Purification of Organosilicon Waste Silicon Powder with Hydrometallurgy
Abstract
:1. Introduction
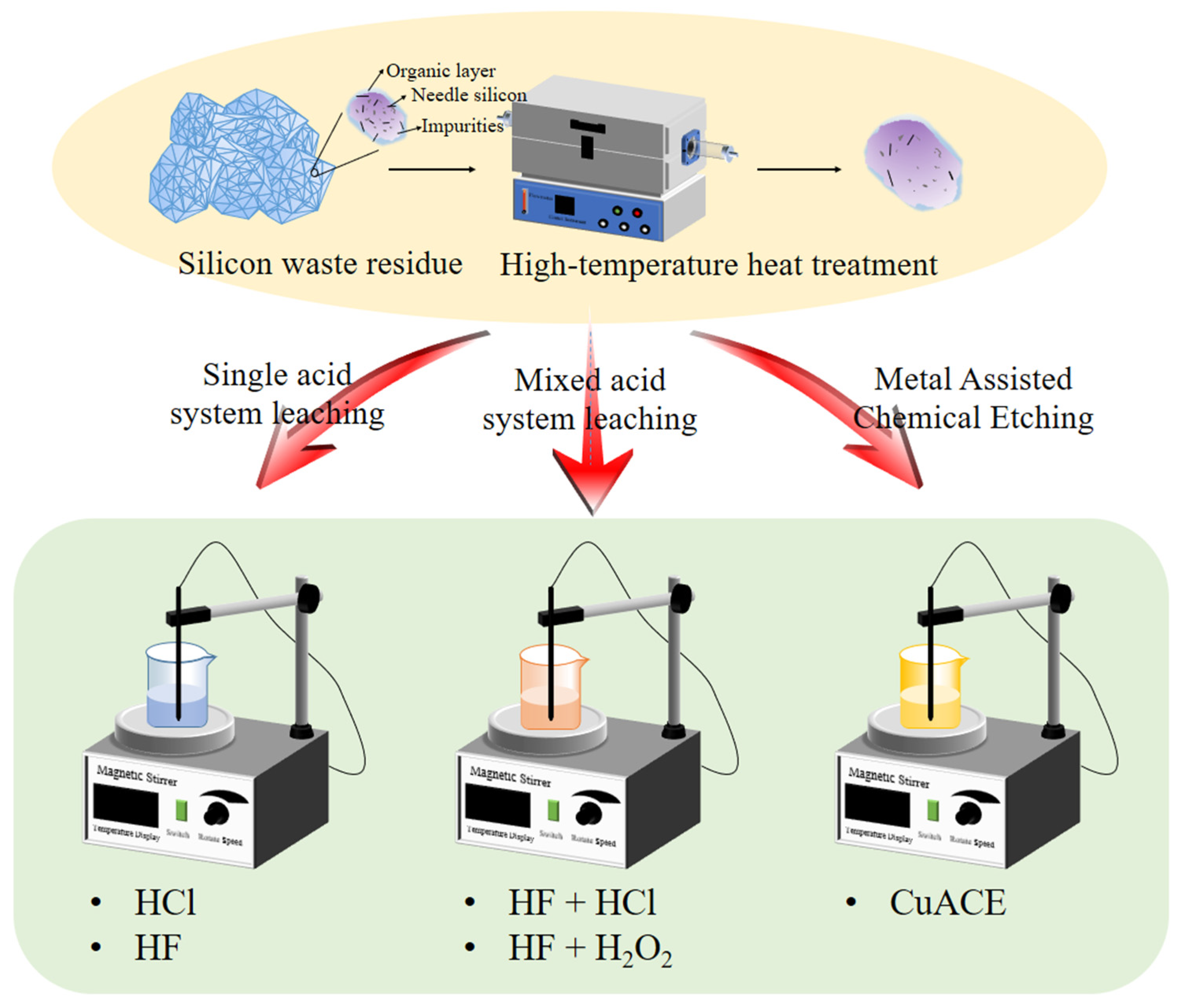
2. Experiments
3. Results and Discussion
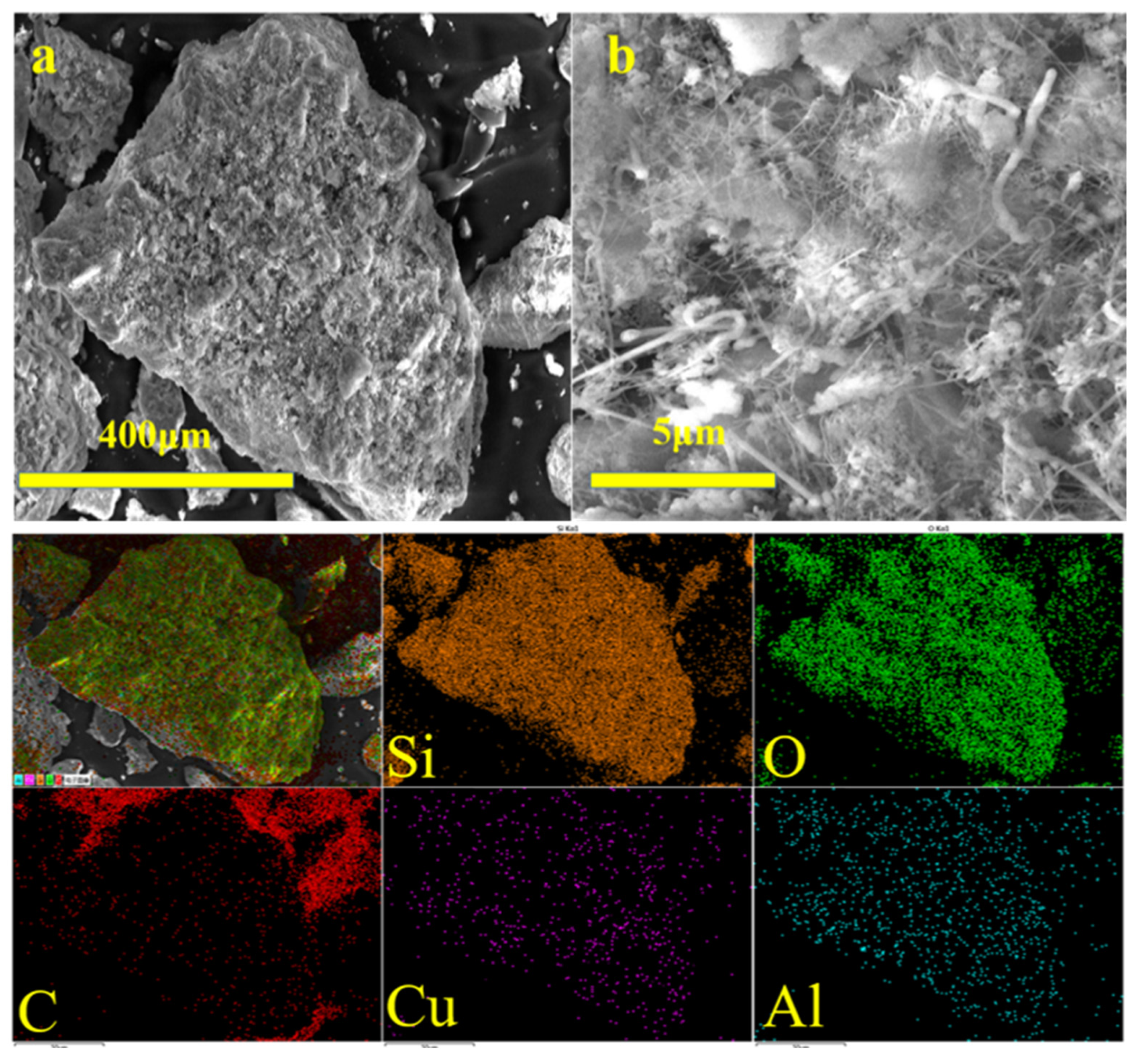
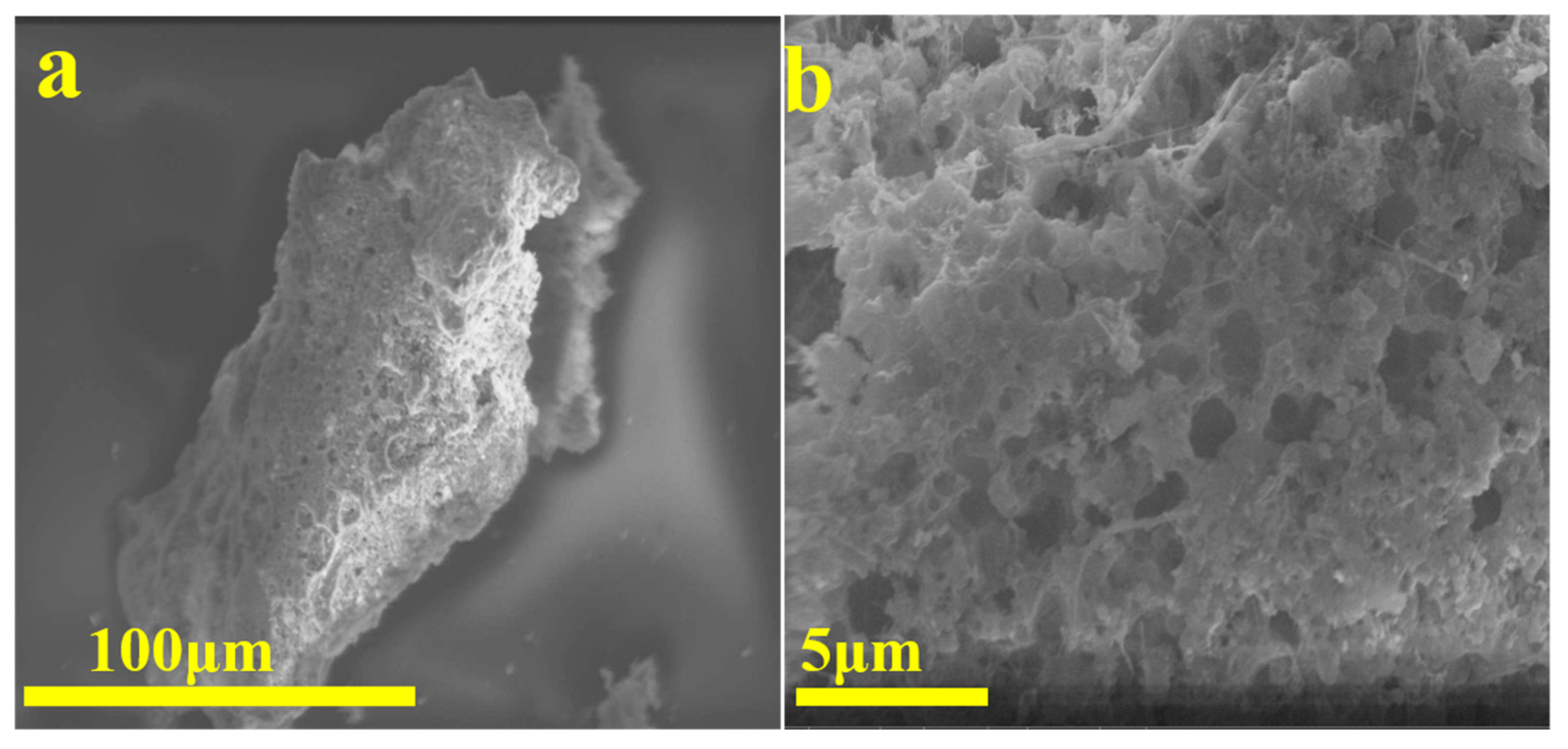
3.1. Morphologies of Waste Silicon Powders
3.2. Impurities Removal from Waste Silicon Powder through Traditional Leaching Process
Si(s) + xFe2+ (aq) + yAl3+ (aq) + zCu2+ (aq) + (3x + 3y + 2z)/2H2↑
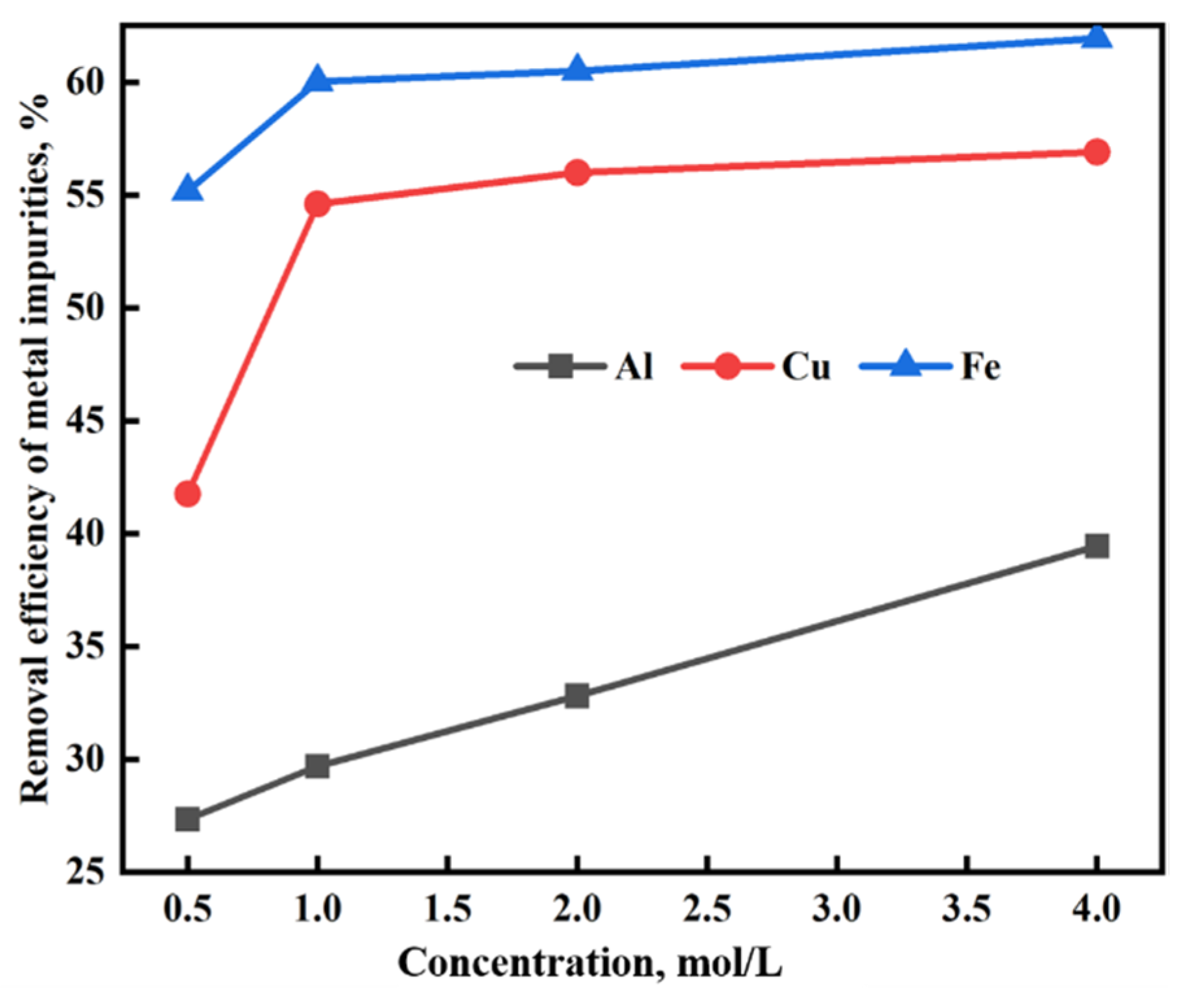
3.2.1. Hydrochloric Acid Leaching Experiments
3.2.2. Hydrofluoric Acid Leaching
3.2.3. Mixed Acid System Leaching Experiments
3.3. Enhancing Removal Rate of Impurity from Waste Silicon Powder Using CuACE
3.4. Comparison of Removing Impurities from Silicon with Various Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandra, G. Organosilicon Materials; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zuo, Y.; Liang, X.; Yin, J.; Gou, Z.; Lin, W. Understanding the significant role of SiOSi bonds: Organosilicon materials as powerful platforms for bioimaging. Coord. Chem. Rev. 2021, 447, 214166. [Google Scholar] [CrossRef]
