The Synthesis of Lead-Bearing Jarosite and Its Occurrence Characteristic and Leaching Toxicity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Simulated Jarosite
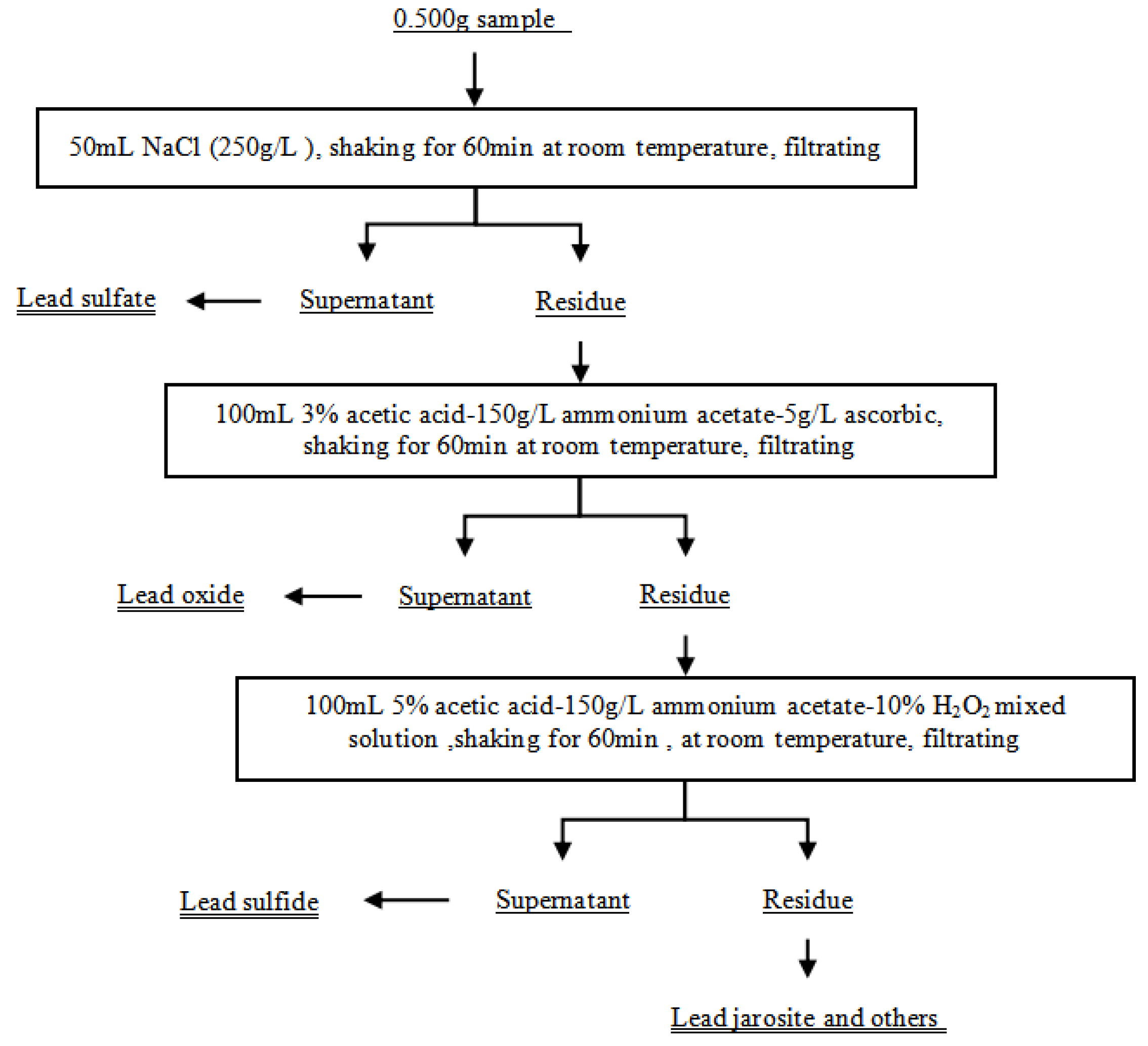
2.3. Leaching Test
2.4. Analysis
2.4.1. Determination of Elemental Composition
2.4.2. Determination of Phase Composition
2.4.3. Others Analysis
3. Results and Discussion
3.1. Chemical Composition
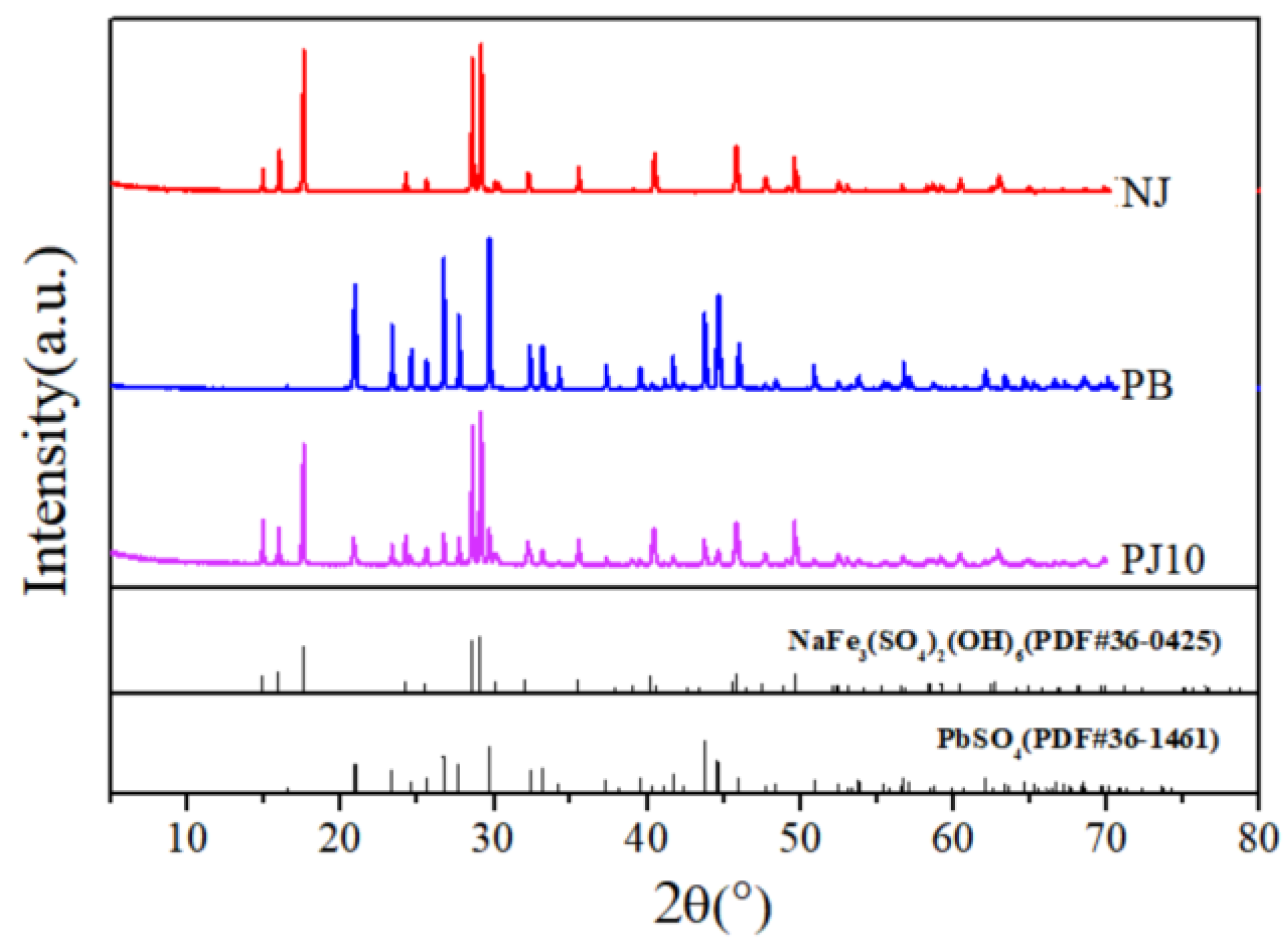
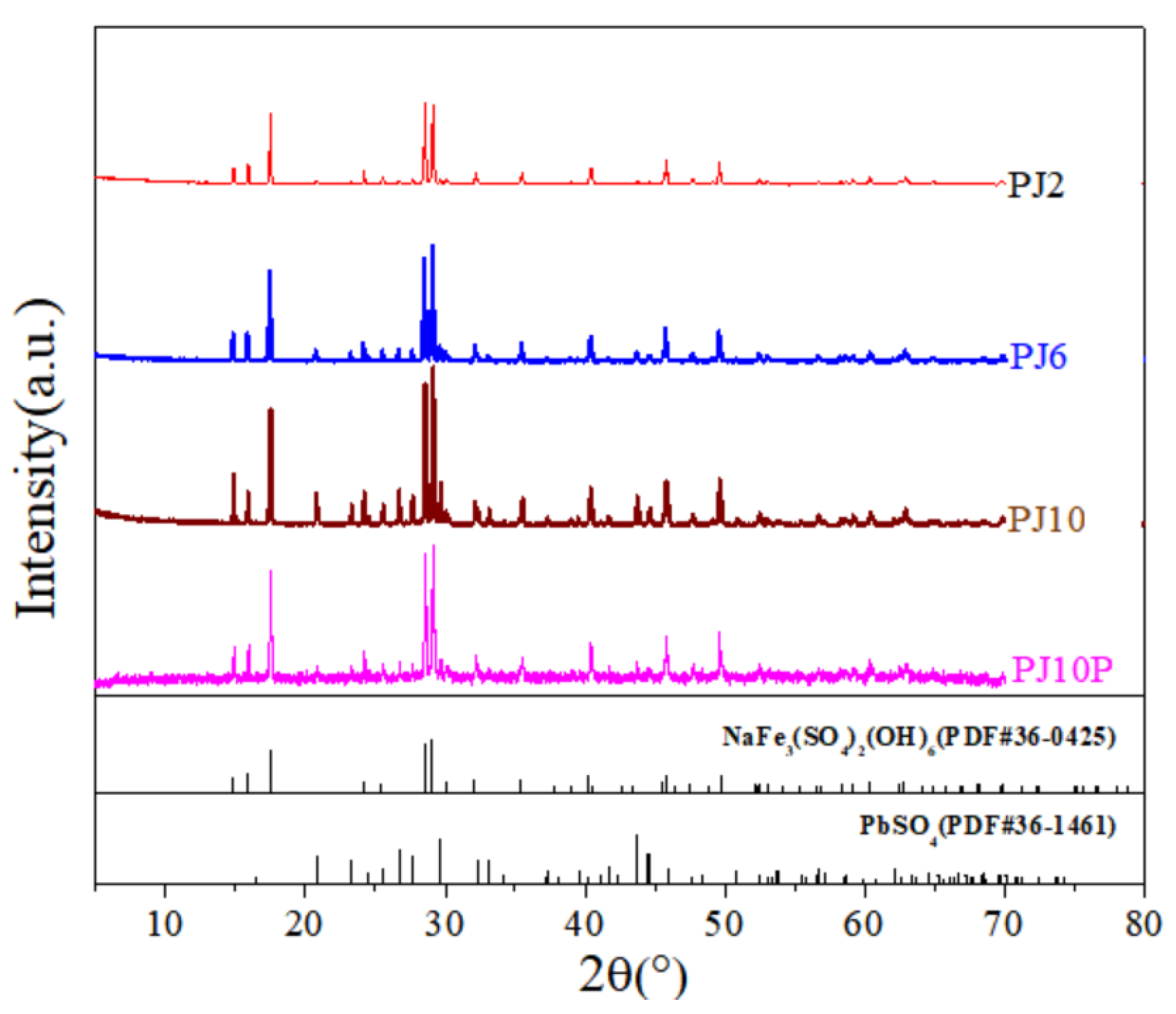
3.2. Phase Composition
3.3. Structural Feature
3.4. Molecular Bonding Structure
3.5. Surface Performance
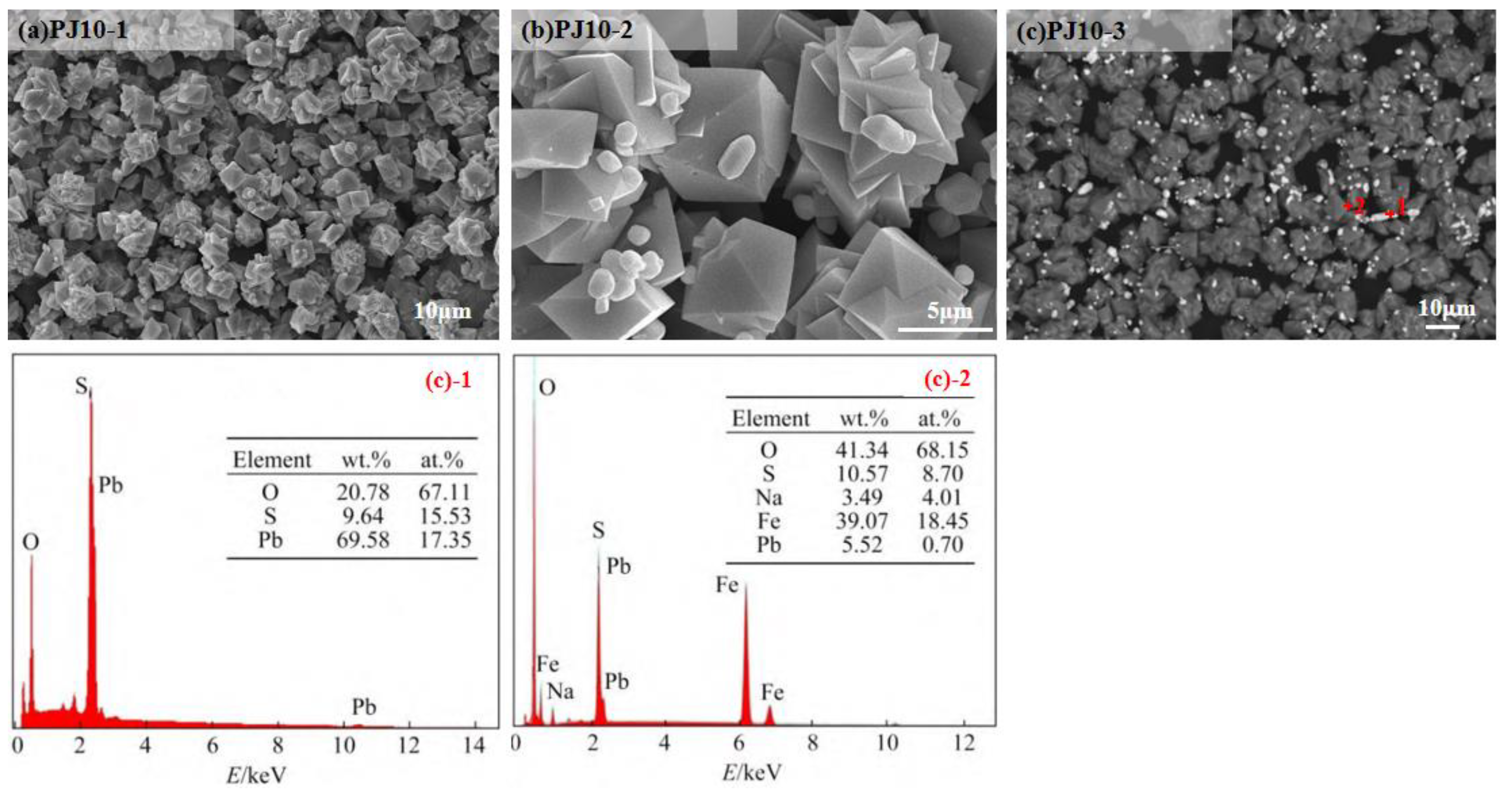
3.6. Particle Size Features
3.7. Environmental Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahamed, A.M.; Pons, M.N.; Ricoux, Q.; Issa, S.; Goettmann, F.; Lapicque, F. New pathway for utilization of jarosite, an industrial waste of zinc hydrometallurgy. Miner. Eng. 2021, 170, 107030. [Google Scholar] [CrossRef]
- Kushwaha, P.; Agarwal, M.; Ghosh, A. Value-added products from jarosite hazardous waste: A review. Mater. Today Proc. 2023, 76, 201–205. [Google Scholar] [CrossRef]
- Hoeber, L.; Steinlechner, S. A comprehensive review of processing strategies for iron precipitation residues from zinc hydrometallurgy. Clean. Eng. Technol. 2021, 4, 100214. [Google Scholar] [CrossRef]
- Cruells, M.; Roca, A. Jarosites: Formation, Structure, Reactivity and Environmental. Metals 2022, 12, 802. [Google Scholar] [CrossRef]
- Shi, M.; Min, X.; Tian, C.; Hao, T.; Zhu, S.; Ge, Y.; Wang, Q.; Yan, X.; Lin, Z. Mechanisms of Pb (II) coprecipitation with natrojarosite and its behavior during acid dissolution. J. Environ. Sci. 2022, 122, 128–137. [Google Scholar] [CrossRef]
