Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Techniques
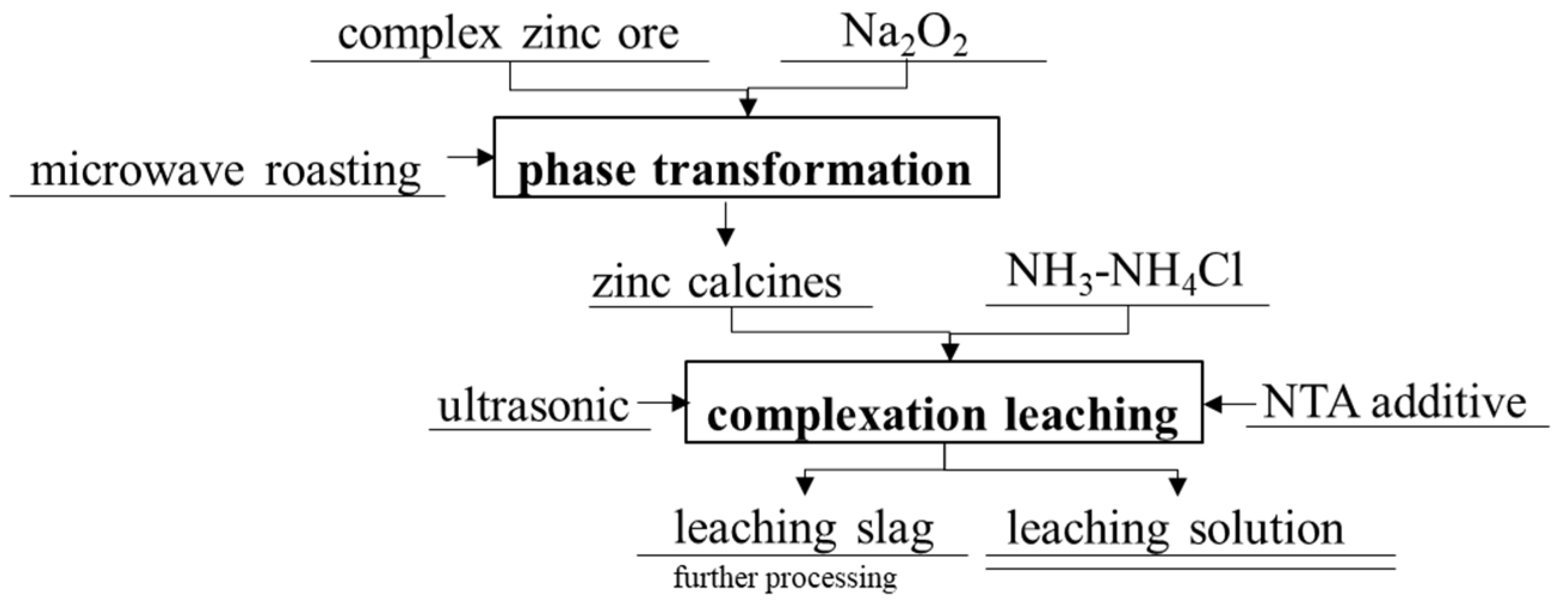
2.3. Experimental Method
2.4. Thermodynamic Simulation Method
3. Results and Discussion
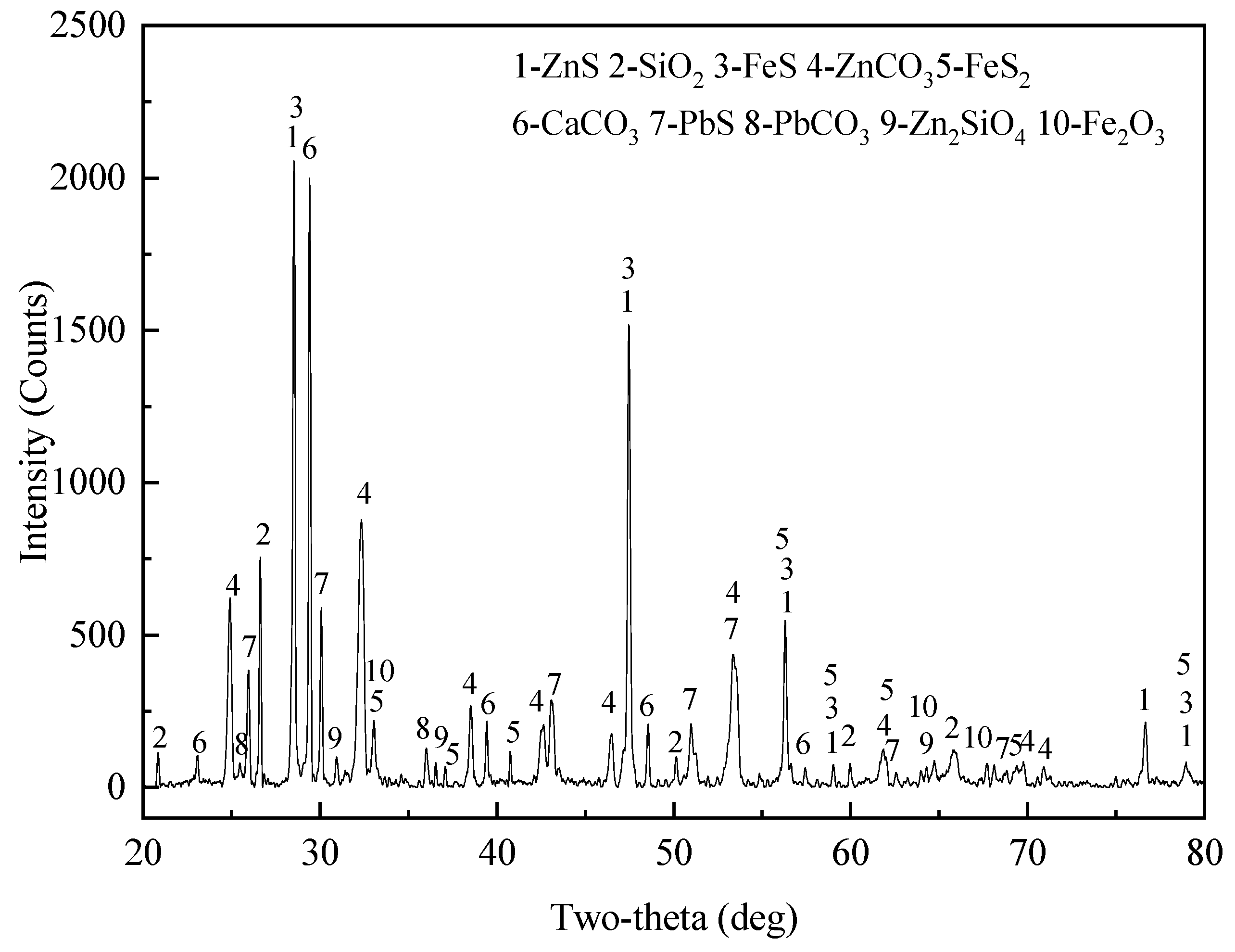
3.1. The Mineralogical Characteristics of Complex Zinc Ore
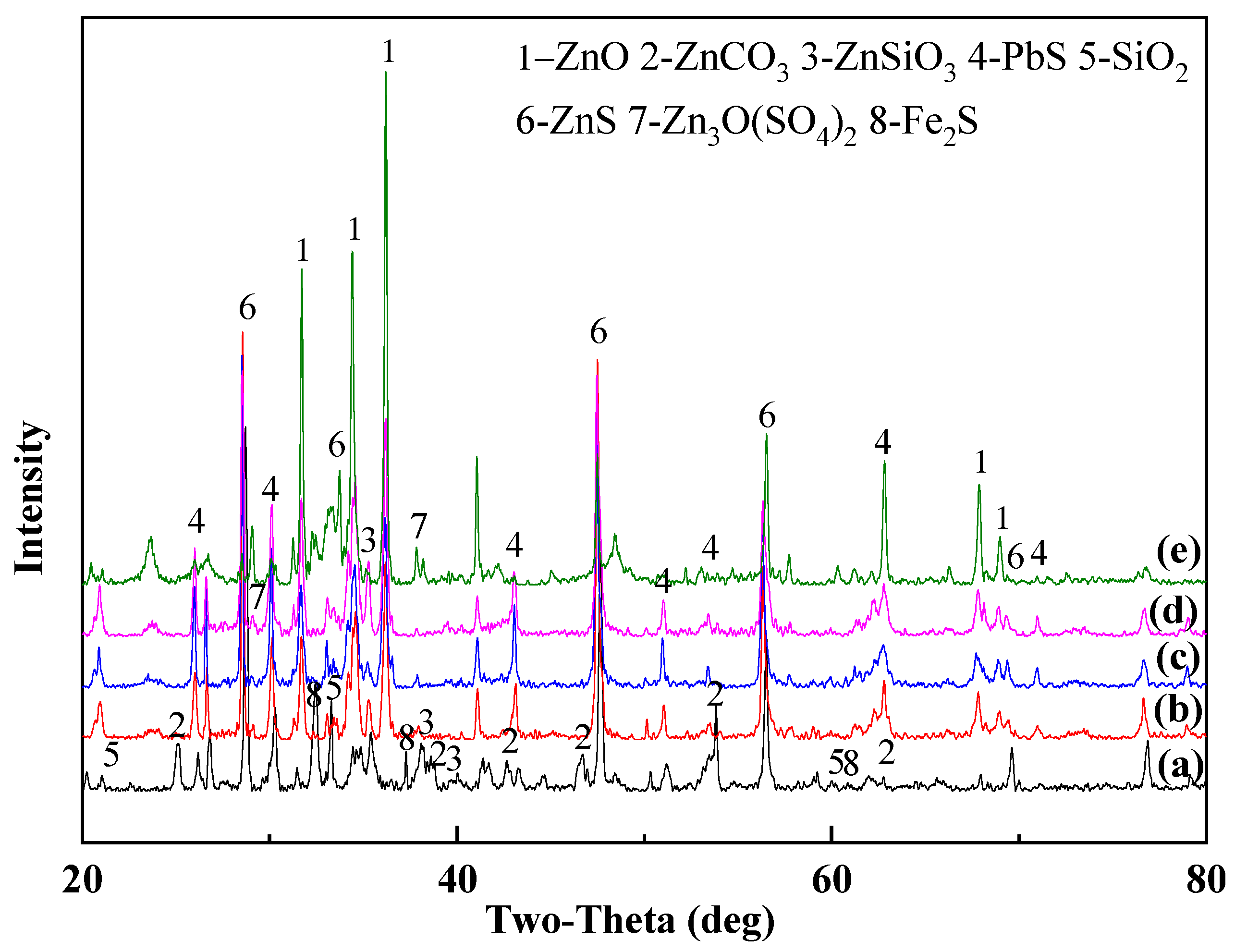