- Hu, J. Progress of silicone industry in China in 2020. Silicone Master 2021, 35, 63–86. [Google Scholar]
- Vu, N.D.; Boulegue-Mondière, A.; Durand, N.; Raynaud, J.; Monteil, V. Back-to-cyclic-monomers: Chemical recycling of silicone wastes using a [polydentate ligand-potassium silanolate] complex. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Wang, W.; Fan, H.; Song, L.; Wang, Z.; Li, H.; Xiang, J.; Chen, X. Organosilicon leather coating technology based on carbon peak strategy. J. Leather Sci. Eng. 2022, 4, 27. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Ludin, N.A.; Mustafa, N.I.; Hanafiah, M.M.; Ibrahim, M.A.; Teridi, M.A.M.; Sepeai, S.; Sopian, K. Prospects of life cycle assessment of renewable energy from solar photovoltaic technologies: A review. Renew. Sustain. Energy Rev. 2018, 96, 11–28. [Google Scholar] [CrossRef]
- Mower, T.M. Thermomechanical behavior of aerospace-grade RTV (silicone adhesive). Int. J. Adhes. Adhes. 2018, 87, 64–72. [Google Scholar] [CrossRef]
- Council, G.S. Socio-Economic Evaluation of the Global Silicones Industry; Final Report; Global Silicones Council: 2016. Available online: https://dokumen.tips/documents/socio-economic-evaluation-of-the-global-silicones-industry-final-.html?page=1 (accessed on 4 January 2023).