- Chen, J.Y. Separation and Utilization of Iron in Metallurgy; Metallurgical Industry Press: Beijing, China, 1991. [Google Scholar]
- Leiva, C.A.; Gálvez, M.E.; Fuentes, G.E.; Acuña, C.A.; Alcota, J.A. Effects of Various Precipitants on Iron Removal from a Zinc Concentrate Pressure Leaching Solution. Minerals 2022, 12, 84. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Dinardo, O. The co-precipitation of copper and zinc with lead jarosite. Hydrometallurgy 1983, 11, 61–78. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Dinardo, O.; Kaiman, S. Factors affecting lead jarosite formation. Hydrometallurgy 1980, 5, 305–324. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Kaiman, S. Synthesis and properties of jarosite-type compounds. Can. Mineral. 1976, 14, 151–158. [Google Scholar]
- Dutrizac, J.E. The Behaviour of Impurities during Jarosite Precipitation. In Hydrometallurgical Process Fundamentals; Springer: Berlin/Heidelberg, Germany, 1984; pp. 125–169. [Google Scholar]
- Dutrizac, J.E. The Behaviour of the Rare Earths during the Precipitation of Sodium, Potassium, and Lead Jarosites. Hydrometallurgy 2004, 73, 11–30. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Chen, T.T. Factors Affecting the Incorporation of Cobalt and Nickel in Jarosite. Can. Metall. Q. 2004, 43, 305–320. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. Behaviour of cesium and lithium during the precipitation of jarosite-type compounds. Hydrometallurgy 1987, 17, 251–265. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Hardy, D.J.; Chen, T.T. The behaviour of cadmium during jarosite precipitation. Hydrometallurgy 1996, 41, 269–285. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Chen, T.T.; Beauchemin, S. The behaviour of thallium (III) during jarosite precipitation. Hydrometallurgy 2005, 79, 138–153. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Kaiman, S. Rubidium. jarosite and thallium jarosite—New synthetic jarosite-type compounds and their structures. Hydrometallurgy 1975, 1, 51–59. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. The behaviour of arsenic during jarosite precipitation: Arsenic precipitation at 97 °C from sulphate or chloride media. Can. Metall. Q. 1987, 26, 91–101. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Chen, T.T. The behaviour of gallium during jarosite precipitation. Can. Metall. Q. 2000, 39, 1–14. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Chen, T.T. Synthesis and characterization of the chromium (III) analogues of jarosite-type compounds. Can. Metall. Q. 2006, 45, 249–260. [Google Scholar] [CrossRef]
- Getskin, L.S.; Margulis, E.V.; Beiskeeva, L.I.; Yaroslavtsev, A.S. Investigation of the hydrolytic deposition of iron in the form of jarosites from sulphate solutions. Izv. Yvssh. Uchebn. Zaved. Tsvetn. Metall. 1975, 6, 40–44. [Google Scholar]
- Jun, P.; Wei, Y.J.; Shi, M.Q.; Wu, J.H.; Wang, Q.W.; Zhang, L.; Hui, L.; Xu, Y. Spontaneous separation of Pb from PbSO4-coprecipitated jarosite using freeze-thaw cycling with thiourea. Trans. Nonferrous Met. Soc. China 2022, 32, 1019–1030. [Google Scholar]
