Effect of Silicon Wafer Surface Stains on Copper-Assisted Chemical Etching
Abstract
:1. Introduction
2. Materials and Methods
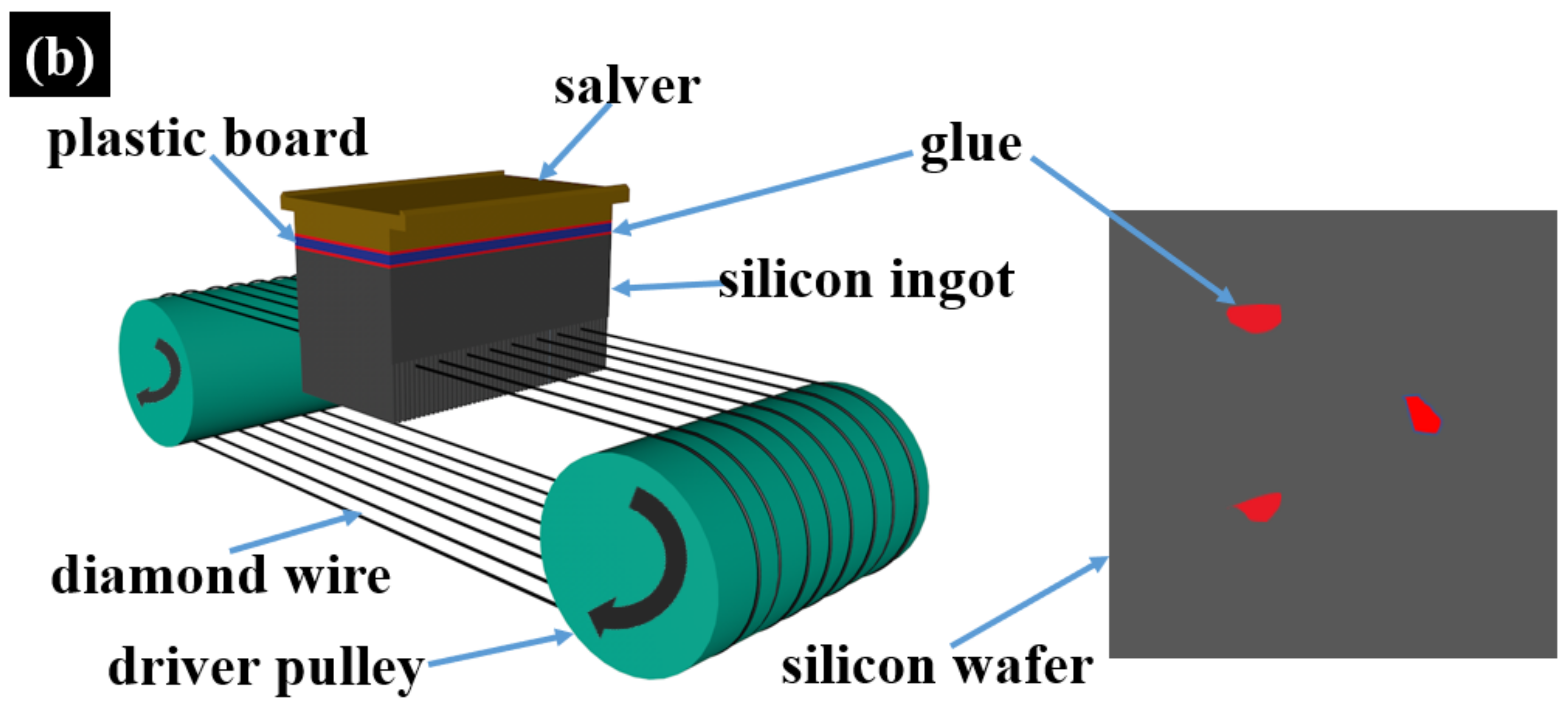
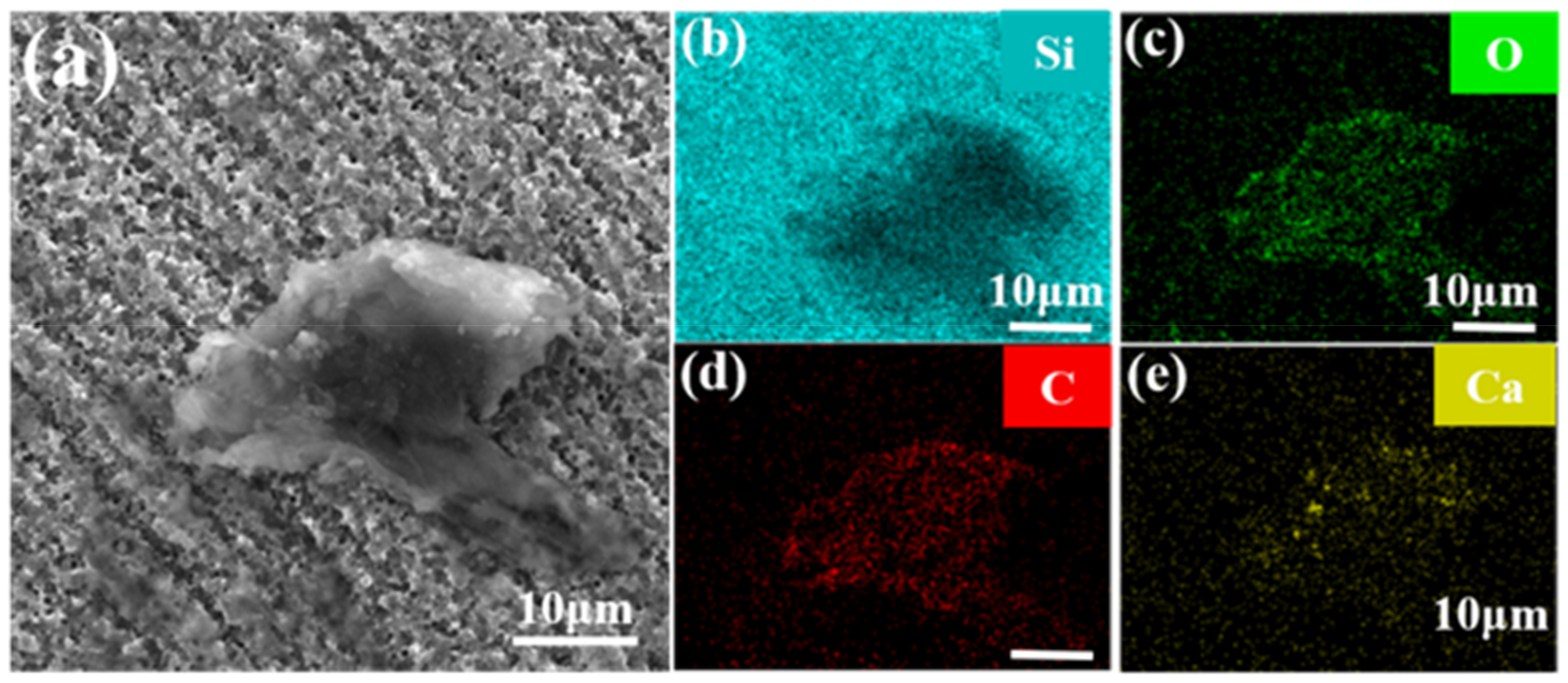
2.1. Testing and Analysis of White-Spot-Stained Silicon Wafers
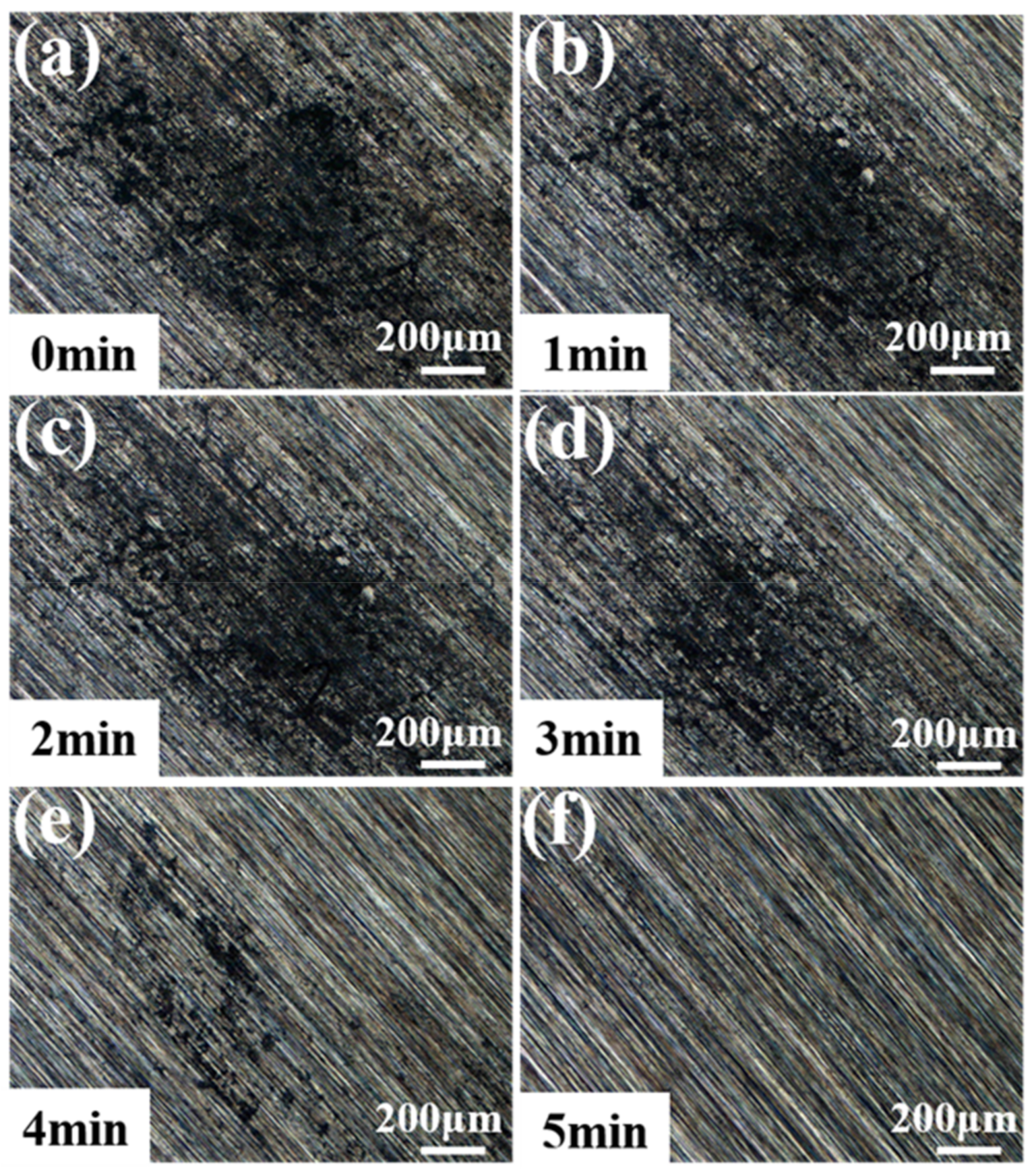
2.2. Deposition and Etching
2.3. Wafer Cleaning
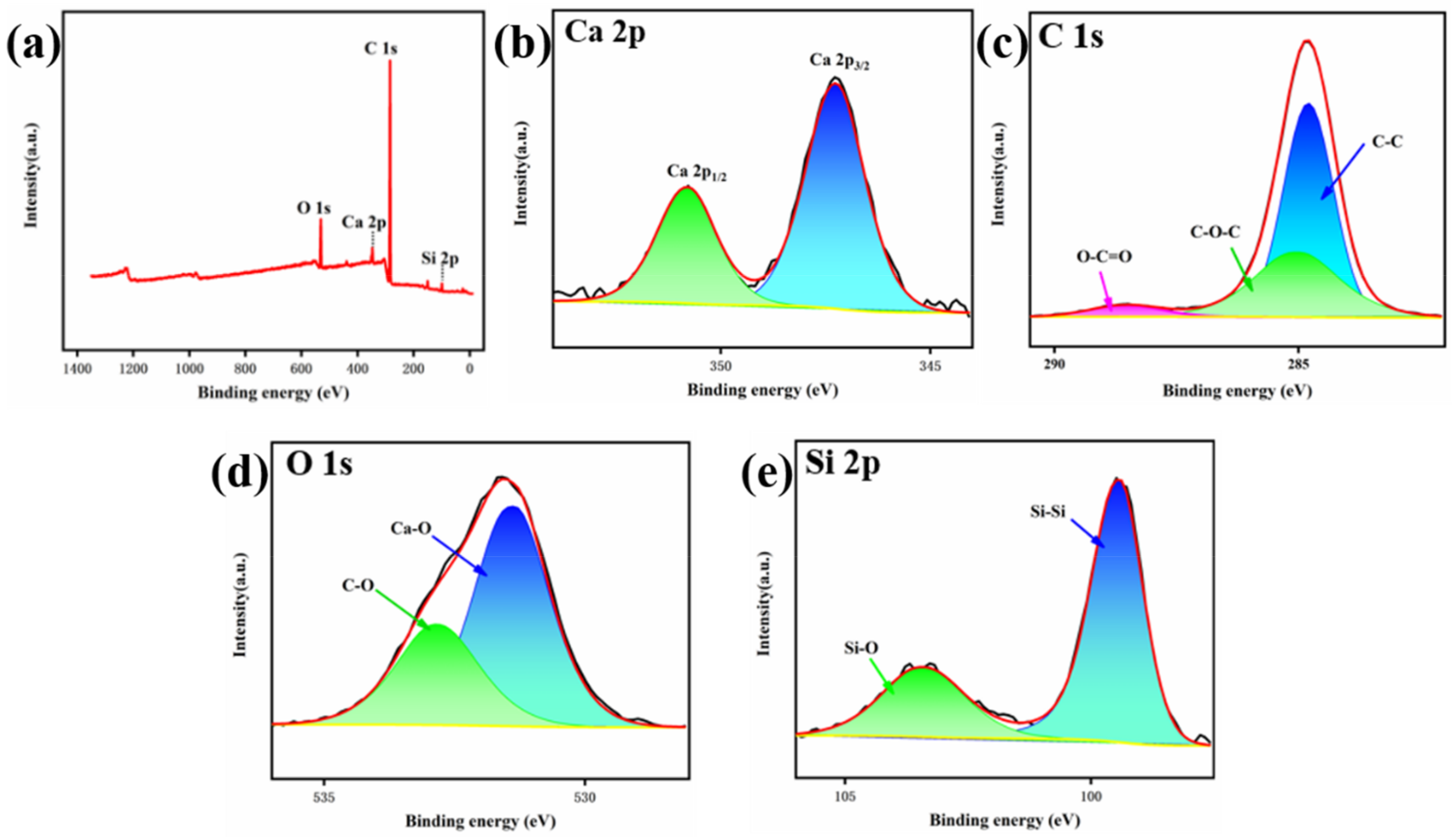
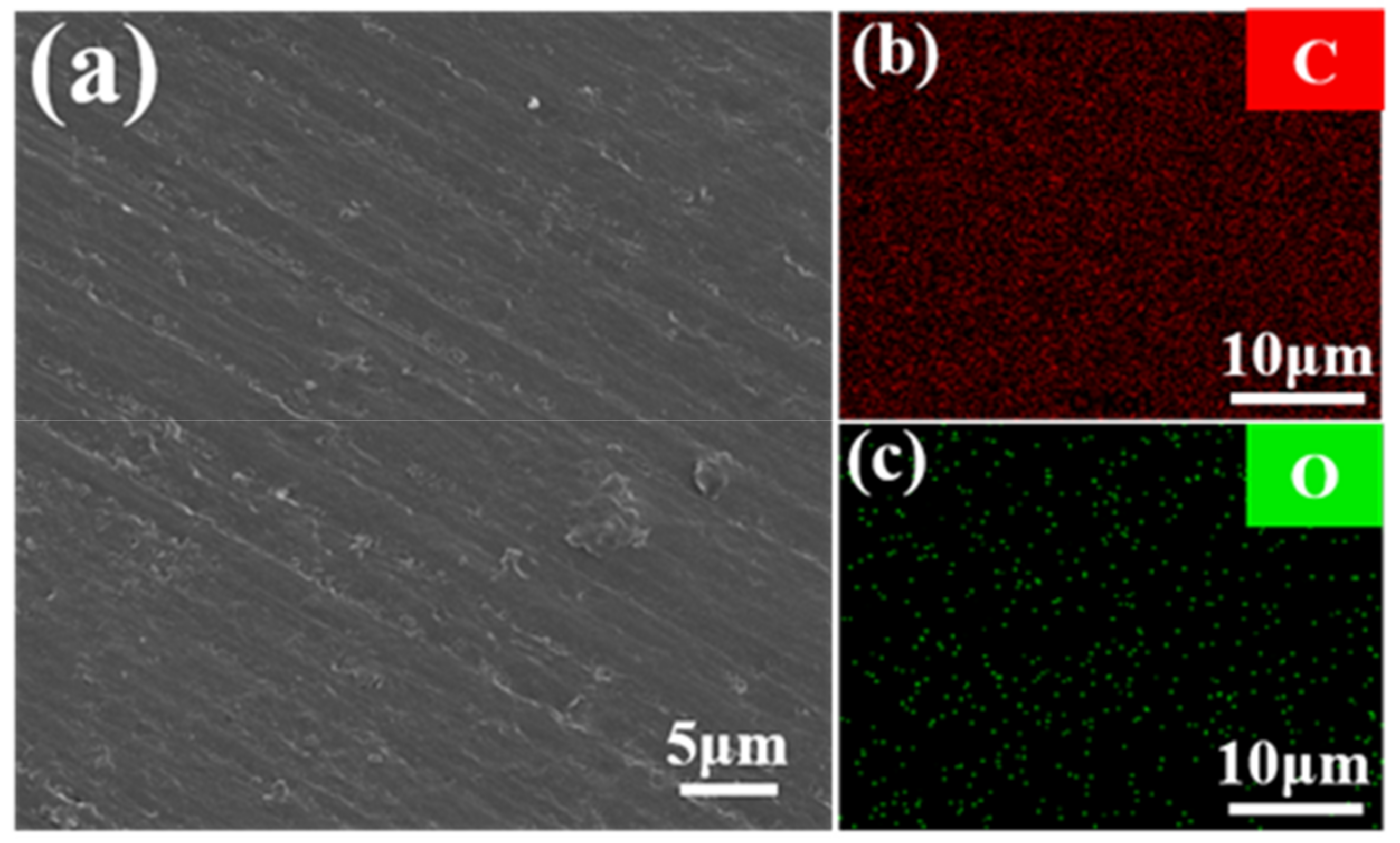
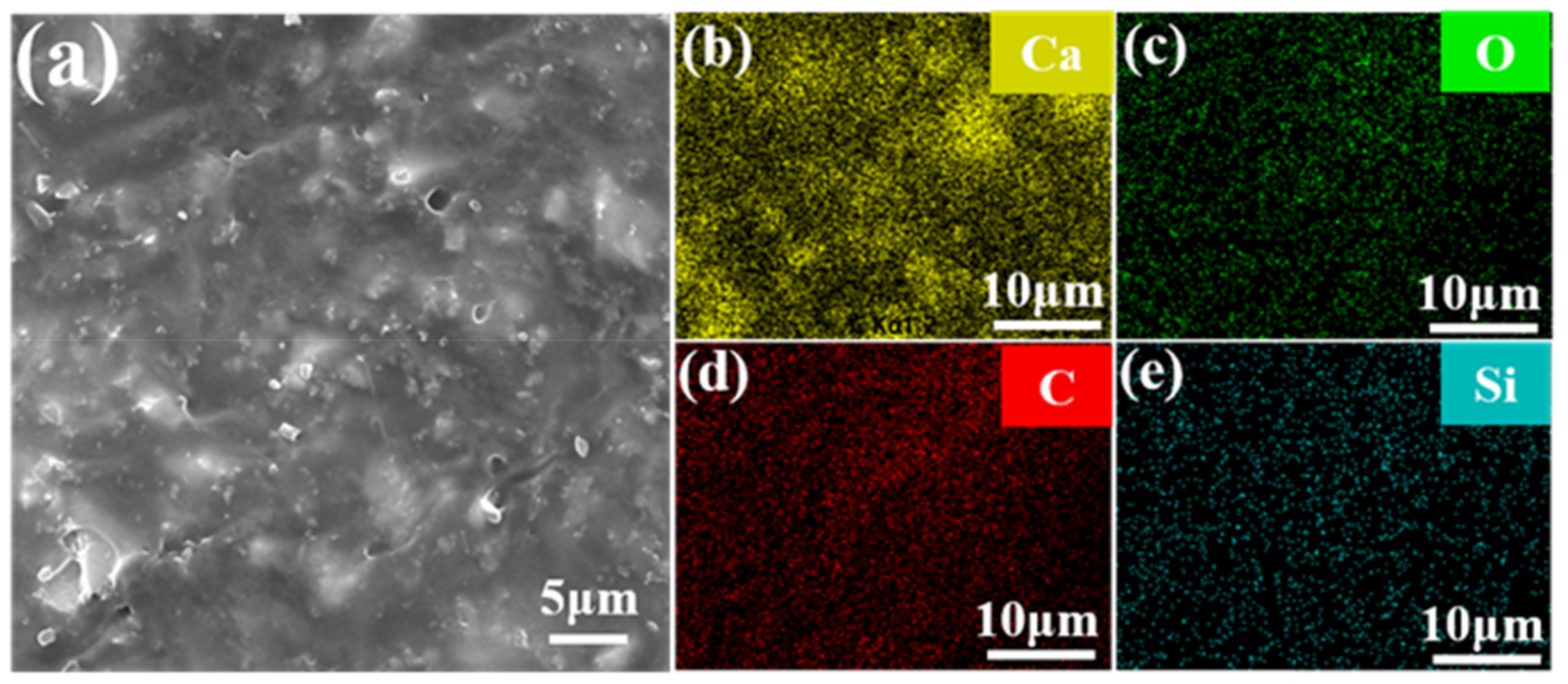
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IRENA_World_Energy_Transitions_Outlook_2022. Available online: https://www.irena.org/publications/2022/Mar/World-Energy-Transitions-Outlook-2022 (accessed on 10 December 2022).
- Xi, F.; Li, S.; Ma, W.; Chen, Z.; Wei, K.; Wu, J. A review of hydrometallurgy techniques for the removal of impurities from metallurgical-grade silicon. Hydrometallurgy 2021, 201, 105553. [Google Scholar] [CrossRef]
- Jäger-Waldau, A. Snapshot of photovoltaics—February 2022. EPJ Photovolt. 2022, 13, 9. [Google Scholar] [CrossRef]
- Global Solar Cells Market Report 2022. Available online: https://www.cognitivemarketresearch.com/solar-cells-market-report (accessed on 10 December 2022).
