Technologies of Recycling REEs and Iron from NdFeB Scrap
Abstract
:1. Introduction
2. Composition Characteristics and Recovery Potential
2.1. Chemical Composition
2.2. Phase Composition
2.3. Recycle Value
3. Recycle Technologies
3.1. Direct Reuse Routes
3.2. Hydrometallurgy
3.3. Pyrometallurgy
3.4. Electrochemistry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dushyantha, N.; Batapola, N.; Ilankoon, I.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122. [Google Scholar] [CrossRef]
- Sasai, R.; Shimamura, N.; Fujimura, T. Eco-Friendly Rare-Earth-Metal Recovering Process with High Versatility from Nd-Fe-B Magnets. ACS Sustain. Chem. Eng. 2020, 8, 1507–1512. [Google Scholar] [CrossRef]
- Rollat, A.; Guyonnet, D.; Planchon, M.; Tuduri, J. Prospective analysis of the flows of certain rare earths in Europe at the 2020 horizon. Waste Manag. 2016, 49, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Coey, J.M.D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- Lixandru, A.; Venkatesan, P.; Jönsson, C.; Poenaru, I.; Hall, B.; Yang, Y.; Walton, A.; Güth, K.; Gauß, R.; Gutfleisch, O. Identification and recovery of rare-earth permanent magnets from waste electrical and electronic equipment. Waste Manag. 2017, 68, 482–489. [Google Scholar] [CrossRef]
- Jin, H.; Afiuny, P.; McIntyre, T.; Yih, Y.; Sutherland, J.W. Comparative Life Cycle Assessment of NdFeB Magnets: Virgin Production versus Magnet-to-Magnet Recycling. Procedia CIRP 2016, 48, 45–50. [Google Scholar] [CrossRef]
- Ali, S. Social and Environmental Impact of the Rare Earth Industries. Resources 2014, 3, 123–134. [Google Scholar] [CrossRef]
- Bonfante, M.C.; Raspini, J.P.; Fernandes, I.B.; Fernandes, S.; Campos, L.M.S.; Alarcon, O.E. Achieving Sustainable Development Goals in rare earth magnets production: A review on state of the art and SWOT analysis. Renew. Sustain. Energy Rev. 2021, 137, 110616. [Google Scholar] [CrossRef]
- Venkatesan, P.; Vander Hoogerstraete, T.; Binnemans, K.; Sun, Z.; Sietsma, J.; Yang, Y. Selective Extraction of Rare-Earth Elements from NdFeB Magnets by a Room-Temperature Electrolysis Pretreatment Step. ACS Sustain. Chem. Eng. 2018, 6, 9375–9382. [Google Scholar] [CrossRef]
- Yue, M.; Yin, X.; Liu, W.; Lu, Q. Progress in recycling of Nd–Fe–B sintered magnet wastes. Chin. Phys. B 2019, 28, 77506. [Google Scholar] [CrossRef]
- Elwert, T.; Goldmann, D.; Roemer, F.; Schwarz, S. Recycling of NdFeB Magnets from Electric Drive Motors of (Hybrid) Electric Vehicles. J. Sustain. Met. 2017, 3, 108–121. [Google Scholar] [CrossRef]
- Tipre, D.R.; Khatri, B.R.; Thacker, S.C.; Dave, S.R. The brighter side of e-waste—A rich secondary source of metal. Environ. Sci. Pollut. R. 2021, 28, 10503–10518. [Google Scholar] [CrossRef]
- Habib, K. A product classification approach to optimize circularity of critical resources—the case of NdFeB magnets. J. Clean. Prod. 2019, 230, 90–97. [Google Scholar] [CrossRef]
- Prokofev, P.A.; Kolchugina, N.B.; Skotnicova, K.; Burkhanov, G.S.; Kursa, M.; Zheleznyi, M.V.; Dormidontov, N.A.; Cegan, T.; Bakulina, A.S.; Koshkidko, Y.S.; et al. Blending Powder Process for Recycling Sintered Nd-Fe-B Magnets. Materials 2020, 13, 3049. [Google Scholar] [CrossRef]
- Abbasalizadeh, A.; Seetharaman, S.; Venkatesan, P.; Sietsma, J.; Yang, Y. Use of iron reactive anode in electrowinning of neodymium from neodymium oxide. Electrochim. Acta 2019, 310, 146–152. [Google Scholar] [CrossRef]
- Sagawa, M.; Fujimura, S.; Togawa, N.; Yamamoto, H.; Matsuura, Y. New material for permanent magnets on a base of Nd and Fe (invited). J. Appl. Phys. 1984, 55, 2083–2087. [Google Scholar] [CrossRef]
- Croat, J.J.; Herbst, J.F.; Lee, R.W.; Pinkerton, F.E. Pr-Fe and Nd-Fe-based materials: A new class of high-performance permanent magnets. J. Appl. Phys. 1984, 55, 2078–2082. [Google Scholar] [CrossRef]
- Sprecher, B.; Kleijn, R.; Kramer, G.J. Recycling Potential of Neodymium: The Case of Computer Hard Disk Drives. Environ. Sci. Technol. 2014, 48, 9506–9513. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, C.; Jiang, T. Hydrometallurgical Recovery of REEs from NdFeB Permanent Magnet Scrap: A Review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Du, C.; Ma, S.; Xie, M.; Yang, F.; Zhao, Z.; Chen, Y.; Ma, Y. Recovery of high-value rare earth elements from waste NdFeB by the water-soluble ammonium salt [Hbet]cl. Sep. Purif. Technol. 2023, 308, 122946. [Google Scholar] [CrossRef]
