||
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||
| Matte | 3450 | 100 | 0.17 | 100 | 0.67 | 100 |
| Received: | ||||||
| Distillation residue | 2520 | 73.04 | 0.11 | 47.26 | 0.18 | 19.62 |
| Condensation | 900 | 26.09 | 0.31 | 47.57 | 1.59 | 61.91 |
| Cyclone dust and losses | 30 | 0.87 | - | 5.17 | - | 18.47 |
| Products | Mass | Cd | Bi | |||
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||
| Matte | 3450 | 100 | 0.23 | 100 | 1 × 10−2 | 100 |
| Received: | ||||||
| Distillation residue | 2520 | 73.04 | 5 × 10−3 | 1.59 | 1 × 10−3 | 7.30 |
| Condensation | 900 | 26.09 | 0.73 | 82.80 | 3 × 10−2 | 78.26 |
| Cyclone dust and losses | 30 | 0.87 | - | 15.61 | - | 14.44 |
| Products | Mass | Sb | As | Cd | ||||
|---|---|---|---|---|---|---|---|---|
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||||
| Matte from the reduction smelting | 850 | 31.48 | 0.30 | 17.95 | 0.65 | 39.89 | 0.23 | 100 |
| Matte from the copper-smelting plant | 1850 | 68.52 | 0.63 | 82.05 | 0.45 | 60.11 | - | - |
| Total: | 2700 | 100 | 0.53* | 100 | 0.51 * | 100 | 7 × 10−2 * | 100 |
| Received: | ||||||||
| Distillation residue | 2255 | 83.52 | 6 × 10−2 | 9.53 | 0.13 | 21.17 | 4 × 10−3 | 1.28 |
| Condensation | 345 | 12.78 | 3.14 | 76.26 | 2.23 | 55.55 | 0.47 | 82.94 |
| Cyclone dust and losses | 100 | 3.70 | - | 14.21 | - | 23.28 | - | 15.78 |
| Products | Mass | Sb | As | Bi | ||||
|---|---|---|---|---|---|---|---|---|
| kg | % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | |
| Loaded: | ||||||||
| Matte | 230 | 100 | 5.21 | 100 | 0.12 | 100 | 6 ×10−3 | 100 |
| Received: | ||||||||
| Distillation residue | 201.4 | 87.57 | 0.55 | 9.24 | 5 × 10−2 | 36.49 | 7 × 10−4 | 10.22 |
| Condensation | 12.5 | 5.43 | 71.57 | 74.66 | 1.02 | 46.20 | 8 × 10−2 | 72.46 |
| Cyclone dust and losses | 16.1 | 7.00 | - | 16.10 | - | 17.31 | - | 17.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volodin, V.; Nitsenko, A.; Linnik, X.; Trebukhov, S. Distribution of Rare Elements in Distillation Processing of Polymetallic Matte. Metals 2023, 13, 1934. https://doi.org/10.3390/met13121934
Volodin V, Nitsenko A, Linnik X, Trebukhov S. Distribution of Rare Elements in Distillation Processing of Polymetallic Matte. Metals. 2023; 13(12):1934. https://doi.org/10.3390/met13121934
Chicago/Turabian StyleVolodin, Valeriy, Alina Nitsenko, Xeniya Linnik, and Sergey Trebukhov. 2023. "Distribution of Rare Elements in Distillation Processing of Polymetallic Matte" Metals 13, no. 12: 1934. https://doi.org/10.3390/met13121934