- Gao, Y.F.; Ge, P.Q.; Hou, Z.J. Study on Removal Mechanism of Fixed-Abrasive Diamond Wire Saw Slicing Monocrystalline Silicon. Key Eng. Mater. 2007, 359–360, 450–454. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, Y.; Li, X.; Pu, T.; Wang, L. Experimental study on slicing photovoltaic polycrystalline silicon with diamond wire saw. Mater. Sci. Semicond. Process. 2020, 106, 104779. [Google Scholar] [CrossRef]
- Luna-sánchez, R.; González-Martínez, I. Metal contamination from process materials used for wet cleaning of silicon wafer. ECS Trans. 2006, 2, 91–98. [Google Scholar] [CrossRef]
- Habuka, H.; Ishiwari, S.; Kato, H.; Shimada, M.; Okuyama, K. Airborne organic contamination behavior on silicon wafer surface. J. Electrochem. Soc. 2003, 150, 148–154. [Google Scholar] [CrossRef]
- Joly, J.-P. Metallic contamination assessment of silicon wafers. Microelectron Eng. 1998, 40, 285–294. [Google Scholar] [CrossRef]
- Wu, L.; Yu, B.; Zhang, P.; Feng, C.; Chen, P.; Deng, L.; Gao, J.; Chen, S.; Jiang, S.; Qian, L. Rapid identification of ultrathin amorphous damage on monocrystalline silicon surface. Phys. Chem. Chem. Phys. 2020, 22, 12987–12995. [Google Scholar] [CrossRef]