- Wu, J.; Wang, D.; Ye, C.; Wang, Z.; Hu, X. Selective extraction and separation of REEs from NdFeB magnets scrap using co-chlorination and water leaching. Sep. Purif. Technol. 2023, 306, 122452. [Google Scholar] [CrossRef]
- Bian, Y.Y.; Guo, S.Q.; Jiang, L.; Liu, J.; Tang, K.; Ding, W.Z. Recovery of Rare Earth Elements from NdFeB Magnet by VIM-HMS Method. ACS Sustain. Chem. Eng. 2016, 4, 810–818. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.Q.; Yue, M.; Liu, Y.Q.; Zhang, D.T.; Zuo, T.Y. Waste Nd-Fe-B Sintered Magnet Recycling by Doping With Rare Earth Rich Alloys. IEEE Trans. Magn. 2014, 50, 2105403. [Google Scholar] [CrossRef]
- Perigo, E.A.; Takiishi, H.; Motta, C.C.; Faria, R.N. On the Squareness Factor Behavior of RE-FeB (RE = Nd or Pr) Magnets Above Room Temperature. IEEE Trans. Magn. 2009, 45, 4431–4434. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, F.G.; Gao, Y.; Jiang, P.; Liu, L.M.; Zhao, M.J.; Ren, S.Q.; Pei, W.L. Dy evolution and coercivity improvement mechanism of sintered NdFeB magnets in thermal diffusion process. J. Magn. Magn. Mater. 2022, 563, 169943. [Google Scholar] [CrossRef]
- Zhou, T.; Guo, Y.; Xie, G.; Rehman, S.U.; Liu, R.; Liu, J.; Qu, P.; Li, M. Coercivity and thermal stability enhancement of NdFeB magnet by grain boundary diffusion Tb80Al20 alloys. Intermetallics 2021, 138, 107335. [Google Scholar] [CrossRef]
- Cui, J.; Kramer, M.; Zhou, L.; Liu, F.; Gabay, A.; Hadjipanayis, G.; Balasubramanian, B.; Sellmyer, D. Current progress and future challenges in rare-earth-free permanent magnets. Acta Mater. 2018, 158, 118–137. [Google Scholar] [CrossRef]
- Yang, Y.X.; Walton, A.; Sheridan, R.; Guth, K.; Gauss, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Met. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Zakotnik, M.; Tudor, C.O.; Peiro, L.T.; Afiuny, P.; Skomski, R.; Hatch, G.P. Analysis of energy usage in Nd-Fe-B magnet to magnet recycling. Environ. Technol. Innov. 2016, 5, 117–126. [Google Scholar] [CrossRef]
- Rehman, S.U.; Jiang, Q.Z.; He, L.K.; Xiong, H.D.; Liu, K.; Wang, L.; Yang, M.A.; Zhong, Z.C. Microstructure and magnetic properties of NdFeB alloys by co-doping alnico elements. Phys. Lett. A 2019, 383, 125878. [Google Scholar] [CrossRef]
- Liu, X.B.; Altounian, Z. The Role of Cu in Sintered Nd-Fe-B Magnets: Ab initio Study. IEEE Ttans. Magn. 2012, 48, 3144–3146. [Google Scholar] [CrossRef]
- Liu, X.B.; Yue, M.; Xiao, Y.F.; Zhang, Z.G.; Wang, R. Effect of cobalt on HDDR anisotropic NdFeB. J. Rare Earth 2002, 20, 579–582. [Google Scholar]
- Yang, S.; Song, X.P.; Wang, X.H.; Sun, Z.B.; Sun, J. Effect of Al and Al/Mo addition on microstructure and magnetic properties of sintered Nd22Fe71B7 magnets. Trans. Nonferr. Met. Soc. 2000, 10, 606–609. [Google Scholar]
- Wang, F.Q.; Chen, K.H.; Du, J.; Zhang, J. Simultaneous increase in remanence and coercivity during grain refining of Nd-Fe-B deformed magnets. J. Rare Earth 2022, 40, 1763–1771. [Google Scholar] [CrossRef]
- Yu, L.Q.; Zhang, Y.P.; Fu, Q.T. Effect of Boron on Magnetic Properties and Corrosion Resistance of High Energy Magnets. Adv. Mater. Res. 2009, 79–82, 1043–1046. [Google Scholar] [CrossRef]
- Schultz, L.; El-Aziz, A.M.; Barkleit, G.; Mummert, K. Corrosion behavior of Nd-Fe-B permanent magnetic alloys. Mater. Sci. Eng. A Struct. 1999, 267, 307–313. [Google Scholar] [CrossRef]
- Pan, S.M.; Zhao, Z.B.; Li, Z.W.; Ma, R.Z. Dynamic observation on microstructure of the B-rich and Nd2Fe14B phases in Nd-Fe-B alloy by means of HVEM and Mossbauer effect analysis. Rare Met. 2011, 30, 666–670. [Google Scholar] [CrossRef]
- Wang, G.P.; Wu, S.; Liu, W.Q.; Huang, Y.L.; Li, Q.L.; Liu, M.L. Mechanical properties and the composition of Nd-rich phase in sintered Nd-Fe-B magnets prepared by spark plasma sintering. J. Magn. Magn. Mater. 2019, 486, 165261. [Google Scholar] [CrossRef]
- Makarova, I.; Soboleva, E.; Osipenko, M.; Kurilo, I.; Laatikainen, M.; Repo, E. Electrochemical leaching of rare-earth elements from spent NdFeB magnets. Hydrometallurgy 2020, 192, 105264. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, T.; Liu, X.; Liu, P.; Jin, J.; Zou, J.; Yan, M. Coercivity enhancement of Nd–Fe–B sintered magnets with intergranular adding (Pr, Dy, Cu)−Hx powders. J. Magn. Magn. Mater. 2016, 399, 159–163. [Google Scholar] [CrossRef]
- Yu, L.Q.; Wen, Y.H.; Yan, M. Effects of Dy and Nb on the magnetic properties and corrosion resistance of sintered NdFeB. J. Magn. Magn. Mater. 2004, 283, 353–356. [Google Scholar] [CrossRef]
- Bala, H.; Szymura, S.; Pawłowska, G.; Rabinovich, Y.M. Effect of impurities on the corrosion behaviour of neodymium. J. Appl. Electrochem. 1993, 23, 1017–1024. [Google Scholar] [CrossRef]