- Zhao, C.; Diao, S.; Yuan, Y.; Wang, M. Preparation and properties of hollow glass microsphere/silicone rubber composite material with the transition layer of silicone resin. Silicon 2021, 13, 517–522. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Khan, F.U.; Haroon, M.; Cheng, L. Modified silicone oil types, mechanical properties and applications. Polym. Bull. 2019, 76, 2129–2145. [Google Scholar] [CrossRef]
- Köhler, T.; Gutacker, A.; Mejía, E. Industrial synthesis of reactive silicones: Reaction mechanisms and processes. Org. Chem. Front. 2020, 7, 4108–4120. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, X.; Cui, C.; Xi, Z.; Sun, J. Simultaneous process parameters and heat integration optimization for industrial organosilicon production. Sep. Purif. Technol. 2021, 265, 118520. [Google Scholar] [CrossRef]
- Matsumoto, K.; Huang, J.; Naganawa, Y.; Guo, H.; Beppu, T.; Sato, K.; Nakajima, Y. Direct Silyl–Heck Reaction of Chlorosilanes. Organic letters. 2018, 20, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Du, S.; He, Y.; Lv, G.; Ma, W.; Xing, A.; Huang, J. Effects of opening design of gas distribution plate on fluidization of the synthesis process of organosilicon monomer. Korean J. Chem. Eng. 2022, 39, 2034–2043. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Chen, G.; Wang, W.; Li, J. Waste minimization and efficient disposal of particles in optimized organic silicon production. J. Clean. Prod. 2020, 242, 118445. [Google Scholar] [CrossRef]
- Huang, M.; Hu, J.; Xu, C.; Chen, B. Study on the composition of abandoned organosilicon catalyst. Sichuan Chem. 2021, 24, 31–34. [Google Scholar]
- de Souza Ribeiro, N.; Barros, R.M.; dos Santos, I.F.S.; Tiago Filho, G.L.; da Silva, S.P.G. Electric energy generation from biogas derived from municipal solid waste using two systems: Landfills and anaerobic digesters in the states of Sao Paulo and Minas Gerais, Brazil. Sustain. Energy Technol. Assess. 2021, 48, 101552. [Google Scholar] [CrossRef]
- Wang, G.; Ye, Z.; Dong, S.; Wang, J. Harmless process of organic matter in organosilicon waste residue by fluidization-like DDBD reactor: Temperature action and mechanism. Chemosphere 2023, 322, 138116. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Xing, P.; Wang, S.; Guo, Y.; Zhuang, Y. Kinetic mechanism of copper extraction from methylchlorosilane slurry residue using hydrogen peroxide as oxidant. Chin. J. Chem. Eng. 2023, in press. [Google Scholar] [CrossRef]
- Yang, S.; Wan, X.; Wei, K.; Ma, W.; Wang, Z. Dissolution and mineralization behavior of metallic impurity content in diamond wire saw silicon powder during acid leaching. J. Clean. Prod. 2020, 248, 119256. [Google Scholar] [CrossRef]
- Tomono, K.; Miyamoto, S.; Ogawa, T.; Furuya, H.; Okamura, Y.; Yoshimoto, M.; Nakayama, M. Recycling of kerf loss silicon derived from diamond-wire saw cutting process by chemical approach. Sep. Purif. Technol. 2013, 120, 304–309. [Google Scholar] [CrossRef]
- Yang, S.; Wei, K.; Ma, W.; Xie, K.; Wu, J.; Lei, Y. Kinetic mechanism of aluminum removal from diamond wire saw powder in HCl solution. J. Hazard. Mater. 2019, 368, 1–9. [Google Scholar] [CrossRef]
- Kong, J.; Jin, X.; Liu, Y.; Wei, D.; Jiang, S.; Gao, S.; Luo, X. Study on the kinetics of iron removal from silicon diamond-wire saw cutting waste: Comparison between heterogeneous and homogeneous reaction methods. Sep. Purif. Technol. 2019, 221, 261–268. [Google Scholar] [CrossRef]
