The Efficiency of Thermite-Assisted Underwater Wet Flux-Cored Arc Welding Process: Electrical Dependence, Microstructural Changes, and Mechanical Properties
Abstract
1. Introduction
2. Experimental Materials and Procedures
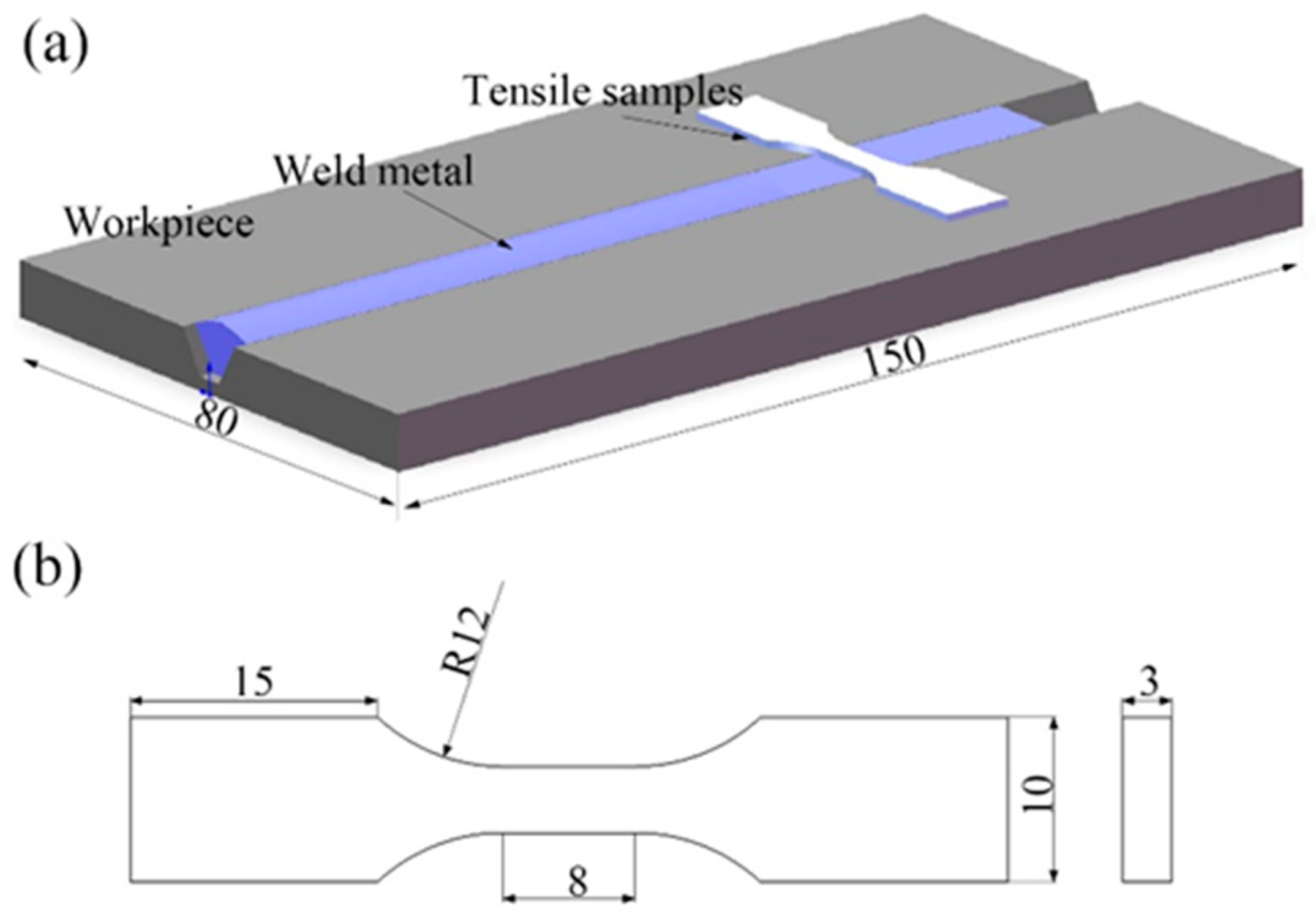
2.1. Underwater Wet Welding Process
2.2. Materials