- Mummert, K.; El-Aziz, A.M.; Barkleit, G.; Rodewald, W.; Schultz, L. Korrosionsverhalten von Nd-Fe-B-Dauermagneten. Mater. Corros. 2000, 51, 13–19. [Google Scholar] [CrossRef]
- Mahesh, B.V.; Raman, R. Role of Nanostructure in Electrochemical Corrosion and High Temperature Oxidation: A Review. Met. Mater. Trans. A 2014, 45, 5799–5822. [Google Scholar] [CrossRef]
- Prakash, V.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. Chapter 22—Simultaneous Electrochemical Recovery of REEs and Iron from Magnet Scrap: A Theoretical Analysis. In Rare Earths Industry; Borges De Lima, I., Leal Filho, W., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 335–346. ISBN 978-0-12-802328-0. [Google Scholar]
- Xu, X.; Sturm, S.; Samardzija, Z.; Vidmar, J.; Scancar, J.; Rozman, K.Z. Direct Recycling of Nd-Fe-B Magnets Based on the Recovery of Nd2Fe14B Grains by Acid-free Electrochemical Etching. Chemsuschem 2019, 12, 4754–4758. [Google Scholar] [CrossRef]
- Dong, S.Z.; Li, W.; Chen, H.S.; Han, R. The status of Chinese permanent magnet industry and R&D activities. AIP Adv. 2017, 7, 056237. [Google Scholar] [CrossRef]
- Rademaker, J.H.; Kleijn, R.; Yang, Y. Recycling as a Strategy against Rare Earth Element Criticality: A Systemic Evaluation of the Potential Yield of NdFeB Magnet Recycling. Environ. Sci. Technol. 2013, 47, 10129–10136. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergoric, M.; Ekberg, C.; Retegan, T. Reclaiming REEs from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Reck, B.K.; Graedel, T.E. Challenges in Metal Recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Chowdhury, N.A.; Deng, S.D.; Jin, H.Y.; Prodius, D.; Sutherland, J.W.; Nlebedim, I.C. Sustainable Recycling of Rare-Earth Elements from NdFeB Magnet Swarf: Techno-Economic and Environmental Perspectives. ACS Sustain. Chem. Eng. 2021, 9, 15915–15924. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, C.; Wang, Y.; Zhao, Z.; Li, B. Recycling of ultrafine NdFeB waste by the selective precipitation of rare earth and the electrodeposition of iron in hydrofluoric acid. Sep. Purif. Technol. 2020, 230, 115870. [Google Scholar] [CrossRef]
- Padhan, E.; Nayak, A.K.; Sarangi, K. Recovery of neodymium and dysprosium from NdFeB magnet swarf. Hydrometallurgy 2017, 174, 210–215. [Google Scholar] [CrossRef]
- Haider, S.K.; Kim, D.; Kang, Y.S. Four-step eco-friendly energy efficient recycling of contaminated Nd2Fe14B sludge and coercivity enhancement by reducing oxygen content. Sci. Rep. 2021, 11, 22255. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Castro, F.D.; Prasad, S.; Rtimi, S. Emerging technologies for the recovery of REEs (REEs) from the end-of-life electronic wastes: A review on progress, challenges, and perspectives. Environ. Sci. Pollut. R. 2020, 27, 36052–36074. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Zhou, H.P.; Li, W.Q.; Luo, X.P.; Wang, J.L.; Liu, F.P. Separation and coextraction of REEs and Fe from NdFeB sludge by co-leaching and stepwise precipitation. Sep. Purif. Technol. 2022, 282, 119795. [Google Scholar] [CrossRef]
- Nlebedim, I.C.; King, A.H. Addressing Criticality in REEs via Permanent Magnets Recycling. JOM 2018, 70, 115–123. [Google Scholar] [CrossRef]
- Munchen, D.D.; Stein, R.T.; Veit, H.M. REEs Recycling Potential Estimate Based on End-of-Life NdFeB Permanent Magnets from Mobile Phones and Hard Disk Drives in Brazil. Minerals 2021, 11, 1190. [Google Scholar] [CrossRef]
- Reimer, M.; Schenk-Mathes, H.; Hoffmann, M.; Elwert, T. Recycling Decisions in 2020, 2030, and 2040—When Can Substantial NdFeB Extraction be Expected in the EU? Metals 2018, 8, 867. [Google Scholar] [CrossRef]
- Cho, B.; Cho, Y.; Lee, J.; Yoo, K. Korea’s metal resources recycling research project—Valuable recycling. Geosystem. Eng. 2019, 22, 48–58. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, B.X.; Gao, F.; Chen, W.J.; Nie, Z.R. Life cycle assessment of regeneration technology routes for sintered NdFeB magnets. Int. J. Life Cycle Assess. 2022, 27, 1044–1057. [Google Scholar] [CrossRef]
- Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2010. Available online: https://www.qualenergia.it/sites/default/files/articolo-doc/Critical_raw_materials_for_the_Eu.pdf (accessed on 1 January 2023).
- Critical Materials Strategy; US Department of Energy: Washington, DC, USA, 2010. Available online: https://energy.gov/sites/prod/files/DOE_CMS2011_FINAL_Full.pdf (accessed on 3 January 2023).