- Kumar, A.; Kaminski, S.; Melkote, S.N.; Arcona, C. Effect of wear of diamond wire on surface morphology, roughness and subsurface damage of silicon wafers. Wear 2016, 364, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Haapalinna, A.; Nevas, S.; Pähler, D. Rotational grinding of silicon wafers—Sub-surface damage inspection. Mater. Sci. Eng. C 2004, 107, 321–331. [Google Scholar] [CrossRef]
- Tan, B.-m.; Li, W.-w.; Niu, X.-h.; Wang, S.-l.; Liu, Y.-l. Effect of surfactant on removal of particle contamination on Si wafers in ULSI. Trans. Nonferrous Met. Soc. China 2006, 16, 195–198. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Cao, Y. Investigation on Adsorption State of Surface Adsorbate on Silicon Wafer. Rare Met. 1999, 18, 27–33. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Wang, F.; Han, Y. Study on the cleaning of silicon after CMP in ULSI. Microelectron Eng. 2003, 66, 433–437. [Google Scholar] [CrossRef]
- Chen, G.; Kashkoush, I. Effect of pre-cleaning on texturization of c-Si wafers in a KOH/IPA mixture. ECS Trans. 2010, 25, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Soha, M.; Braun, M.; Takáts, V.; Hakl, J.; Fodor, T.; Braun, Á.; Szabó, I.; Haslinger, M.; John, J.; Vad, K. Investigation of ppb-level surface contamination of n-type silicon solar cells. Appl. Surf. Sci. 2020, 520, 146299. [Google Scholar] [CrossRef]