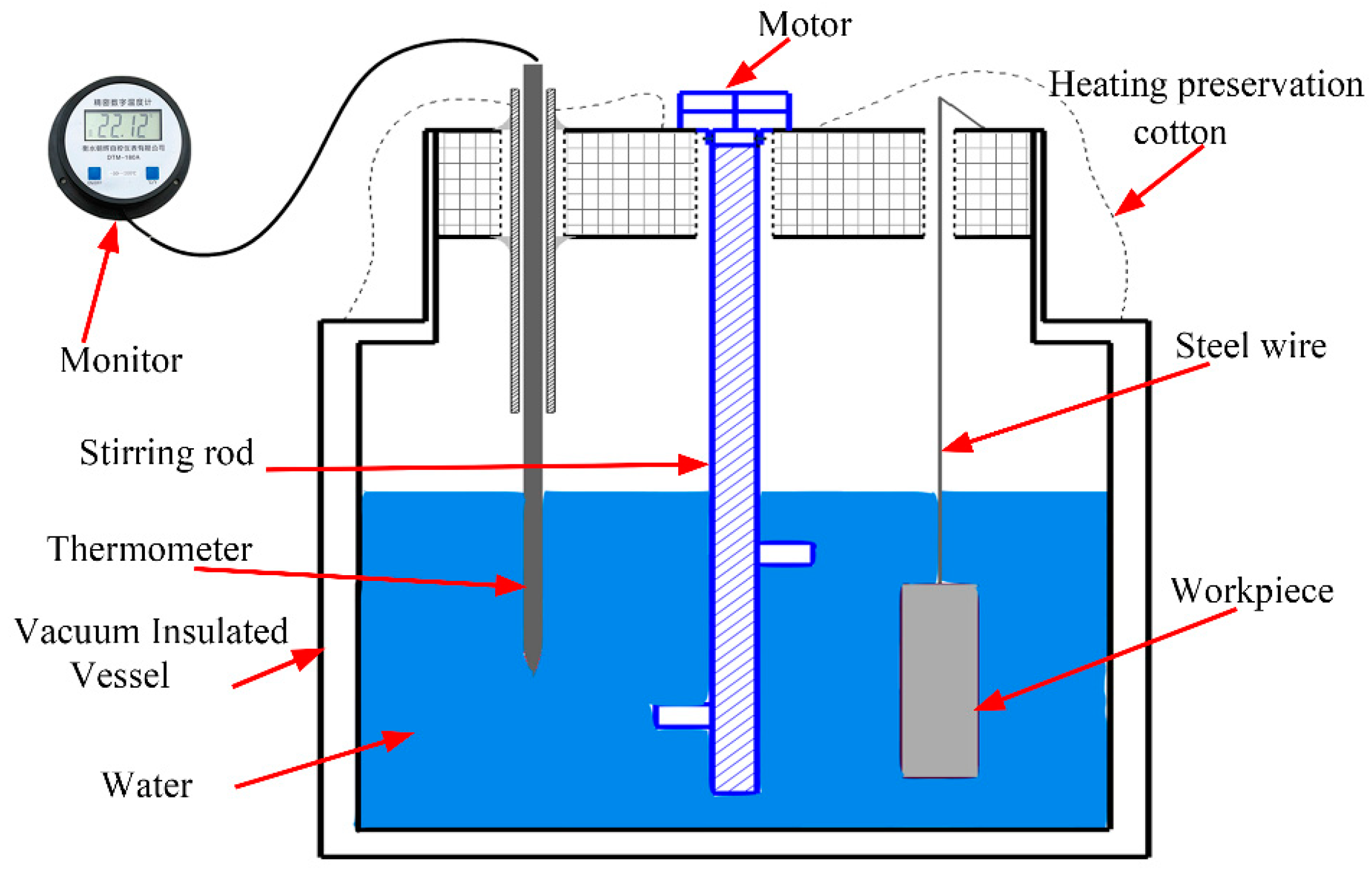
2.3. Experimental Methods
3. Results and Discussion
3.1. Weld Appearances and Slag
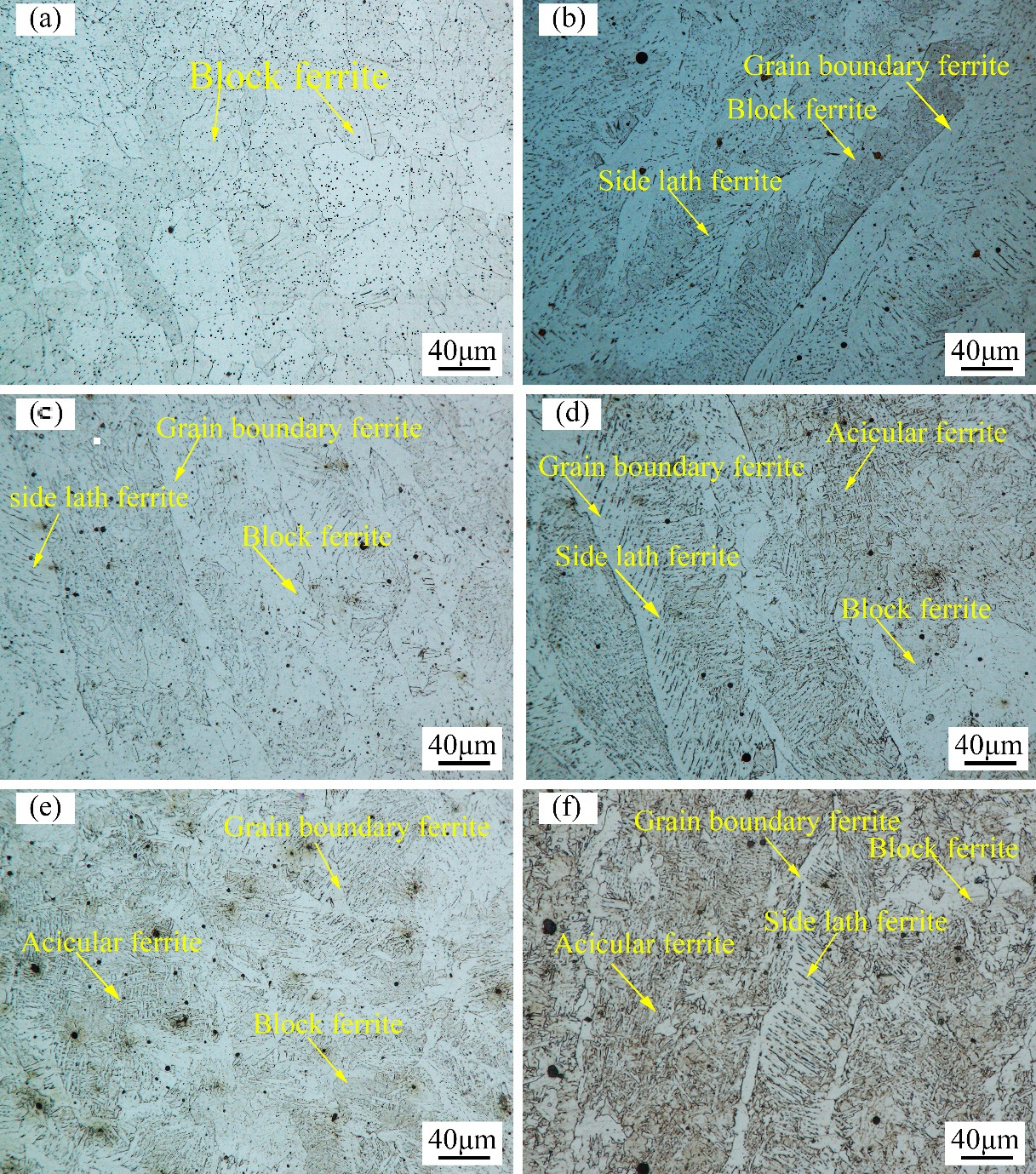
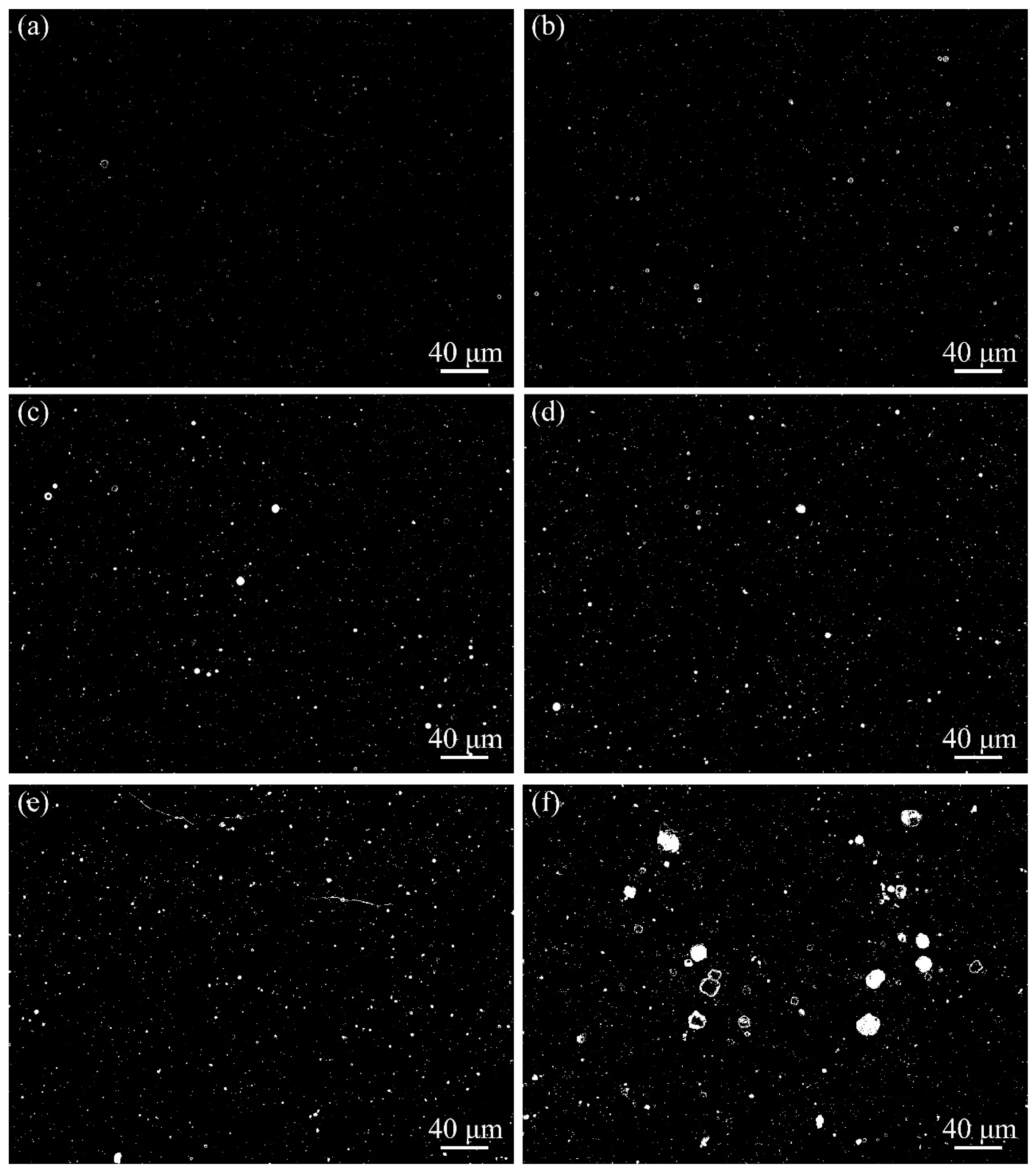
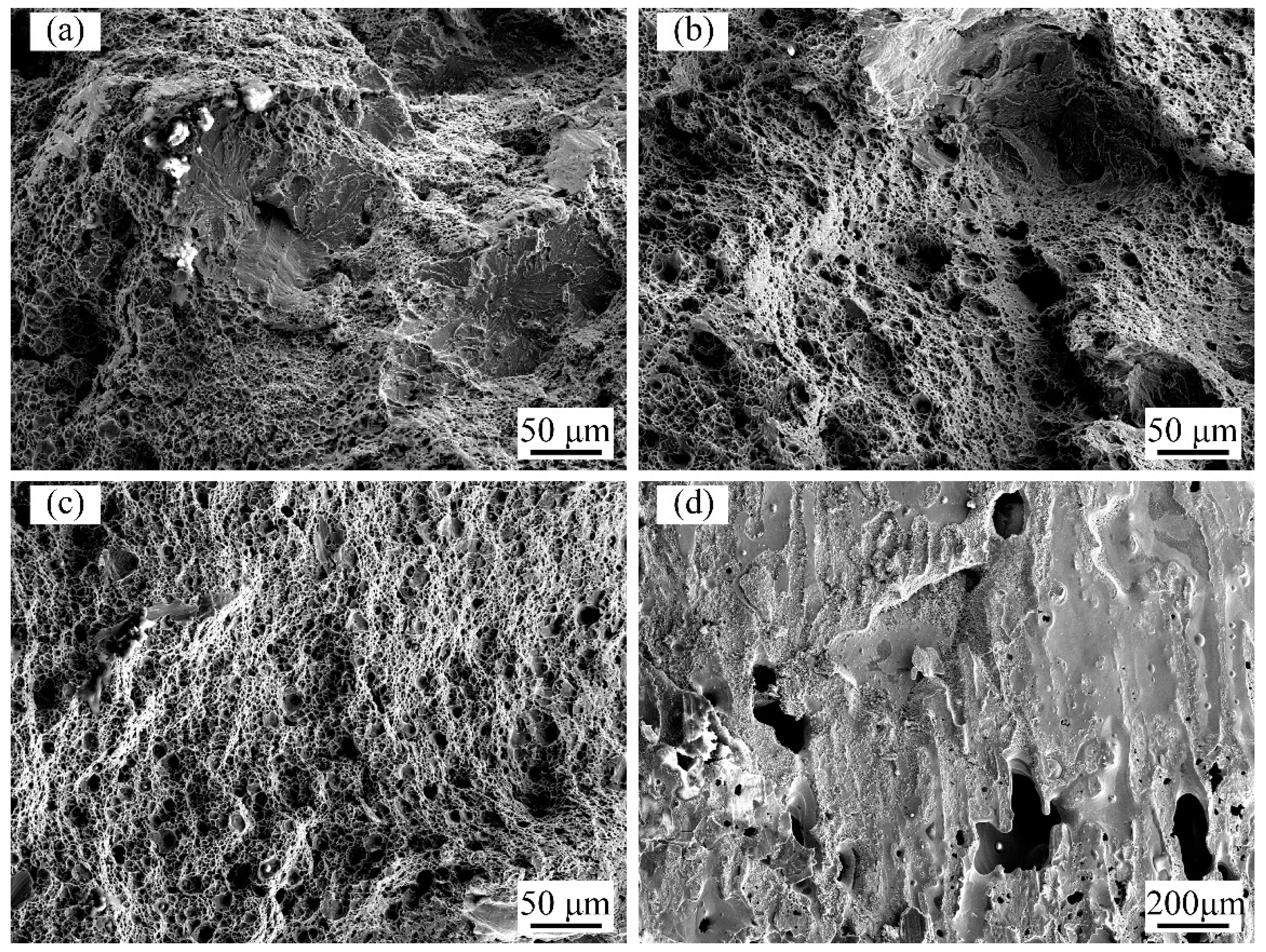
3.2. Microstructure Characteristics of Welded Joints with Different Thermite Contents