- Yan, Y.; Wan, B.; Mansor, M.; Wang, X.; Zhang, Q.; Kappler, A.; Feng, X. Co-sorption of metal ions and inorganic anions/organic ligands on environmental minerals: A review. Sci. Total Environ. 2022, 803, 149918. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Zhang, H.Y.; Shi, Y.J.; Ping, Z.; Huan, L.I.; Wu, D.L.; Liu, L. Simulation on release of heavy metals Cd and Pb in sediments. Trans. Nonferrous Met. Soc. China 2021, 31, 277–287. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, S.; Wang, Z.; Ding, Y.; Lu, G.; Lin, Z.; Dang, Z.; Shi, Z. Ferrihydrite transformation under the impact of humic acid and Pb: Kinetics, nanoscale mechanisms, and implications for C and Pb dynamics. Environ. Sci. Nano 2019, 6, 747–762. [Google Scholar] [CrossRef]
- Sowers, T.D.; Blackmon, M.D.; Bone, S.E.; Kirby, A.M.; Jerden, M.L.; Noerpel, M.R.; Bradham, K.D. Successful Conversion of Pb-Contaminated Soils to Low-Bioaccessibility Plumbojarosite Using Potassium-Jarosite at Ambient Temperature. Environ. Sci. Technol. 2022, 56, 15718–15727. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Lai, J.; Wang, Y.; Fang, Z.; Su, Y.; Alessi, D.S.; Wang, H. Effect of fulvic acid co-precipitation on biosynthesis of Fe (III) hydroxysulfate and its adsorption of lead. Environ. Pollut. 2022, 295, 118669. [Google Scholar] [CrossRef]
- Shi, M.; Min, X.; Ke, Y.; Lin, Z.; Yang, Z.; Wang, S.; Peng, N.; Yan, X.; Luo, S.; Wu, J.; et al. Recent progress in understanding the mechanism of heavy metals retention by iron(oxyhydr) oxides. Sci. Total Environ. 2021, 752, 141930. [Google Scholar] [CrossRef]