- Richter, A.; Benick, J.; Fell, A.; Hermle, M.; Glunz, S.W. Impact of bulk impurity contamination on the performance of high-efficiencyn-type silicon solar cells. Prog. Photovolt. 2018, 26, 342–350. [Google Scholar] [CrossRef]
- Sugimoto, F.; Okamura, S. Adsorption behavior of organic contaminants on a silicon wafer surface. J. Electrochem. Soc. 1999, 146, 2725. [Google Scholar] [CrossRef]
- Choi, S.; Jang, B.; Kim, J.; Song, H.; Han, M. Cu-contamination of single crystalline silicon wafers with thickness of 100 μm during multi-wire sawing process. Sol. Energy 2016, 125, 198–206. [Google Scholar] [CrossRef]
- Watanabe, M.; Hamano, M.; Harazono, M. The role of atmospheric oxygen and water in the generation of water marks on the silicon surface in cleaning processes. Mater. Sci. Eng. C 1989, 4, 401–405. [Google Scholar] [CrossRef]
- Burdick, G.M.; Berman, N.S.; Beaudoin, S.P. A Theoretical Evaluation of Hydrodynamic and Brush Contact Effects on Particle Removal during Brush Scrubbing. J. Electrochem. Soc. 2003, 150, G658–G665. [Google Scholar] [CrossRef]