3.3. Mechanical Properties of Welded Joints with Different Thermite Contents
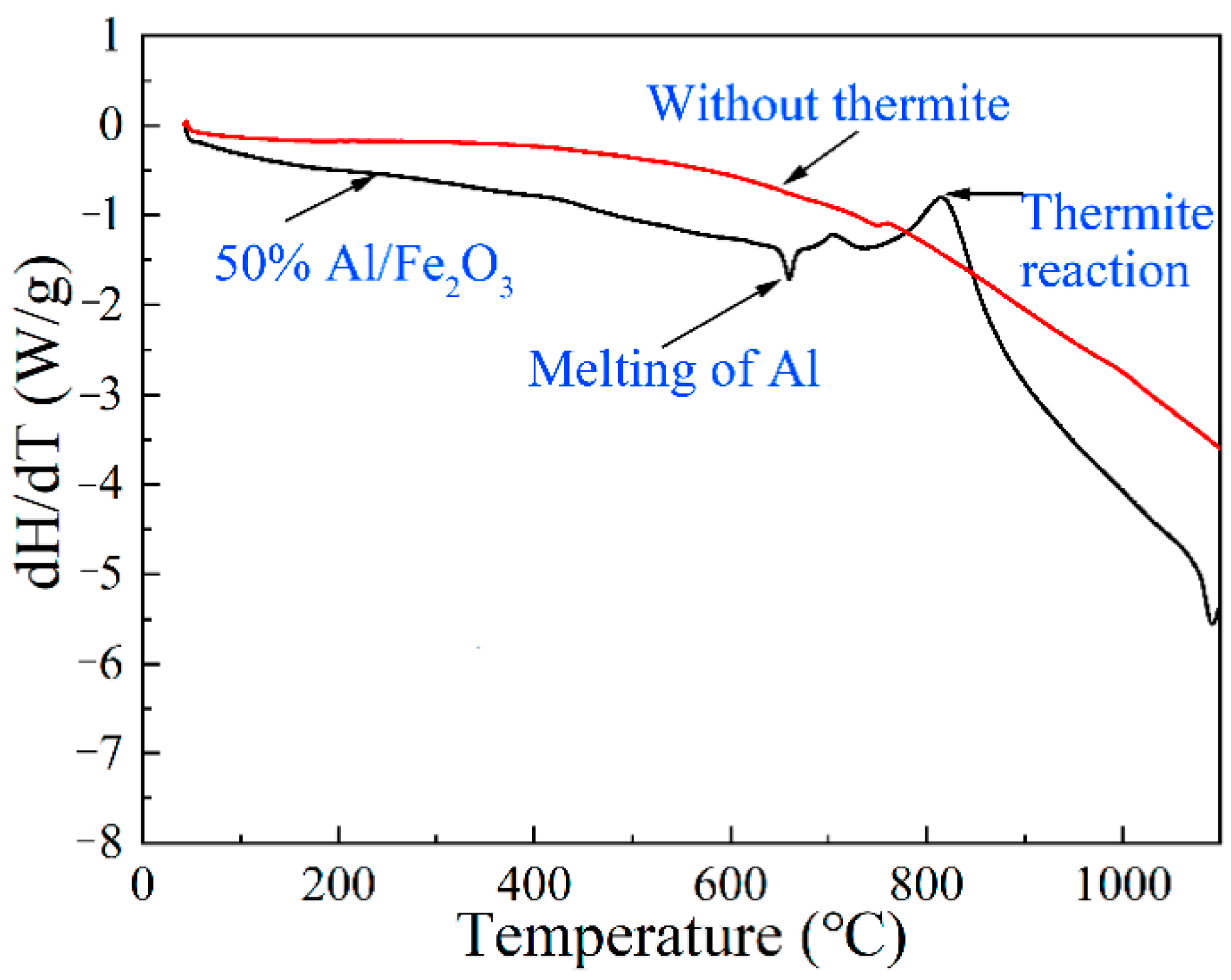
3.4. Effect of Thermite on the Heat Transfer Mechanism of the Welding Process
4. Conclusions
- The addition of the exothermic flux improved the underwater wet weld’s