- Gao, K.; Jiang, M.; Guo, C.; Zeng, Y.; Fan, C.; Zhang, J.; Reinfelder, J.R.; Huang, W.; Lu, G.; Dang, Z. Reductive dissolution of jarosite by a sulfate reducing bacterial community: Secondary mineralization and microflora development. Sci. Total Environ. 2019, 690, 1100–1109. [Google Scholar] [CrossRef]
- Hua, J.; Sun, J.; Chen, M.; Liu, C.; Wu, F. Aqueous Fe (II)-catalyzed iron oxide recrystallization: Fe redox cycling and atom exchange, mineralogical recrystallization and contributing factor. Rev. Environ. Sci. Bio/Technol. 2023, 22, 55–78. [Google Scholar] [CrossRef]
- Deng, H.; Tu, Y.; Wang, H.; Wang, Z.; Li, Y.; Chai, L.; Lin, Z. Environmental behavior, human health effect and pollution control of heavy metal (loid) s towards full life cycle processes. Eco-Environ. Health 2022, 1, 229–243. [Google Scholar] [CrossRef]
- Wu, J.; Chai, L.; Lin, Z.; Wei, Y.; Shi, M.; Peng, J.; Peng, N.; Yan, X. Fe(II)-induced transformation of Jarosite residues generated from zinc hydrometallurgy: Influence on metals behaviors during acid washing. Hydrometallurgy 2021, 200, 105523. [Google Scholar] [CrossRef]
- Smeaton, C.M.; Walshe, G.E.; Smith, A.M.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K.; Beale, A.M.; Fryer, B.J.; Weisener, C.G. Simultaneous release of Fe and As during the reductive dissolution of Pb-As jarosite by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2012, 46, 12823–12831. [Google Scholar] [CrossRef]
- HJ/T299-2007; Soild Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric Acid Method. Ministry of Economy and Environment: Beijing, China, 2007.
- HJ/T 300-2007; Soild Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method. Ministry of Economy and Environment: Beijing, China, 2007.