- Celler, G.K. Etching of Silicon by the RCA Standard Clean 1. ECS Solid State Lett. 1999, 3, 47–49. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.M.; Cho, S.H.; Kim, T.H. Acoustic emission monitoring during laser shock cleaning of silicon wafers. Opt. Lasers Eng. 2005, 43, 1010–1020. [Google Scholar] [CrossRef]
- Seike, Y.; Miyachi, K.; Shibata, T.; Kobayashi, Y.; Kurokawa, S.; Doi, T. Silicon Wafer Cleaning Using New Liquid Aerosol with Controlled Droplet Velocity and Size by Rotary Atomizer Method. Jpn. J. Appl. Phys. 2010, 49, 066701. [Google Scholar] [CrossRef]
- Hong, S.; Zou, Y.; Ma, L.; Chen, X.; Li, S.; Ma, W.; Chang, Y. Surface Texturing Behavior of Nano-Copper Particles under Copper-Assisted Chemical Etching with Various Copper Salts System. Silicon 2022, 14, 7169–7177. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Chen, W.; Wu, J.; Chen, Q.; Tang, H.; Wang, Y.; Du, X. Regulation of surface texturization through copper-assisted chemical etching for silicon solar cells. Sol. Energy 2020, 201, 461–468. [Google Scholar] [CrossRef]
- Zha, J.; Wang, T.; Pan, C.; Chen, K.; Hu, F.; Pi, X.; Su, X. Constructing submicron textures on mc-Si solar cells via copper-catalyzed chemical etching. Appl. Phys. Lett. 2017, 110, 93901. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, T.; Dang, B.; Wang, H.; Xiong, Y.; Yao, Q.; Wang, C.; Sun, Q.; Jin, C. The properties of fibreboard based on nanolignocelluloses/CaCO3/PMMA composite synthesized through mechano-chemical method. Sci. Rep. 2018, 8, 12633. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Wang, X.; Sheng, G.; Wang, S.; Ai, Y.; Ji, Y.; Liu, Y.; Hayat, T.; Wang, X. Glycerol-modified binary layered double hydroxide nanocomposites for uranium immobilization via extended X-ray absorption fine structure technique and density functional theory calculation. Adv. Synth. Catal. 2017, 5, 3583–3595. [Google Scholar] [CrossRef]
- Ravizza, E.; Spadoni, S.; Piagge, R.; Comite, P.; Wiemer, C. XPS composition study of stacked Si oxide/Si nitride/Si oxide nano-layers. Surf. Interface Anal. 2012, 44, 1209–1213. [Google Scholar] [CrossRef]
- Dukarska, D.; Czarnecki, R. Fumed silica as a filler for MUPF resin in the process of manufacturing water-resistant plywood. Eur. J. Wood Wood Prod. 2015, 74, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Rudawska, A.; Frigione, M. Cold-Cured Bisphenolic Epoxy Adhesive Filled with Low Amounts of CaCO3: Effect of the Filler on the Durability to Aqueous Environments. Materials 2021, 14, 1324. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, X.; Ge, R.; Zhang, S.; Wang, H. Formation mechanism of wire bow and its influence on diamond wire saw process and wire cutting capability. Int. J. Mech. Sci. 2020, 185, 105851. [Google Scholar] [CrossRef]
- Orhan, G.; Gezgin, G. Effect of electrolysis parameters on the morphologies of copper powder obtained at high current densities. J. Serb. Chem. Soc. 2012, 77, 651–665. [Google Scholar] [CrossRef]
- Yang, J.; Shen, H.; Jiang, Y.; Sun, L. Controllable fabrication and mechanism study of textured ultra-thin silicon wafers via one-step Cu-assisted chemical etching. Mater. Sci. Semicond. Process. 2019, 100, 79–86. [Google Scholar] [CrossRef]
- Wu, X.; Tan, Y.; Li, J.; Cai, M.; Li, P. Surface damage and metal-catalyzed chemical etching investigation of multicrystalline silicon by diamond wire sawing. Sol. Energy 2020, 207, 609–617. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Li, M.; Yin, C.; Zhou, L. On the nature and removal of saw marks on diamond wire sawn multicrystalline silicon wafers. Mater. Sci. Semicond. Process. 2014, 27, 220–227. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yang, L.; Chen, W.; Du, X.; Kuznetsov, A. Micro-structured inverted pyramid texturization of Si inspired by self-assembled Cu nanoparticles. Nanoscale 2017, 9, 907–914. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Zhang, X.; Chen, X.; Xi, F.; Li, S.; Ma, W.; Chang, Y. Performance Improvement of Graphene/Silicon Solar Cells via Inverted Pyramid Texturation Array. Silicon 2022, 14, 10485–10493. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Yang, L.; Wu, J.; Chen, Q.; Zhao, Y.; Wang, Y.; Du, X. Difference in anisotropic etching characteristics of alkaline and copper based acid solutions for single-crystalline Si. Sci. Rep. 2018, 8, 3408. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Chen, X.; Tang, C.; Li, S.; Xi, F.; Lan, H.; Ma, W.; Chang, Y. Effect of Silicon Wafer Surface Stains on Copper-Assisted Chemical Etching. Metals 2023, 13, 742. https://doi.org/10.3390/met13040742
Ma L, Chen X, Tang C, Li S, Xi F, Lan H, Ma W, Chang Y. Effect of Silicon Wafer Surface Stains on Copper-Assisted Chemical Etching. Metals. 2023; 13(4):742. https://doi.org/10.3390/met13040742
Chicago/Turabian StyleMa, Liang, Xiuhua Chen, Chenggui Tang, Shaoyuan Li, Fengshuo Xi, Huayan Lan, Wenhui Ma, and Yuanchih Chang. 2023. "Effect of Silicon Wafer Surface Stains on Copper-Assisted Chemical Etching" Metals 13, no. 4: 742. https://doi.org/10.3390/met13040742