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Hogberg, S.; Holboll, J.; Mijatovic, N.; Jensen, B.B.; Bendixen, F.B. Direct Reuse of Rare Earth Permanent Magnets-Coating Integrity. IEEE Trans. Magn. 2017, 53, 8000609. [Google Scholar] [CrossRef]
- Walton, A.; Yi, H.; Rowson, N.A.; Speight, J.D.; Mann, V.; Sheridan, R.S.; Bradshaw, A.; Harris, I.R.; Williams, A.J. The use of hydrogen to separate and recycle neodymium-iron-boron-type magnets from electronic waste. J. Clean. Prod. 2015, 104, 236–241. [Google Scholar] [CrossRef]
- Sheridan, R.S.; Sillitoe, R.; Zakotnik, M.; Harris, I.R.; Williams, A.J. Anisotropic powder from sintered NdFeB magnets by the HDDR processing route. J. Magn. Magn. Mater. 2012, 324, 63–67. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kawamoto, Y.; Ono, T.; Tsubota, M.; Kitagawa, J. Hydrogenation of Nd-Fe-B magnet powder under a high pressure of hydrogen. Results Phys. 2015, 5, 99–100. [Google Scholar] [CrossRef]
- Liu, X.B.; Kesler, M.S.; Besser, M.F.; Kramer, M.J.; McGuire, M.A.; Nlebedim, I.C. Effect of Processing Hydrogen Pressure on Magnetic Properties of HDDR Nd-Fe-B Magnet. IEEE Trans. Magn. 2021, 57, 2100604. [Google Scholar] [CrossRef]
- Sheridan, R.S.; Harris, I.R.; Walton, A. The development of microstructure during hydrogenation-disproportionation-desorption-recombination treatment of sintered neodymium-iron-boron-type magnets. J. Magn. Magn. Mater. 2016, 401, 455–462. [Google Scholar] [CrossRef]
- Poskovic, E.; Ferraris, L.; Franchini, F.; Grande, M.A.; Pallavicini, E. A different approach to rare-earth magnet recycling. In Proceedings of the 2018 IEEE International Conference on Environment and Electrical Engineering and 2018 IEEE Industrial and Commercial Power Systems Europe (EEEIC/I&CPS Europe), Palermo, Italy, 12–15 June 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yin, W.Z.; Ding, G.F.; Ju, J.Y.; Tang, X.; Chen, R.J.; Yuan, J.H.; Yan, A.R. Preparation of anisotropic (Ce, Nd, Pr)-Fe-B powder with HDDR method from wasted sintered magnets. J. Magn. Magn. Mater. 2022, 562, 169745. [Google Scholar] [CrossRef]
- Michalski, B.; Szymanski, M.; Gola, K.; Zygmuntowicz, J.; Leonowicz, M. Experimental evidence for the suitability of the hydrogen decomposition process for the recycling of Nd-Fe-B sintered magnets. J. Magn. Magn. Mater. 2022, 548, 168979. [Google Scholar] [CrossRef]
- Delogu, M.; Berzi, L.; Del Pero, F.; Dattilo, C.A. Definition and sustainability assessment of recycling processes for bonded rare earths permanent magnets used on wind generators. Adv. Mater. Process. Technol. 2022, 1–47. [Google Scholar] [CrossRef]
- Zakotnik, M.; Harris, I.R.; Williams, A.J. Multiple recycling of NdFeB-type sintered magnets. J. Alloys Compd. 2009, 469, 314–321. [Google Scholar] [CrossRef]
- Zakotnik, M.; Tudor, C.O. Commercial-scale recycling of NdFeB-type magnets with grain boundary modification yields products with ‘designer properties’ that exceed those of starting materials. Waste Manag. 2015, 44, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Liu, X.; Jin, W.; Fu, S.; Yang, E.; Liu, X.; Zhao, L.; Zhang, X.; Yan, M. Effect of Cu grain boundary modification on microstructure and corrosion resistance in recycled Nd-Fe-B sintered magnets. J. Magn. Magn. Mater. 2022, 550, 169109. [Google Scholar] [CrossRef]
- Liu, W.Q.; Li, C.; Zakotnik, M.; Ming, Y.; Zhang, D.; Huang, X. Recycling of waste Nd-Fe-B sintered magnets by doping with dysprosium hydride nanoparticles. J. Rare Earth 2015, 33, 846–849. [Google Scholar] [CrossRef]