appearance. The FeO content in the slag decreased, and the Al2O3 content increased with increasing thermite content. The slag’s basicity fell from 1.77 to 0.73 because of the formation of Al2O3.
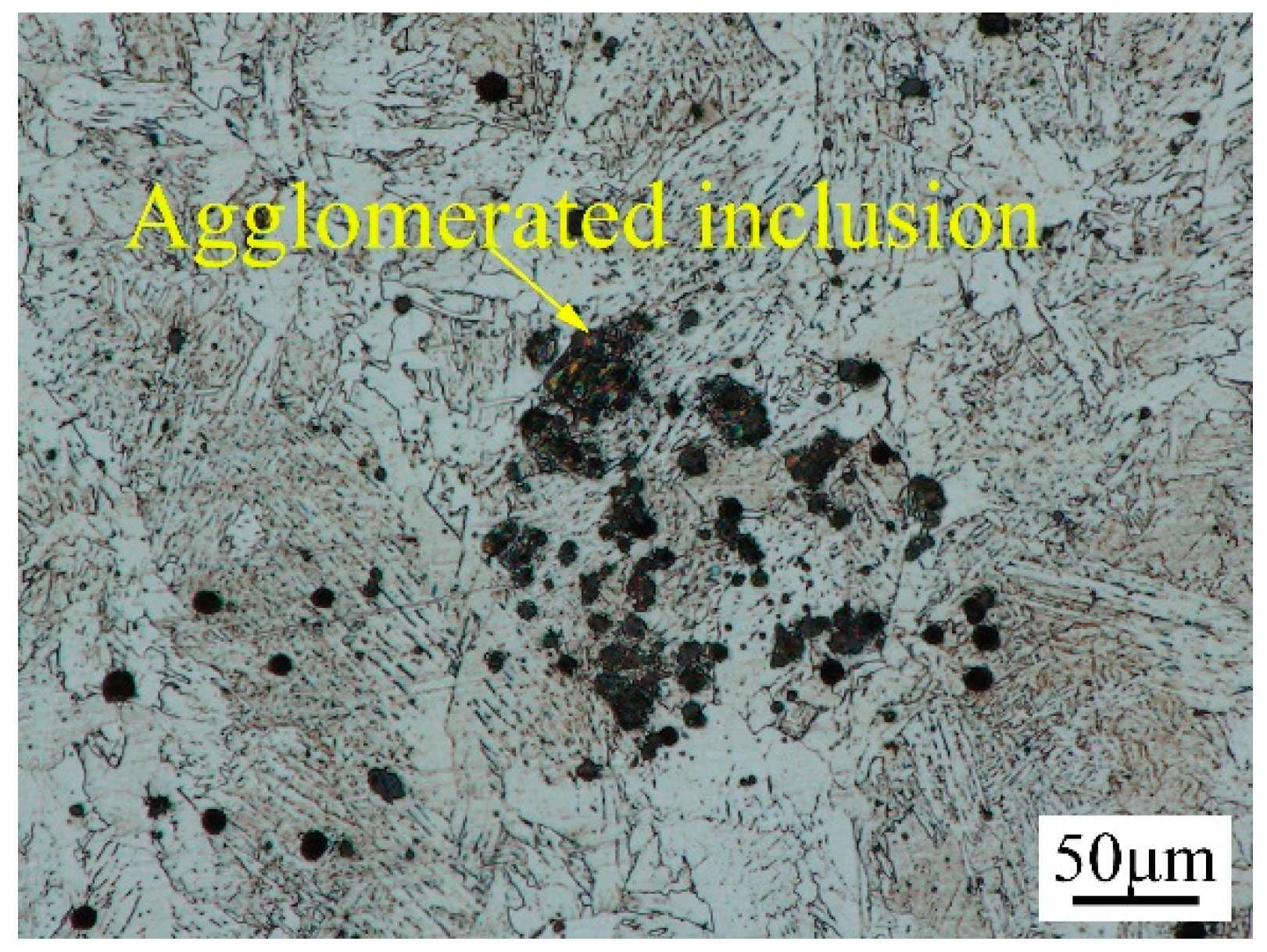
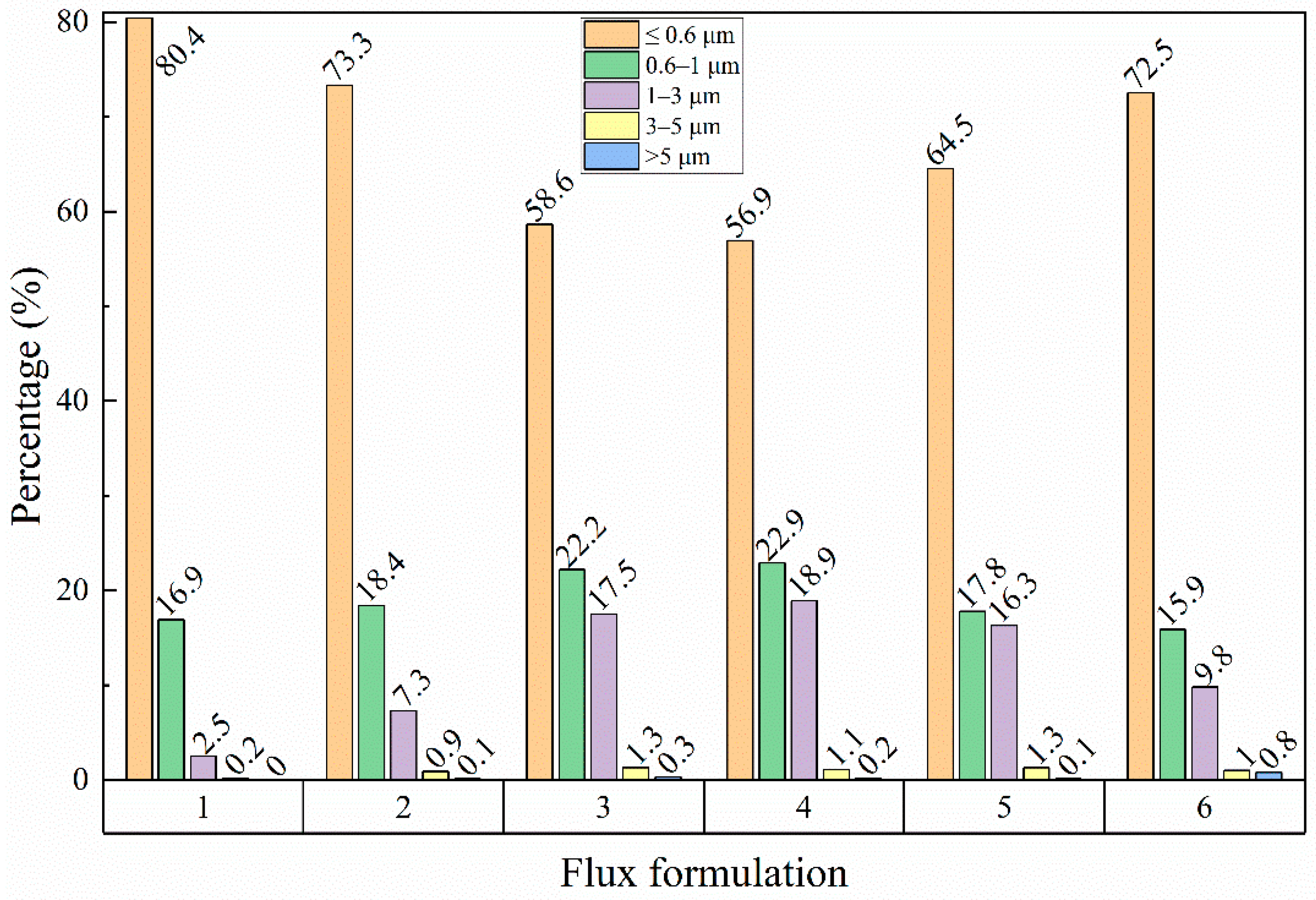
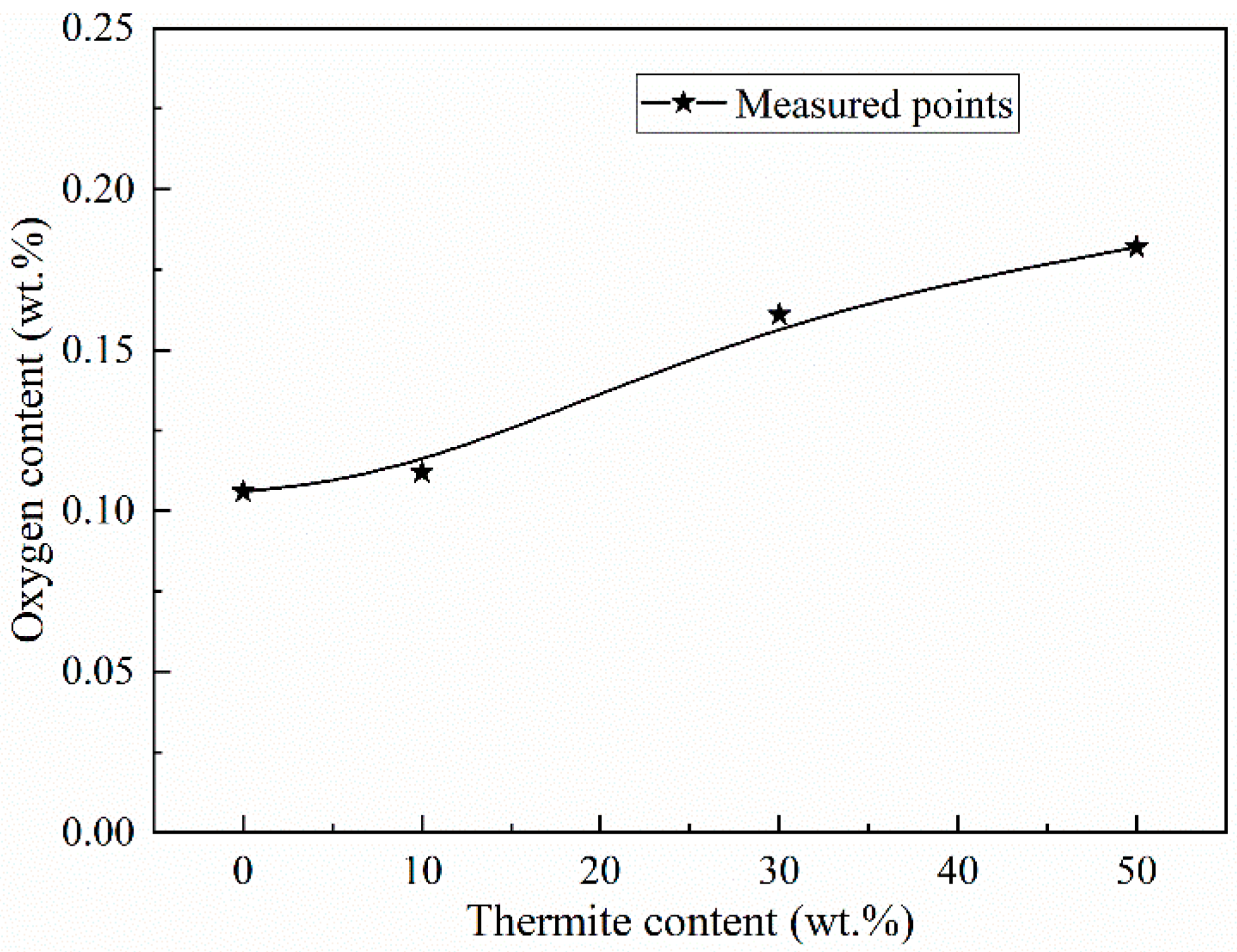