3.2. Microwave-Assisted Phase Transformation
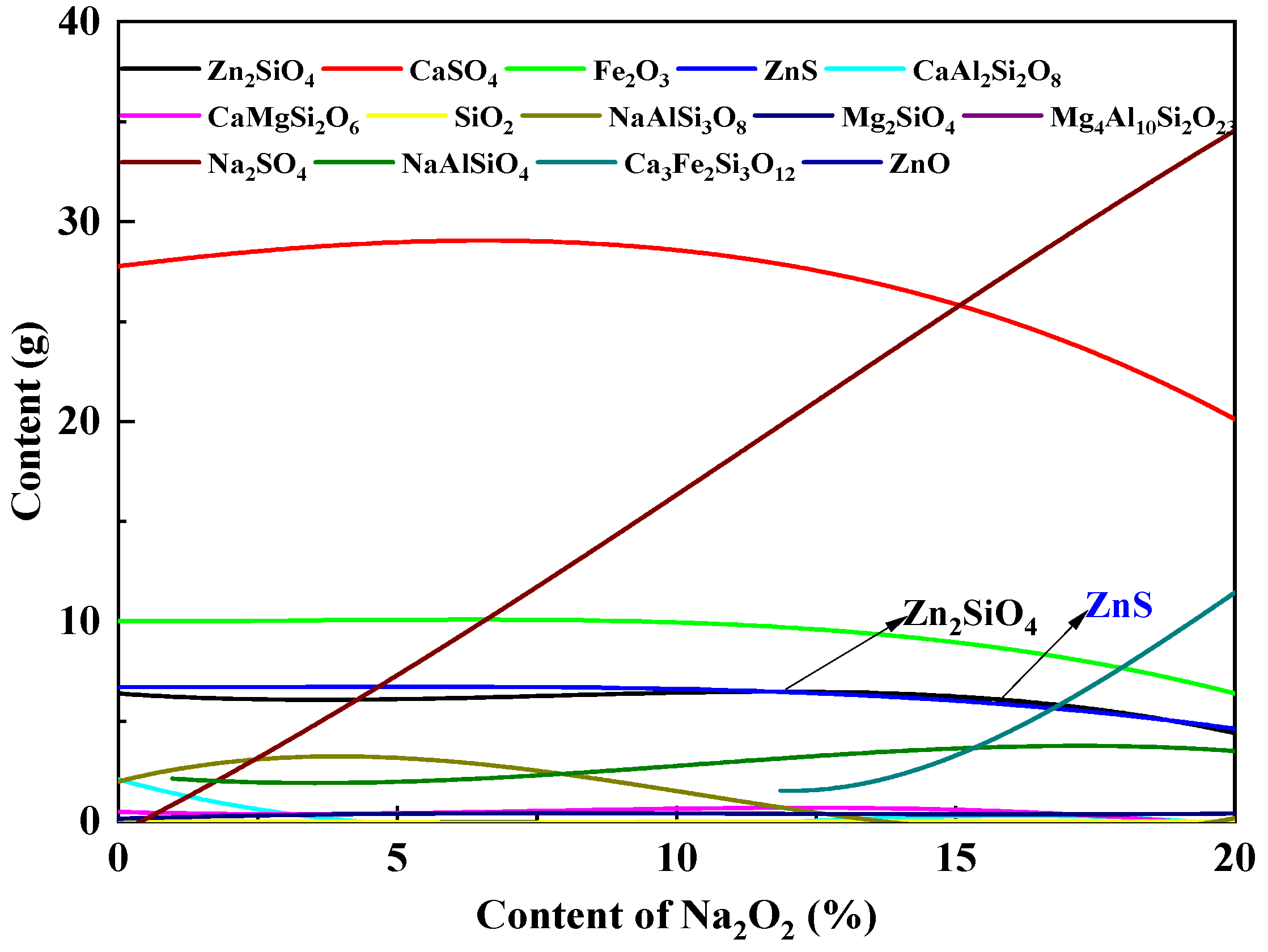
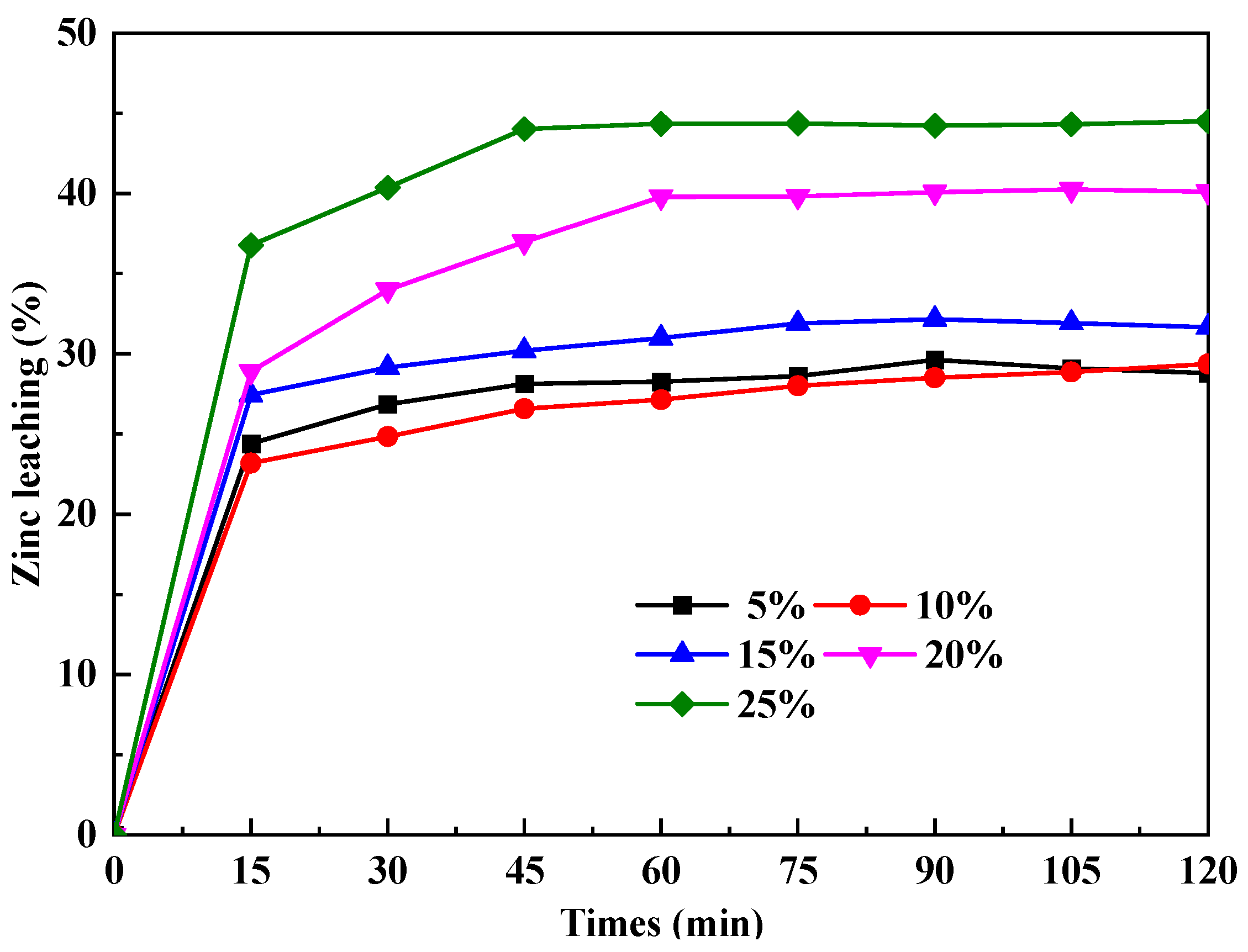
3.2.1. Effect of Na2O2 Additive Doping on Phase Transformation
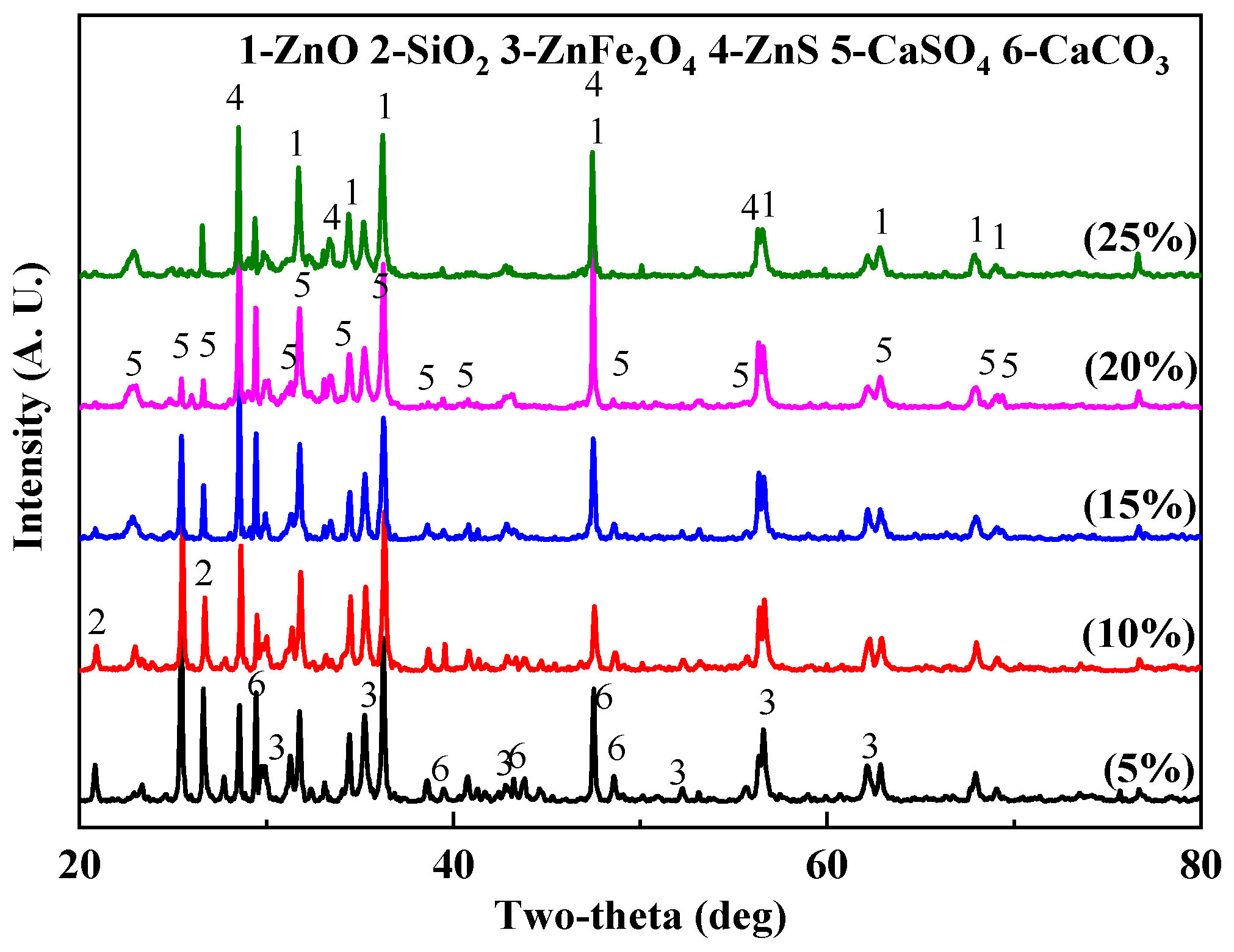
3.2.2. Effect of Microwave-Assisted Heating on Phase Transformation
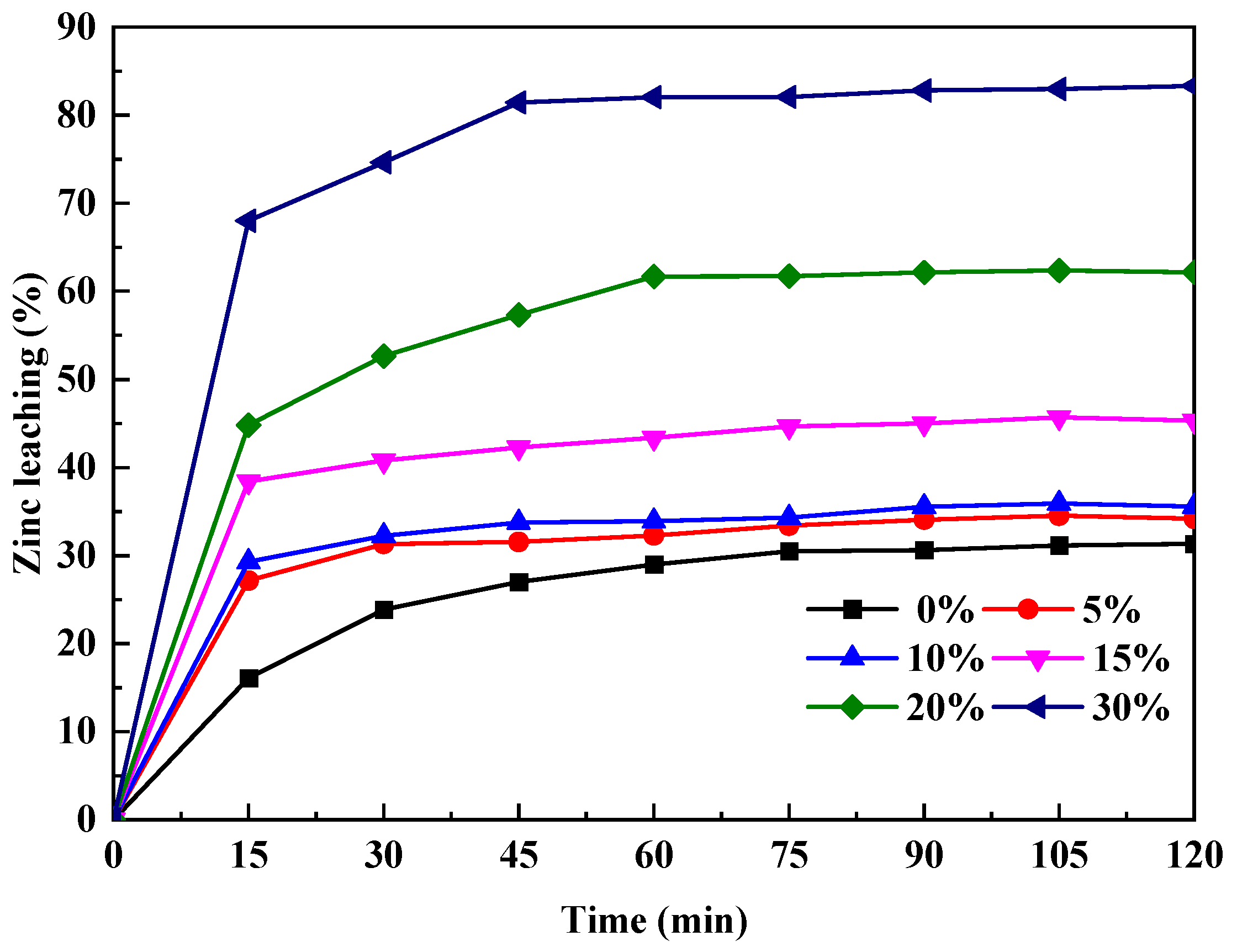