- Ikram, A.; Mehmood, M.F.; Samardzija, Z.; Sheridan, R.S.; Awais, M.; Walton, A.; Sturm, S.; Kobe, S.; Rozman, K.Z. Coercivity Increase of the Recycled HDDR Nd-Fe-B Powders Doped with DyF3 and Processed via Spark Plasma Sintering & the Effect of Thermal Treatments. Materials 2019, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Masuda, M.; Suzuki, S.; Machida, K.I. Recycling of rare earth sintered magnets as isotropic bonded magnets by melt-spinning. J. Alloys Compd. 2004, 374, 393–396. [Google Scholar] [CrossRef]
- Li, L.; Post, B.; Kunc, V.; Elliott, A.M.; Paranthaman, M.P. Additive manufacturing of near-net-shape bonded magnets: Prospects and challenges. Scr. Mater. 2017, 135, 100–104. [Google Scholar] [CrossRef]
- Paranthaman, M.P.; Shafer, C.S.; Elliott, A.M.; Siddel, D.H.; McGuire, M.A.; Springfield, R.M.; Martin, J.; Fredette, R.; Ormerod, J. Binder Jetting: A Novel NdFeB Bonded Magnet Fabrication Process. JOM 2016, 68, 1978–1982. [Google Scholar] [CrossRef]
- Gandha, K.; Ouyang, G.Y.; Gupta, S.; Kunc, V.; Paranthaman, M.P.; Nlebedim, I.C. Recycling of additively printed rare-earth bonded magnets. Waste Manag. 2019, 90, 94–99. [Google Scholar] [CrossRef]
- Kumari, R.; Samadder, S.R. A critical review of the pre-processing and metals recovery methods from e-wastes. J. Environ. Manag. 2022, 320, 115887. [Google Scholar] [CrossRef] [PubMed]
- Takeda, O.; Lu, X.; Zhu, H.M. Recent Trend on the Studies of Recycling Technologies of Rare Earth Metals. In REWAS 2022: Developing Tomorrow’s Technical Cycles, Volume I; The Minerals, Metals & Materials Series; Lazou, A., Daehn, K., Fleuriault, C., Gökelma, M., Olivetti, E., Meskers, C., Eds.; Springer: Cham, Switzerland, 2022; pp. 259–266. [Google Scholar] [CrossRef]
- Papagianni, S.; Moschovi, A.M.; Sakkas, K.M.; Chalaris, M.; Yakoumis, I. Preprocessing and Leaching Methods for Extraction of REE from Permanent Magnets: A Scoping Review. Appl. Chem. 2022, 2, 199–212. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward Sustainability for Recovery of Critical Metals from Electronic Waste: The Hydrochemistry Processes. ACS Sustain. Chem. Eng. 2017, 5, 21–40. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, Y.J.; Liao, C.H.; Popuri, S.; Tsai, S.L.; Hung, C.E. Selective Leaching Process for Neodymium Recovery from Scrap Nd-Fe-B Magnet. Metall. Mater. Trans. A 2013, 44, 5825–5833. [Google Scholar] [CrossRef]
- Gergoric, M.; Barrier, A.; Retegan, T. Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Glycolic, Maleic, and Ascorbic Acids Followed by Solvent Extraction. J. Sustain. Met. 2019, 5, 85–96. [Google Scholar] [CrossRef]
- Stein, R.T.; Kasper, A.C.; Veit, H.M. Recovery of REEs Present in Mobile Phone Magnets with the Use of Organic Acids. Minerals 2022, 12, 668. [Google Scholar] [CrossRef]
- Ding, A.T.; Liu, C.Y.; Zhang, X.W.; Lei, L.C.; Xiao, C.L. ZnCl2: A Green Bronsted Acid for Selectively Recovering REEs from Spent NdFeB Permanent Magnets. Environ. Sci. Technol. 2022, 56, 4404–4412. [Google Scholar] [CrossRef]
- Forte, F.; Riano, S.; Binnemans, K. Dissolution of noble metals in highly concentrated acidic salt solutions. Chem. Commun. 2020, 56, 8230–8232. [Google Scholar] [CrossRef]
- Klemettinen, A.; Żak, A.; Chojnacka, I.; Matuska, S.; Leśniewicz, A.; Wełna, M.; Adamski, Z.; Klemettinen, L.; Rycerz, L. Leaching of Rare Earth Elements from NdFeB Magnets without Mechanical Pretreatment by Sulfuric (H2SO4) and Hydrochloric (HCl) Acids. Minerals 2021, 11, 1374. [Google Scholar] [CrossRef]
- Opare, E.O.; Struhs, E.; Mirkouei, A. A comparative state-of-technology review and future directions for rare earth element separation. Renew. Sustain. Energ. Rev. 2021, 143, 110917. [Google Scholar] [CrossRef]