- Al/Fe2O3 thermite addition increased the inclusion density and area fraction in the underwater wet weld metal. The excessive addition of Al/Fe2O3 thermite led to agglomerated inclusions in the weld metal. The inclusion types changed from Fe-O to SiO2-Al2O3 mixtures.
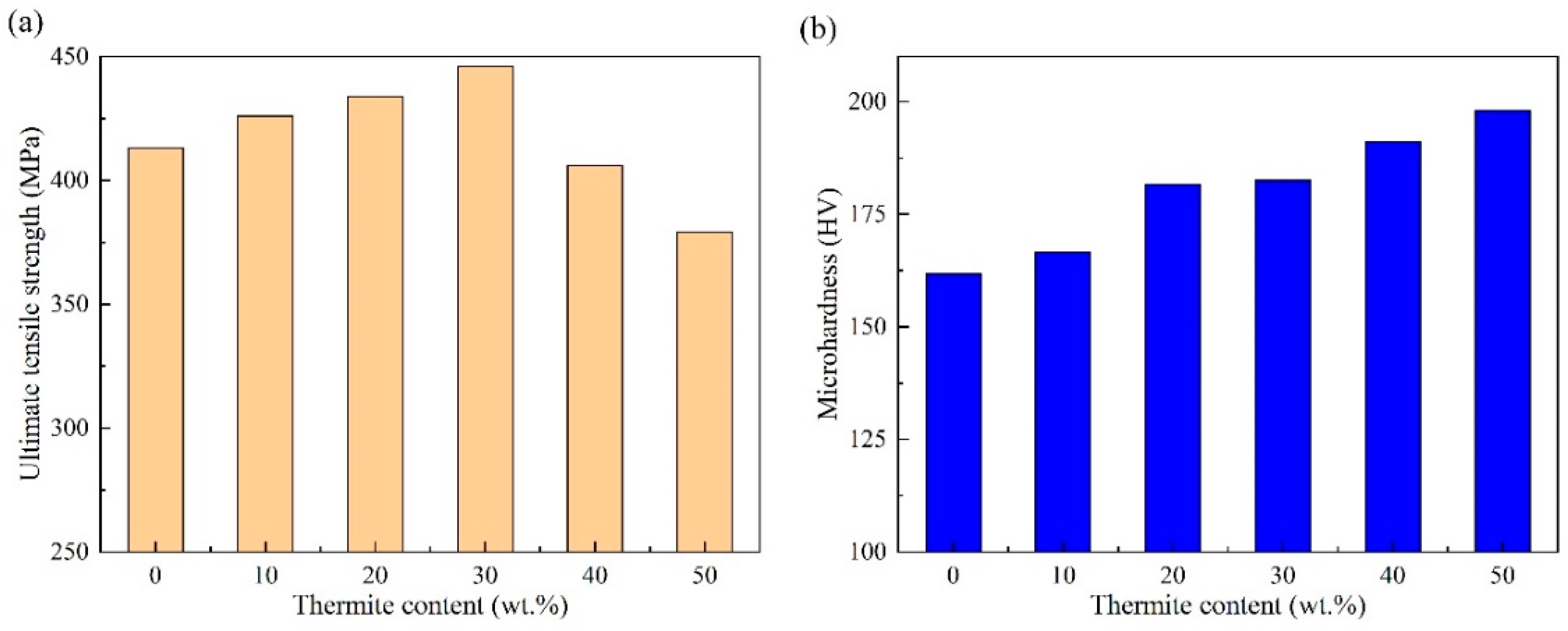
- An obvious change in microstructure was observed due to the addition of thermite caused by the de-oxidation products of the thermite process. The ultimate tensile strength first increased and then declined with the addition of thermite. A maximum of 446 MPa was obtained with a thermite percentage of 30 wt.%.