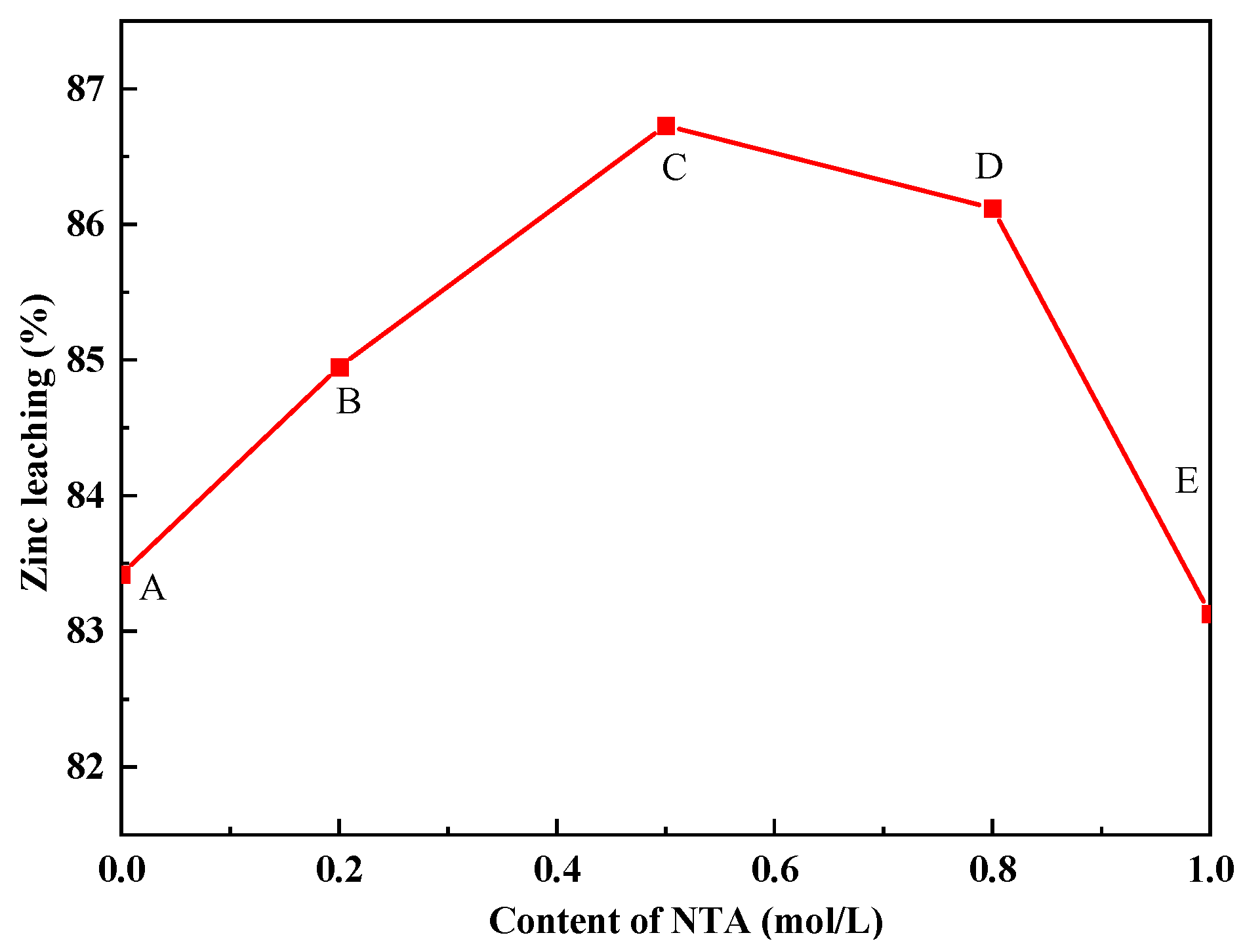
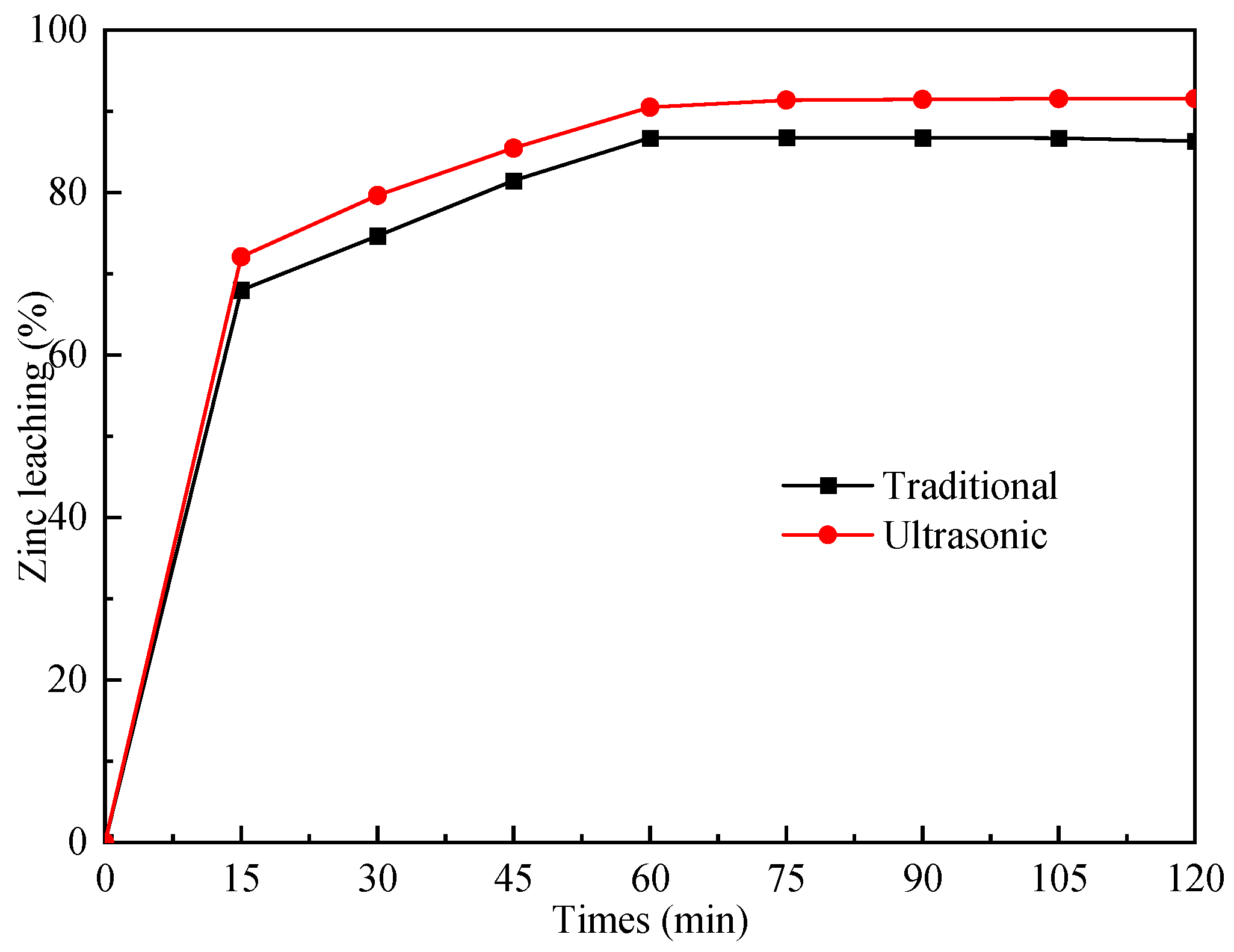
3.3. Ultrasonic-Assisted Complexation Leaching
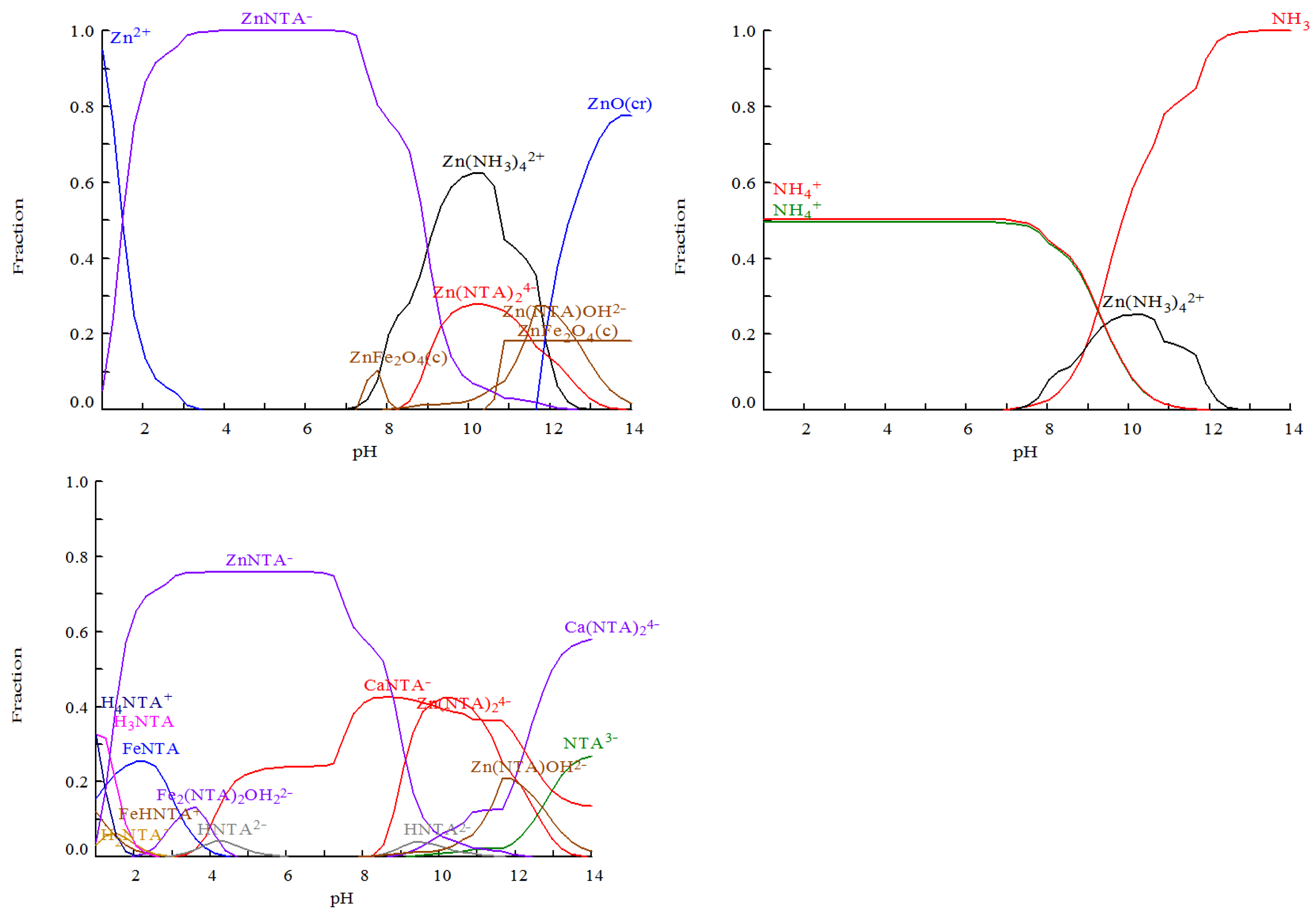
3.3.1. The Complexation Leaching Equilibrium under Zn(II)-NH4+-NH3-NTA3−-H2O System
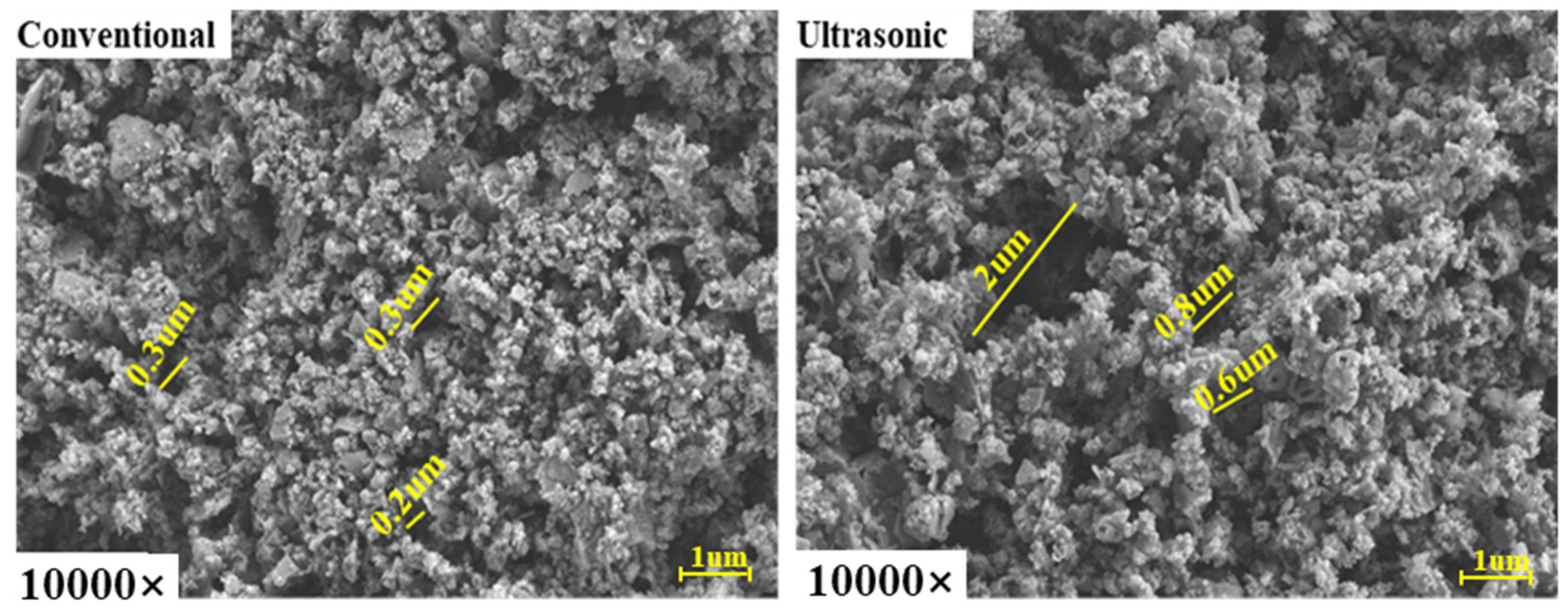