- Pietrantonio, M.; Pucciarmati, S.; Sebastianelli, L.; Forte, F.; Fontana, D. Materials recovery from end-of-life wind turbine magnets. Int. J. Environ. Sci. Technol. 2022, 19, 8019–8026. [Google Scholar] [CrossRef]
- Pavón, S.; Fortuny, A.; Coll, M.T.; Sastre, A.M. Neodymium recovery from NdFeB magnet wastes using Primene 81R·Cyanex 572 IL by solvent extraction. J. Environ. Manag. 2018, 222, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, M.; Makarova, I.; Sainio, T.; Repo, E. Selective acid leaching of rare earth elements from roasted NdFeB magnets. Sep. Purif. Technol. 2021, 278, 119571. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Kim, Y.H.; Lee, M.S. Selective Dissolution of Nd2O3 from the Mixture with Fe2O3 and Ga2O3 by Using Inorganic Acid Solutions Containing Ethylene Glycol. Metals 2022, 12, 1268. [Google Scholar] [CrossRef]
- Stopic, S.; Friedrich, B. Advances in Understanding of the Application of Unit Operations in Metallurgy of Rare Earth Elements. Metals 2021, 11, 978. [Google Scholar] [CrossRef]
- Akcil, A.; Ibrahim, Y.A.; Meshram, P.; Panda, S.; Abhilashb. Hydrometallurgical recycling strategies for recovery of REEs from consumer electronic scraps: A review. J. Chem. Technol. Biot. 2021, 96, 1785–1797. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes-a review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Omodara, L.; Pitkaaho, S.; Turpeinen, E.M.; Saavalainen, P.; Oravisjarvi, K.; Keiski, R.L. Recycling and substitution of light REEs, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Sun, H.; Wang, T.Y.; Li, C.X.; Yang, Y.S. Recycling rare earth from ultrafine NdFeB waste by capturing fluorine ions in wastewater and preparing them into nano-scale neodynium oxyfluoride. J. Rare Earth 2022, 40, 815–821. [Google Scholar] [CrossRef]
- Liu, Q.S.; Tu, T.; Guo, H.; Cheng, H.J.; Wang, X.Z. High-efficiency simultaneous extraction of rare earth elements and iron from NdFeB waste by oxalic acid leaching. J. Rare Earth 2021, 39, 323–330. [Google Scholar] [CrossRef]
- Van Loy, S.; Önal, M.A.R.; Binnemans, K.; Van Gerven, T. Recovery of valuable metals from NdFeB magnets by mechanochemically assisted ferric sulfate leaching. Hydrometallurgy 2020, 191, 105154. [Google Scholar] [CrossRef]
- He, L.; Xu, Q.; Li, W.; Dong, Q.; Sun, W. One-step separation and recovery of rare earth and iron from NdFeB slurry via phosphoric acid leaching. J. Rare Earth 2022, 40, 338–344. [Google Scholar] [CrossRef]
- Bi, M.X.; Xu, C.; Wattoo, A.G.; Bagheri, R.; Chen, Y.X.; Mao, S.D.; Lv, Z.S.; Yang, L.J.; Song, Z.L. Corrosion behavior of sintered CeNdFeB magnets in different solutions. J. Alloys Compd. 2017, 703, 232–241. [Google Scholar] [CrossRef]
- Xu, X.; Sturm, S.; Samardzija, Z.; Scancar, J.; Markovic, K.; Rozman, K.Z. A facile method for the simultaneous recovery of rare-earth elements and transition metals from Nd-Fe-B magnets. Green Chem. 2020, 22, 1105–1112. [Google Scholar] [CrossRef]
- Lister, T.E.; Meagher, M.; Strauss, M.L.; Diaz, L.A.; Rollins, H.W.; Das, G.; Lencka, M.M.; Anderko, A.; Riman, R.E.; Navrotsky, A. Recovery of Rare Earth Elements from Recycled Hard Disk Drive Mixed Steel and Magnet Scrap. Rare Met. Technol. 2021, 139–154. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, E.Y. A Multi-stage Process for Recovery of Neodymium (Nd) and Dysprosium (Dy) from Spent Hard Disc Drives (HDDs). Min. Proc. Ext. Met. Rev. 2021, 42, 90–101. [Google Scholar] [CrossRef]