- The addition of Al/Fe2O3 thermite to flux-cored wire slightly enhanced the thermal efficiency of the welding process. The maximum chemical heat produced by the exothermic reaction accounted for 7% of the heat obtained by the workpiece. Future research on thermite-aided underwater wet welding should aim to develop a new type of thermite and improve the efficiency of the underwater wet welding process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omajene, J.E.; Martikainen, J.; Kah, P.; Pirinen, M. Fundamental difficulties associated with underwater wet welding. Int. J. Eng. Res. Appl. 2014, 4, 26–31. [Google Scholar]
- Fydrych, D.; Łabanowski, J.; Rogalski, G.; Haras, J.; Tomków, J.; Świerczyńska, A.; Jakóbczak, P.; Kostro, Ł. Weldability of S500MC steel in underwater conditions. Adv. Mater. Sci. 2014, 14, 37–45. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.; Hou, S.; Zhang, T.; Jin, P.; Feng, J. Dynamic control of current and voltage waveforms and droplet transfer for ultrasonic-wave-assisted underwater wet welding. Mater. Des. 2019, 181, 108051. [Google Scholar] [CrossRef]
- Liu, S.; Ji, H.; Zhao, W.; Hu, C.; Wang, J.; Li, H.; Wang, J.; Lei, Y. Evaluation of Arc Signals, Microstructure and Mechanical Properties in Ultrasonic-Frequency Pulse Underwater Wet Welding Process with Q345 Steel. Metals 2022, 12, 2119. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.; Pan, Z.; Yang, J.; Feng, J. Effects of welding speed on bubble dynamics and process stability in mechanical constraint-assisted underwater wet welding of steel sheets. J. Mater. Process. Technol. 2019, 264, 389–401. [Google Scholar] [CrossRef]
- Tomków, J.; Landowski, M.; Fydrych, D.; Rogalski, G. Underwater wet welding of S1300 ultra-high strength steel. Mar. Struct. 2022, 81, 103120. [Google Scholar] [CrossRef]
- Brätz, O.; Klett, J.; Wolf, T.; Henkel, K.M.; Maier, H.J.; Hassel, T. Induction Heating in Underwater Wet Welding—Thermal Input, Microstructure and Diffusible Hydrogen Content. Materials 2022, 15, 1417. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, X.; Fu, Y.; Luo, W.; Chen, H.; He, J.L. A novel strategy to prevent hydrogen charging via spontaneously molten-slag-covering droplet transfer mode in underwater wet FCAW. Mater. Des. 2023, 226, 111636. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Fydrych, D.; Rogalski, G. Diffusible hydrogen management in underwater wet self-shielded flux cored arc welding. Int. J. Hydrog. Energy 2017, 42, 24532–24540. [Google Scholar] [CrossRef]
- Chen, H.; Guo, N.; Xu, K.; Xu, C.; Zhou, L.; Wang, G. In-situ observations of melt degassing and hydrogen removal enhanced by ultrasonics in underwater wet welding. Mater. Des. 2020, 188, 108482. [Google Scholar] [CrossRef]
- Pessoa, E.C.P.; Society, A.W.; Liu, S. The state of the art of underwater wet welding practice: Part 2. Weld. J. 2021, 100, 171–182. [Google Scholar] [CrossRef]
- Ma, Q.; Li, H.; Liu, S.; Liu, D.; Wang, P.; Zhu, Q.; Lei, Y. Comparative Evaluation of Self-Shielded Flux-Cored Wires Designed for High Strength Low Alloy Steel in Underwater Wet Welding: Arc Stability, Slag Characteristics, and Joints’ Quality. J. Mater. Eng. Perform. 2022, 31, 5231–5244. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, N.; Xu, C.; Du, Y.; Chen, B.; Feng, J. Influence of CaF2 on microstructural characteristics and mechanical properties of 304 stainless steel underwater wet welding using flux-cored wire. J. Manuf. Process. 2019, 45, 138–146. [Google Scholar] [CrossRef]
- Allen, J.W.; Olson, D.L.; Frost, R.H. Exothermically assisted shielded metal arc welding. Weld. J. 1998, 77, 277. [Google Scholar]
- Malene, S.H.; Park, Y.D.; Olson, D.L. Response of exothermic additions to the flux cored arc welding electrode-Part 1 Effectiveness of exothermically reacting magnesium-type flux additions was investigated with the flux cored arc welding process. Weld. J. 2007, 86. [Google Scholar]
- Li, H.L.; Liu, D.; Guo, N.; Chen, H.; Du, Y.P.; Feng, J.C. The effect of alumino-thermic addition on underwater wet welding process stability. J. Mater. Process. Technol. 2017, 245, 149–156. [Google Scholar] [CrossRef]
- Pessoa, E.C.; Bracarense, A.Q.; Liu, S. Exothermic additions in a tubular covered electrode and oxidizing reactions influence on underwater wet welding. In Proceedings of the ASME 2007 26th International Conference on Offshore Mechanics and Arctic Engineering, San Diego, CA, USA, 10–15 June 2007; Volume 42703, pp. 259–268. [Google Scholar]
- Murzin, V.V.; Russo, V.L. Manual underwater welding structures of steel with higher strength. Weld. Int. 1994, 8, 43–46. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, K. Development of exothermic flux for enhanced penetration in submerged arc welding. J. Adv. Manuf. Syst. 2020, 19, 131–146. [Google Scholar] [CrossRef]
- Trembach, B.; Grin, A.; Subbotina, V.; Vynar, V.; Knyazev, S.; Zakiev, V.; Kabatskyi, O. Effect of exothermic addition (CuO-Al) on the structure, mechanical properties and abrasive wear resistance of the deposited metal during self-shielded flux-cored arc welding. Tribol. Ind. 2021, 43, 452. [Google Scholar] [CrossRef]