3.3.2. The Effect of Ultrasonic-Assisted Complexation Leaching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X. Mineral resources in China: Geological exploration and exploitation. In China’s Energy and Mineral Industries; Routledge: London, UK, 2019; pp. 105–116. [Google Scholar]
- Feng, D.; Bai, L.; Xie, H.; Tong, X. Study on separation of low-grade zinc oxide ore with sulfurization-amination flotation. Physicochem. Probl. Miner. Process. 2019, 55, 1082–1090. [Google Scholar]
- Zupanc, A.; Install, J.; Jereb, M.; Repo, T.J. Sustainable and Selective Modern Methods of Noble Metal Recycling. Angew. Chem. Int. Ed. 2023, 62, e202214453. [Google Scholar] [CrossRef]
- Mishra, G.; Jha, R.; Rao, M.D.; Meshram, A.; Singh, K.K. Recovery of silver from waste printed circuit boards (WPCBs) through hydrometallurgical route: A review. Environ. Chall. 2021, 4, 100073. [Google Scholar] [CrossRef]
- Soni, A.; Smith, J.; Thompson, A.; Brightwell, G. Microwave-induced thermal sterilization-A review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol. 2020, 97, 433–442. [Google Scholar] [CrossRef]
- Singh, C.; Khanna, V.; Singh, S. Sustainability of Microwave Heating in Materials Processing Technologies. Materials Today: Proceedings. 2022. Available online: https://www.sciencedirect.com/science/article/pii/S2214785322048398 (accessed on 25 July 2022).