- Ciro, E.; Alzate, A.; López, E.; Serna, C.; Gonzalez, O. Neodymium recovery from scrap magnet using ammonium persulfate. Hydrometallurgy 2019, 186, 226–234. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, C.; Chung, K.; Kim, S.; Lee, J.; Kumar, J.R. Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: Waste recycling strategies for permanent magnet processing. Hydrometallurgy 2016, 165, 27–43. [Google Scholar] [CrossRef]
- Prusty, S.; Pradhan, S.; Mishra, S. Ionic liquid as an emerging alternative for the separation and recovery of Nd, Sm and Eu using solvent extraction technique-A review. Sustain. Chem. Pharm. 2021, 21, 100434. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H.D. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Traore, M.; Gong, A.J.; Wang, Y.W.; Qiu, L.A.; Bai, Y.Z.; Zhao, W.Y.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.L.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earth 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Wang, J.; Wang, H.; Peng, C.; Wilson, B.P.; Lundström, M. Recovery and separation of rare earths and boron from spent Nd-Fe-B magnets. Min. Eng. 2020, 145, 106097. [Google Scholar] [CrossRef]
- Stopic, S.; Polat, B.; Chung, H.W.; Emil-Kaya, E.; Smiljanic, S.; Gurmen, S.; Friedrich, B. Recovery of REEs through Spent NdFeB Magnet Oxidation (First Part). Metals 2022, 12, 1464. [Google Scholar] [CrossRef]
- Chung, H.W.; Stopic, S.; Emil-Kaya, E.; Gurmen, S.; Friedrich, B. Recovery of REEs from Spent NdFeB-Magnets: Separation of Iron through Reductive Smelting of the Oxidized Material (Second Part). Metals 2022, 12, 1615. [Google Scholar] [CrossRef]
- Shirayama, S.; Okabe, T.H. Selective Extraction and Recovery of Nd and Dy from Nd-Fe-B Magnet Scrap by Utilizing Molten MgCl2. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2018, 49, 1067–1077. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhu, N.W.; Li, Y.; Wu, P.X.; Dang, Z.; Ke, Y.X. Efficient recovery of rare earth elements from discarded NdFeB magnets. Process. Saf. Environ. 2019, 124, 317–325. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Aktan, E.; Borra, C.R.; Blanpain, B.; Van Gerven, T.; Guo, M. Recycling of NdFeB magnets using nitration, calcination and water leaching for REE recovery. Hydrometallurgy 2017, 167, 115–123. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Van Gerven, T. Recycling of NdFeB Magnets Using Sulfation, Selective Roasting, and Water Leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Riaño, S.; Binnemans, K. Alkali baking and solvometallurgical leaching of NdFeB magnets. Hydrometallurgy 2020, 191, 105213. [Google Scholar] [CrossRef]
- Yin, T.; Xue, Y.; Yan, Y.; Ma, Z.; Ma, F.; Zhang, M.; Wang, G.; Qiu, M. Recovery and separation of REEs by molten salt electrolysis. Int. J. Miner. Metall. Mater. 2021, 28, 899–914. [Google Scholar] [CrossRef]
- Jeon, Y.; Hur, J.; Jeong, G.Y.; Sohn, S.; Park, J. Selective and efficient extraction of Nd from NdFeB magnets via ionization in LiCl-KCl-CdCl2 melt. J. Alloys Compd. 2021, 860, 158424. [Google Scholar] [CrossRef]
- Yang, Y.S.; Lan, C.Q.; Guo, L.Y.; An, Z.Q.; Zhao, Z.W.; Li, B.W. Recovery of rare-earth element from rare-earth permanent magnet waste by electro-refining in molten fluorides. Sep. Purif. Technol. 2020, 233, 116030. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Mahanty, B. Direct dissolution of metal oxides in ionic liquids as a smart strategy for separation: Current status and prospective. Sep. Sci. Technol. 2022, 57, 2792–2823. [Google Scholar] [CrossRef]