- Trembach, B.; Balenko, O.; Davydov, V.; Brechko, V.; Trembach, I.; Kabatskyi, O. Prediction the Melting Characteristics of Self-Shielded Flux Cored arc Welding (FCAW-S) with Exothermic Addition (CuO-Al). In Proceedings of the 2022 IEEE 4th International Conference on Modern Electrical and Energy System (MEES), Kremenchuk, Ukraine, 20–23 October 2022; pp. 1–6. [Google Scholar]
- Abson, D.J.; Pargeter, R.J. Factors influencing as-deposited strength, microstructure, and toughness of manual metal arc welds suitable for C-Mn steel fabrications. Int. Met. Rev. 1986, 31, 141–196. [Google Scholar] [CrossRef]
- Ferrante, M.; Farrar, R.A. The role of oxygen rich inclusions in determining the microstructure of weld metal deposits. J. Mater. Sci. 1982, 17, 3293–3298. [Google Scholar] [CrossRef]
- Harrison, P.L.; Farrar, R.A. Influence of oxygen-rich inclusions on the γ→ α phase transformation in high-strength low-alloy (HSLA) steel weld metals. J. Mater. Sci. 1981, 16, 2218–2226. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances: Supplement; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Yasuo, S. The effect of cooling rate on mechanical properties of underwater wet welds in gravity arc welding. Trans. Jpn. Weld. Soc. 1990, 21, 144–149. [Google Scholar]



| Formulation | TiO2 | CaF2 | LiF | SiO2 | MgO | Ti | Fe | Al/Fe2O3 * | Flux/Sheath Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 16 | 2 | 2 | 4 | 2 | 50 | 0 | 30.1 |
| 2 | 24 | 16 | 2 | 2 | 4 | 2 | 40 | 10 | 26.1 |
| 3 | 24 | 16 | 2 | 2 | 4 | 2 | 30 | 20 | 22.0 |
| 4 | 24 | 16 | 2 | 2 | 4 | 2 | 20 | 30 | 18.5 |
| 5 | 24 | 16 | 2 | 2 | 4 | 2 | 10 | 40 | 17.2 |
| 6 | 24 | 16 | 2 | 2 | 4 | 2 | 0 | 50 | 15.6 |
| Formulation | FeO | TiO2 | CaO | SiO2 | Al2O3 | MgO | ZrO2 | CaF2 | Slag Basicity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33.7 | 47.7 | 18.2 | 6.93 | 1.52 | 5.4 | 0.78 | 4.1 | 1.77 |
| 2 | 23 | 46.8 | 18.5 | 8.39 | 8.4 | 6.5 | 0.77 | 4.8 | 1.43 |
| 3 | 18.8 | 43.2 | 17.8 | 10.9 | 14.3 | 6.6 | 0.74 | 4.3 | 1.18 |
| 4 | 17.6 | 39.8 | 15.7 | 13.4 | 18.8 | 5.9 | 0.65 | 4.1 | 0.97 |
| 5 | 12.1 | 38.4 | 16.4 | 15.8 | 23.6 | 6.1 | 0.63 | 4.5 | 0.84 |
| 6 | 13.4 | 33.2 | 14.8 | 19.7 | 26.3 | 5.7 | 0.58 | 4.7 | 0.73 |
| Fe | Ti | Si | O | Mn | Al | |
|---|---|---|---|---|---|---|
| 1 | 49.05 | - | 0.45 | 50.50 | - | - |
| 2 | 27.88 | 2.14 | 5.72 | 57.94 | 3.98 | 2.34 |
| 3 | 17.15 | 3.15 | 7.28 | 62.4 | 4.17 | 5.85 |
| 4 | 15.72 | 2.68 | 8.14 | 60.81 | 2.84 | 9.81 |
| 5 | 14.33 | 2.46 | 10.27 | 56.48 | 1.98 | 14.48 |
| 6 | 7.22 | 3.14 | 8.44 | 56.75 | 2.28 | 22.17 |
| U (V) | I (A) | Mie (%) | Nim (g/mm) | |
|---|---|---|---|---|
| 1 | 26.22 | 143.78 | 0 | 0.7426 |
| 2 | 26.31 | 151.03 | 10 | 0.7426 |
| 3 | 26.27 | 146.65 | 20 | 0.7422 |
| 4 | 26.38 | 146.64 | 30 | 0.7515 |
| 5 | 26.50 | 143.26 | 40 | 0.7525 |
| 6 | 26.52 | 142.67 | 50 | 0.7740 |
| qw (J) | qs (J) | qc (W) | qf (W) | Arc Efficiency (%) | qc/qw (%) | qc/qs (%) | |
|---|---|---|---|---|---|---|---|
| 1 | 75,398 | 55,201 | 0 | 1189 | 73.2 | 0 | 0 |
| 2 | 79,532 | 57,642 | 68 | 1198 | 72.5 | 1.7 | 2.4 |
| 3 | 77,048 | 58,021 | 114 | 1209 | 75.3 | 3.0 | 3.9 |
| 4 | 77,367 | 57,483 | 144 | 1226 | 74.3 | 3.7 | 5.0 |
| 5 | 75,927 | 57,423 | 184 | 1239 | 75.6 | 4.8 | 6.4 |
| 6 | 75,788 | 57,954 | 208 | 1276 | 76.5 | 5.5 | 7.2 |
| Test | U (V) | I (A) | Qc (W) | Qw (W) | Q (W) | Δ*t8/5 | Δ t8/5 |
|---|---|---|---|---|---|---|---|
| 1 | 205.35 | 29.65 | 0 | 6088.63 | 6088.63 | 8.81 | 8.81 |
| 2 | 206.11 | 29.55 | 92.72 | 6090.55 | 6183.28 | 8.82 | 8.96 |
| 3 | 213.73 | 29.50 | 155.45 | 6305.04 | 6460.49 | 9.14 | 9.37 |
| 4 | 215.90 | 29.37 | 196.36 | 6340.98 | 6537.35 | 9.19 | 9.49 |
| 5 | 210.55 | 29.33 | 250.91 | 6175.43 | 6426.34 | 8.94 | 9.32 |
| 6 | 211.17 | 29.27 | 283.64 | 6180.95 | 6464.58 | 8.95 | 9.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, H.; Hu, C.; Wang, Z.; Han, K.; Liu, D.; Wang, J.; Zhu, Q. The Efficiency of Thermite-Assisted Underwater Wet Flux-Cored Arc Welding Process: Electrical Dependence, Microstructural Changes, and Mechanical Properties. Metals 2023, 13, 831. https://doi.org/10.3390/met13050831
Wang J, Li H, Hu C, Wang Z, Han K, Liu D, Wang J, Zhu Q. The Efficiency of Thermite-Assisted Underwater Wet Flux-Cored Arc Welding Process: Electrical Dependence, Microstructural Changes, and Mechanical Properties. Metals. 2023; 13(5):831. https://doi.org/10.3390/met13050831
Chicago/Turabian StyleWang, Jibo, Hongliang Li, Chengyu Hu, Zeyu Wang, Ke Han, Duo Liu, Jianfeng Wang, and Qiang Zhu. 2023. "The Efficiency of Thermite-Assisted Underwater Wet Flux-Cored Arc Welding Process: Electrical Dependence, Microstructural Changes, and Mechanical Properties" Metals 13, no. 5: 831. https://doi.org/10.3390/met13050831
APA StyleWang, J., Li, H., Hu, C., Wang, Z., Han, K., Liu, D., Wang, J., & Zhu, Q. (2023). The Efficiency of Thermite-Assisted Underwater Wet Flux-Cored Arc Welding Process: Electrical Dependence, Microstructural Changes, and Mechanical Properties. Metals, 13(5), 831. https://doi.org/10.3390/met13050831