- Patra, A.C.; Sumesh, C.G.; Mohapatra, S.; Sahoo, S.K.; Tripathi, R.M.; Puranik, V.D. Long-term leaching of uranium from different waste matrices. J. Environ. Manag. 2011, 92, 919–925. [Google Scholar] [CrossRef]
- Zhang, H.B. Chemical Phase Analysis of Ores and Industrial Products; Metallurgical Industry Press: Beijing, China, 1992. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; American Cancer Society: Anchorage, AK, USA; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Wen, L. The Infrared Spectroscopy of Minerals; Chongqing University Press: Chengdu, China, 1989. [Google Scholar]
- Socrates, G. Infrared and Raman characteristic group frequencies. tables and charts. Proteomics 2005, 108, 1–347. [Google Scholar]
- Moulder, J.F.; Chastain, J.; King, R.C. Handbook of x-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corportion: Waltham, MA, USA, 1992. [Google Scholar]
- Crist, B.V. The Elements of Native Oxides. In Handbook of Monochromatic XPS Spectra; XPS International, Inc.: Mountain View, CA, USA, 1999. [Google Scholar]


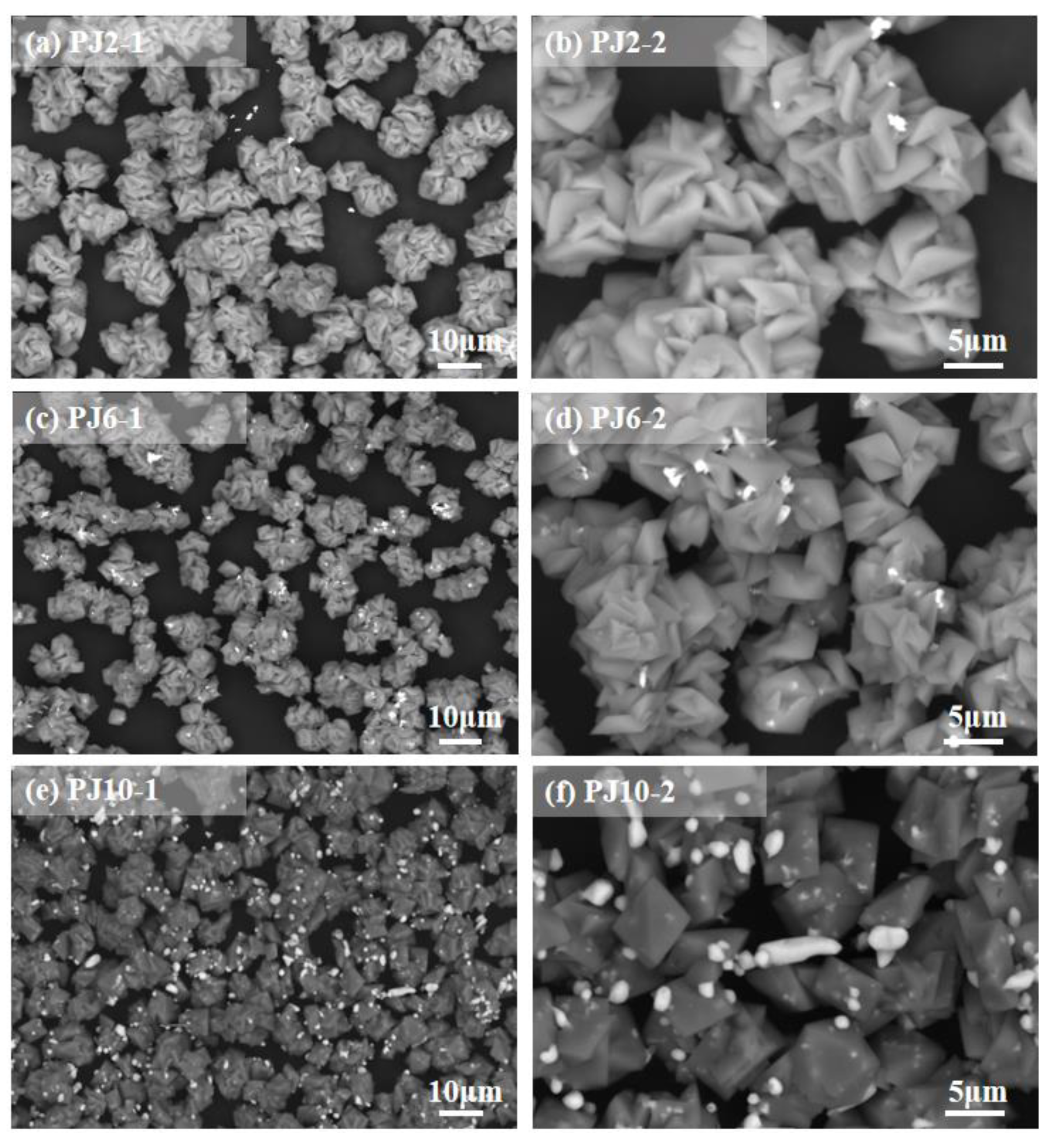
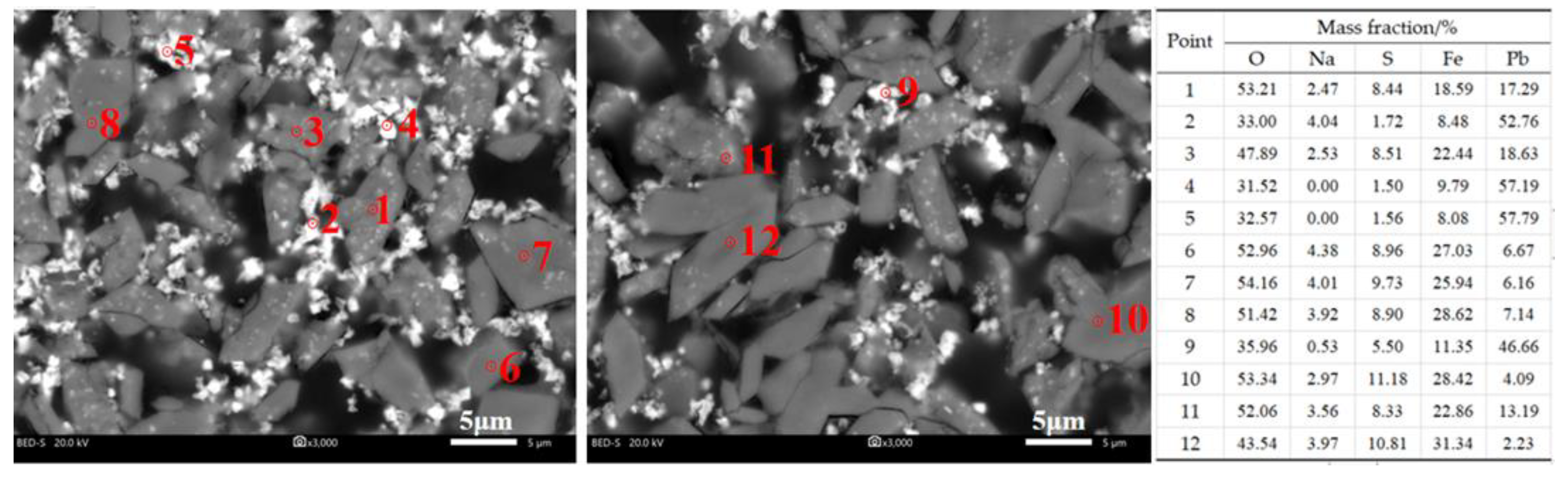



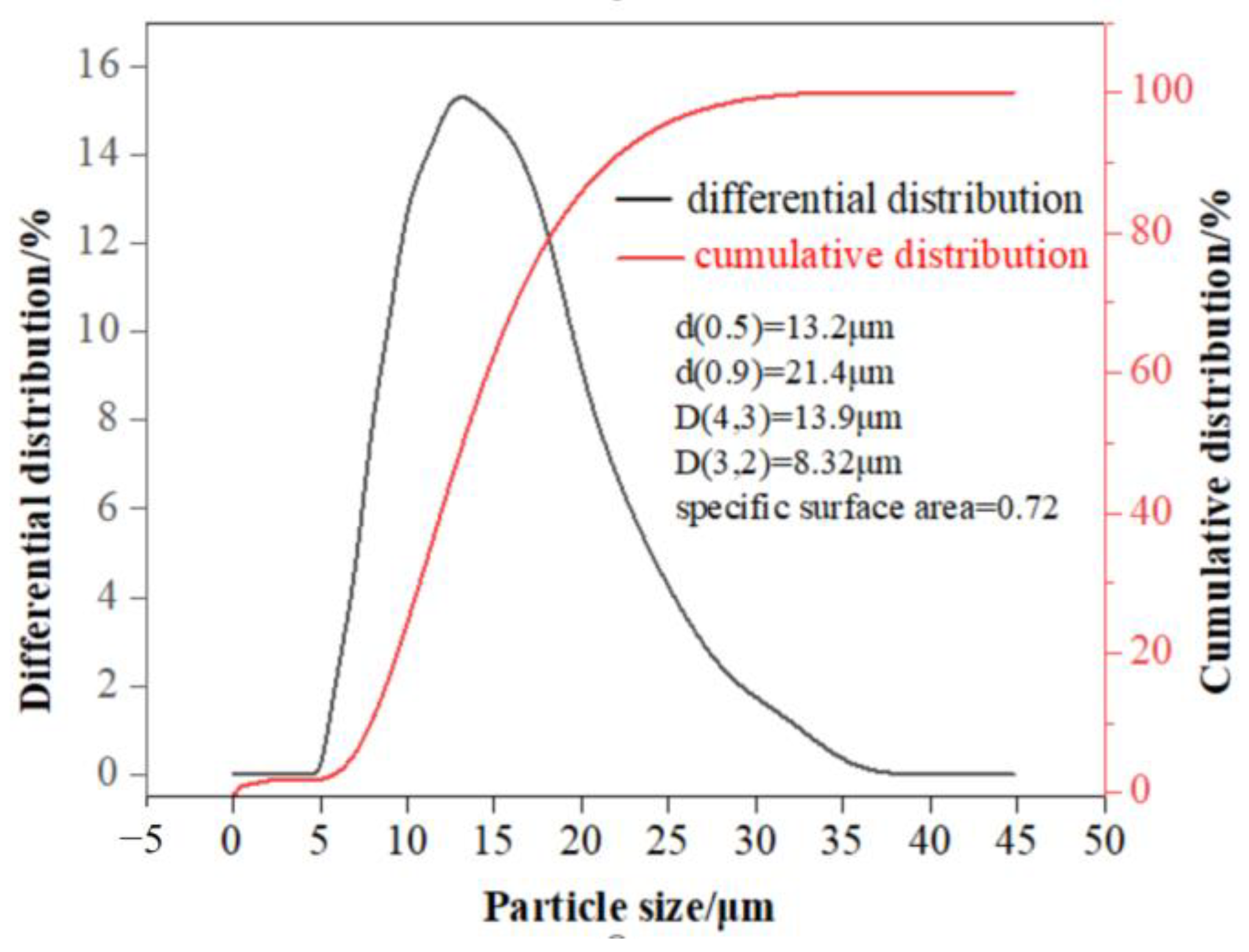
| Method | Leaching Reagent | Oscillation Time |
|---|---|---|
| TCLP | CH3COOH (acetic acid); pH 2.88 ± 0.05 | At room temperature for (18 ± 2) h, 30 r/min |