- Wei, W.; Shao, Z.; Zhang, Y.; Qiao, R.; Gao, J. Fundamentals and applications of microwave energy in rock and concrete processing—A review. Appl. Therm. Eng. 2019, 157, 113751. [Google Scholar] [CrossRef]
- Kamariah, N.; Kalebic, D.; Xanthopoulos, P.; Blannin, R.; Araujo, F.P.; Koelewijn, S.F.; Spooren, J. Conventional versus microwave-assisted roasting of sulfidic tailings: Mineralogical transformation and metal leaching behavior. Miner. Eng. 2022, 183, 107587. [Google Scholar] [CrossRef]
- Kalebic, D.; Dehaen, W.; Spooren, J. Additive-Free Aqueous Extraction of Copper and Zinc from Sulfidic Tailings Using Fast Microwave-Assisted Pre-and Post-Treatments. Ind. Eng. Chem. Res. 2022, 61, 13303–13313. [Google Scholar] [CrossRef]
- Atia, T.A.; Spooren, J. Microwave assisted chloride leaching of zinc plant residues. J. Hazard. Mater. 2020, 398, 122814. [Google Scholar] [CrossRef] [PubMed]
- Al-Harahsheh, M.; Kingman, S.; Hamilton, I. Microwave treatment of electric arc furnace dust with tetrabromobisphenol A: Dielectric characterization and pyrolysis-leaching. J. Anal. Appl. Pyrolysis 2017, 128, 168–175. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Zhang, L.; Peng, J.; Chen, W.; Xie, F.; Ma, A. Microwave roasting and leaching of an oxide-sulphide zinc ore. Hydrometallurgy 2016, 166, 243–251. [Google Scholar] [CrossRef]
- Vereš, J.; Lovás, M.; Jakabský, Š.; Šepelák, V.; Hredzák, S. Characterization of blast furnace sludge and removal of zinc by microwave assisted extraction. Hydrometallurgy 2012, 129, 67–73. [Google Scholar] [CrossRef]
- Scruby, C.B.; Drain, L.E. Laser Ultrasonics: Techniques and Applications; Routledge: London, UK, 2019. [Google Scholar]
- Vargas, S.A.; Delgado-Macuil, R.J.; Ruiz-Espinosa, H.; Rojas-López, M.; Amador-Espejo, G.G. High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: Evaluation of selected functional properties. Ultrason. Sonochem. 2021, 70, 105340. [Google Scholar] [CrossRef] [PubMed]
- Slaczka, A.S. Effect of ultrasound on ammonium leaching of zinc from galmei ore. Ultrasonics 1986, 24, 53–55. [Google Scholar] [CrossRef]
- Beşe, A.V. Effect of ultrasound on the dissolution of copper from copper converter slag by acid leaching. Ultrason. Sonochem. 2007, 14, 790–796. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of binding energy positions with C1s for XPS results. J. Wuhan Univ. Technol.—Mater. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Decterov, S. Thermodynamic database for multicomponent oxide systems. Chim. Techno Acta 2018, 5, 16–48. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Surface and Ground Water, Weathering, and Soils: Treatise on Geochemistry; US Geological Survey: Boulder, CO, USA, 2005; Volume 5, p. 37.
- Yang, K.; Zhang, L.; Zhu, X.; Peng, J.; Li, S.; Ma, A.; Li, H.; Zhu, F. Role of manganese dioxide in the recovery of oxide–sulphide zinc ore. J. Hazard. Mater. 2018, 343, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, S.; Lin, B.; Lu, J.; Lu, Y.; Ye, Q.; Wang, Z.; Hong, Y.; Zhu, X. A fully coupled electromagnetic, heat transfer and multiphase porous media model for microwave heating of coal. Fuel Process. Technol. 2019, 189, 49–61. [Google Scholar] [CrossRef]
- Li, H.X.; Li, B.W.; Deng, L.B.; Xu, P.F.; Du, Y.S.; Ouyang, S.L.; Liu, Z.X. Evidence for non-thermal microwave effect in processing of tailing-based glass-ceramics. J. Eur. Ceram. Soc. 2019, 39, 1389–1396. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Martell, A.E.; Smith, R.M. Critical Stability Constants; Plenum Press: New York, NY, USA, 1974. [Google Scholar]
- Yang, P.; Liang, X.; Wu, C.; Cui, T.; Wang, Y. Improve manganese leaching efficiency by adding different complexants during ammonia leaching of low-grade rhodochrosite. Miner. Eng. 2022, 188, 107834. [Google Scholar] [CrossRef]
- Zulhan, Z.; Adzana, Z.; Munawaroh, M.; Yusro, A.H.; Christian, J.D.; Saputri, A.D.; Hidayat, T. Sulfur Removal and Iron Extraction from Natrojarosite Residue of Laterite Nickel Ore Processing by Reduction Roasting. Metals 2022, 13, 52. [Google Scholar] [CrossRef]



| ZnT | CaO | SiO2 | S | Fe | Pb | Al2O3 | MgO |
|---|---|---|---|---|---|---|---|
| 24.91 | 11.96 | 10.30 | 10.20 | 7.70 | 4.80 | 1.27 | 0.22 |
| Zinc Phases | Sulphide | Carbonate | Silicate | Franklinite et al. | ZnT |
|---|---|---|---|---|---|
| Mass fraction/wt−% | 11.06 | 9.68 | 4.00 | 0.052 | 24.91 |
| Distribution/wt−% | 44.61 | 39.04 | 16.13 | 0.21 | 100 |
| Elements | S | Pb | Fe | Zn | O | Si |
|---|---|---|---|---|---|---|
| A/wt% | 1.85 | 84.56 | – | 4.95 | 8.64 | – |
| B/wt% | 33.89 | 4.73 | 6.61 | 54.76 | – | – |
| C/wt% | 54.20 | 45.80 | – | – | – | – |
| D/wt% | – | – | – | – | 35.51 | 64.49 |
| LogK1 | LogK2 | LogK3 | LogK4 | |
|---|---|---|---|---|
| Ammonia–zinc | 2.37 | 4.81 | 7.31 | 9.46 |
| Hydroxide–zinc | 4.40 | 11.30 | 14.14 | 17.66 |
| Chloride–zinc | 0.43 | 0.61 | 0.43 | 0.20 |
| Nitrilotriacetic–zinc | 10.45 | 13.45 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, S.; Zhang, L.; Yang, K. Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore. Metals 2023, 13, 356. https://doi.org/10.3390/met13020356
Liu J, Li S, Zhang L, Yang K. Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore. Metals. 2023; 13(2):356. https://doi.org/10.3390/met13020356
Chicago/Turabian StyleLiu, Junchang, Shiwei Li, Libo Zhang, and Kun Yang. 2023. "Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore" Metals 13, no. 2: 356. https://doi.org/10.3390/met13020356