- Xu, X.; Sturm, S.; Zavasnik, J.; Rozman, K.Z. Electrodeposition of a Rare-Earth Iron Alloy from an Ionic-Liquid Electrolyte. Chemelectrochem 2019, 6, 2860–2869. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Ehrenburg, M.R.; Arkhipushkin, I.A.; Rudnev, A.V. Interfacial effects in the electro(co)deposition of Nd, Fe, and Nd-Fe from an ionic liquid with controlled amount of water. Electrochim. Acta 2021, 398, 139342. [Google Scholar] [CrossRef]
- Da Costa, J.; Costa, J.M.; Neto, A. Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes. Metals 2022, 12, 2095. [Google Scholar] [CrossRef]
- Rout, A. Electroanalytical Chemistry of Lanthanides/Actinides and the Feasibility of Direct Electrodeposition in Ligand Containing Ionic Liquids: A Comprehensive Review. J. Electrochem. Soc. 2022, 169, 126502. [Google Scholar] [CrossRef]
- Venkatesan, P.; Vander Hoogerstraete, T.; Hennebel, T.; Binnemans, K.; Sietsma, J.; Yang, Y. Selective electrochemical extraction of REEs from NdFeB magnet waste at room temperature. Green Chem. 2018, 20, 165–173. [Google Scholar] [CrossRef]
- Venkatesan, P.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. An environmentally friendly electro-oxidative approach to recover valuable elements from NdFeB magnet waste. Sep. Purif. Technol. 2018, 191, 384–391. [Google Scholar] [CrossRef]
- Makarova, I.; Ryl, J.; Sun, Z.; Kurilo, I.; Górnicka, K.; Laatikainen, M.; Repo, E. One-step recovery of REE oxalates in electro-leaching of spent NdFeB magnets. Sep. Purif. Technol. 2020, 251, 117362. [Google Scholar] [CrossRef]
- Reisdorfer, G.; Bertuol, D.; Tanabe, E.H. Recovery of neodymium from the magnets of hard disk drives using organic acids. Miner. Eng. 2019, 143. [Google Scholar] [CrossRef]
- Kumari, A.; Dipali; Randhawa, N.S.; Sahu, S.K. Electrochemical treatment of spent NdFeB magnet in organic acid for recovery of rare earths and other metal values. J. Clean. Prod. 2021, 309, 127393. [Google Scholar] [CrossRef]
- Makarava, I.; Kasach, A.; Kharytonau, D.; Kurilo, I.; Laatikainen, M.; Repo, E. Enhanced acid leaching of rare earths from NdCeFeB magnets. Min. Eng. 2022, 179, 107446. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y. Green electrochemical redox mediation for valuable metal extraction and recycling from industrial waste. Green Chem. 2020, 22, 6288–6639. [Google Scholar] [CrossRef]
- Kaplan, V.; Feldman, Y.; Gartsman, K.; Leitus, G.; Wachtel, E.; Lubomirsky, I. Electrolytic Hydrogen Decrepitation of NdFeB Magnets Under Ambient Conditions. J. Sustain. Met. 2022, 8, 1290–1298. [Google Scholar] [CrossRef]

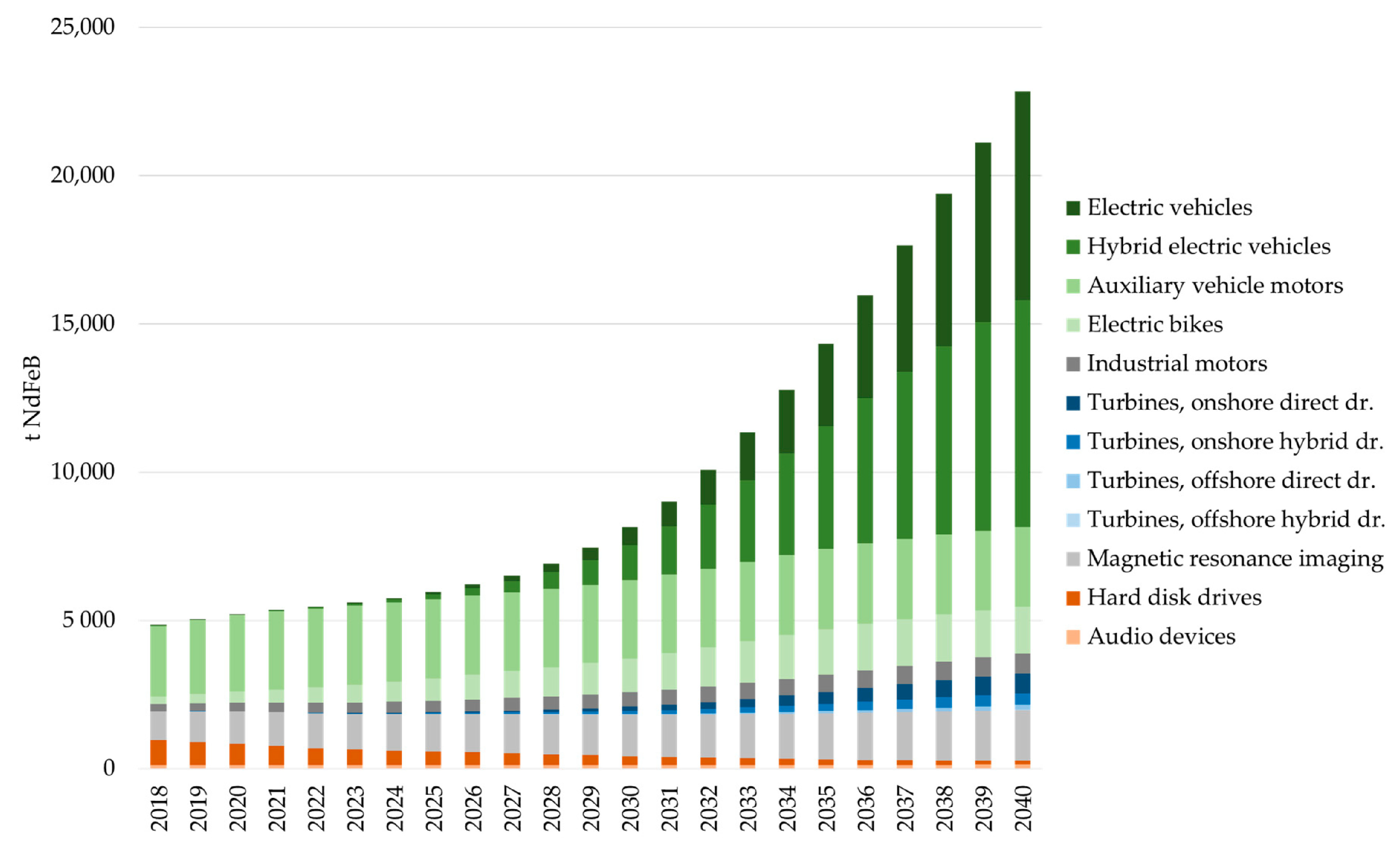




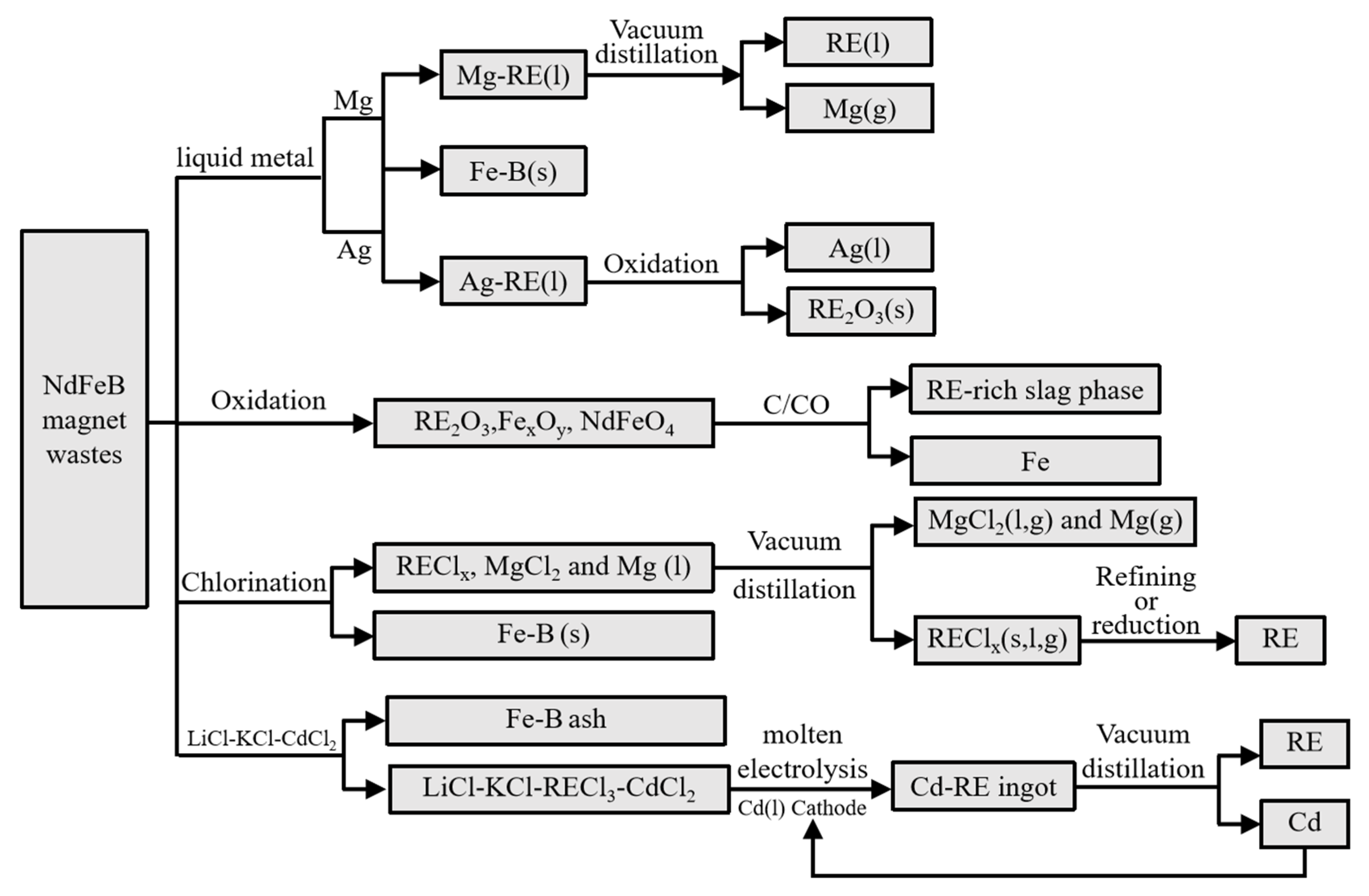

| REEs Content | Nd | Fe | B | Pr | Dy | Co | La | Nb | Gd | Cu | Al | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | 10.70–14.00 | 78.00–79.20 | 5.75–6.00 | ≈2.68 | 0.43–0.60 | ≈0.79 | - | - | - | ≈0.11 | ≈0.19 | - |
| Medium | 21.00–28.00 | 61.09–70.60 | 0.73–1.04 | 0.12–2.62 | 1.00–6.30 | 0.57–3.34 | - | ≈0.37 | ≈0.02 | ≈0.15 | 0.20–0.95 | 1.00–3.00 |
| High | 19.4–30.73 | 58.16–67.15 | 0.96–1.02 | 0.07–7.10 | 0.79–5.93 | 0.42–4.22 | ≈1.58 | ≈0.83 | ≈1.51 | - | 0.34–1.04 | ≈0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Hu, W.; Zhao, J.; Zhu, H. Technologies of Recycling REEs and Iron from NdFeB Scrap. Metals 2023, 13, 779. https://doi.org/10.3390/met13040779
Xiao F, Hu W, Zhao J, Zhu H. Technologies of Recycling REEs and Iron from NdFeB Scrap. Metals. 2023; 13(4):779. https://doi.org/10.3390/met13040779
Chicago/Turabian StyleXiao, Fusheng, Wentao Hu, Jianqi Zhao, and Hongmin Zhu. 2023. "Technologies of Recycling REEs and Iron from NdFeB Scrap" Metals 13, no. 4: 779. https://doi.org/10.3390/met13040779