| CSLT-1 (HJ/T 299-2007) | H2SO4 and HNO3 in a mass ratio of 2:1; pH 3.20 ± 0.05 | At room temperature for (18 ± 2) h, 30 r/min |
| CSLT-2 (HJ/T 300-2007) | CH3COOH(glacial acetic acid); pH 2.64 ± 0.05 | At room temperature for (18 ± 2) h, 30 r/min |
| Sample Name | Main Element Content/% | Na/Fe Molar Ratio | |||
|---|---|---|---|---|---|
| Pb | Fe | Na | S | ||
| PJ2 | 2.27 | 31.8 | 3.45 | 14.4 | 0.26 |
| PJ6 | 5.87 | 31.7 | 3.57 | 14.4 | 0.27 |
| PJ10 | 9.86 | 27.7 | 2.98 | 14.2 | 0.26 |
| PJ10-P * | 7.37 | 31.8 | 3.68 | 14.5 | 0.28 |
| Phase | PJ2 | PJ6 | PJ10 | PJ10-P | ||||
|---|---|---|---|---|---|---|---|---|
| w(Pb)/% | Phase Percentage/% | w(Pb)/% | Phase Percentage/% | w(Pb)/% | Phase Percentage/% | w(Pb)/% | Phase Percentage/% | |
| lead sulfate | 0.71 | 31.3 | 1.88 | 32.1 | 3.38 | 34.3 | 5.99 | 81.3 |
| lead oxide | 0.0036 | 0.16 | 0.011 | 0.18 | 0.011 | 0.11 | 0.015 | 0.20 |
| lead sulfide | 0.0007 | 0.03 | 0.004 | 0.06 | 0.008 | 0.08 | 0.001 | 0.02 |
| lead jarosite and others | 1.55 | 68.5 | 3.97 | 67.7 | 6.46 | 65.6 | 1.37 | 18.5 |
| Total | 2.27 | 100 | 5.87 | 100 | 9.86 | 100 | 7.37 | 100 |
| Element | Regulatory Threshold (China) | Regulatory Threshold (USEPA) | Leaching Concentrations | ||
|---|---|---|---|---|---|
| TCLP Method | CSLT-1 Method | CSLT-2 Method | |||
| Pb | ≤5 | ≤5 | 238 | 73.9 | 246 |
| No. | Time (h) | Concentration of Lead in Solution (mg/L) | ηPb * (%) |
|---|---|---|---|
| 1 | 24 | 243 | 0.25 |
| 2 | 48 | 242 | 0.74 |
| 3 | 72 | 191 | 1.32 |
| 4 | 96 | 188 | 2.08 |
| 5 | 120 | 186 | 3.03 |
| 6 | 144 | 149 | 3.94 |
| 7 | 168 | 117 | 4.77 |
| 8 | 192 | 90.7 | 5.50 |
| 9 | 216 | 87.6 | 6.30 |
| 10 | 240 | 83.2 | 7.15 |
| 11 | 264 | 80.3 | 8.04 |
| 12 | 288 | 78.6 | 9.00 |
| 13 | 312 | 75.3 | 9.99 |
| 14 | 336 | 70.6 | 11.0 |
| 15 | 360 | 72.0 | 12.1 |
| 16 | 384 | 70.9 | 13.2 |
| 17 | 432 | 78.2 | 14.7 |
| 18 | 456 | 75.7 | 16.1 |
| 19 | 480 | 74.7 | 17.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; He, L.; Liu, H.; Sun, Z.; Yan, X. The Synthesis of Lead-Bearing Jarosite and Its Occurrence Characteristic and Leaching Toxicity Evaluation. Metals 2023, 13, 941. https://doi.org/10.3390/met13050941
Peng J, He L, Liu H, Sun Z, Yan X. The Synthesis of Lead-Bearing Jarosite and Its Occurrence Characteristic and Leaching Toxicity Evaluation. Metals. 2023; 13(5):941. https://doi.org/10.3390/met13050941
Chicago/Turabian StylePeng, Jun, Luhua He, Hui Liu, Zhumei Sun, and Xu Yan. 2023. "The Synthesis of Lead-Bearing Jarosite and Its Occurrence Characteristic and Leaching Toxicity Evaluation" Metals 13, no. 5: 941. https://doi.org/10.3390/met13050941