- Huang, L.; Danaei, A.; Fang, M.; Thomas, S.; Luo, X.; Barati, M. A metallurgical route to upgrade silicon kerf derived from diamond-wire slicing process. Vacuum 2019, 163, 164–171. [Google Scholar] [CrossRef]
- Yang, H.L.; Liu, I.T.; Liu, C.E.; Hsu, H.P.; Lan, C.W. Recycling and reuse of kerf-loss silicon from diamond wire sawing for photovoltaic industry. Waste Manag. 2019, 84, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, K.; Zhu, H. Removal of Fe, B and P impurities by enhanced separation technique from silicon-rich powder of the multi-wire sawing slurry. Chem. Eng. J. 2016, 299, 276–281. [Google Scholar] [CrossRef] [Green Version]
- GB/T 14849.4-2014; Methods for Chemical Analysis of Silicon Metal—Part 4:Determination of Impurity Contents—Inductively Coupled Plasma Atomic Emission Spectrometric Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2014.
- Xi, F.; Li, S.; Ma, W.; Chen, Z.; Wei, K.; Wu, J. A review of hydrometallurgy techniques for the removal of impurities from metallurgical-grade silicon. Hydrometallurgy 2021, 201, 105553. [Google Scholar] [CrossRef]
- Lei, Y.; Ma, W.; Lv, G.; Wei, K.; Li, S.; Morita, K. Purification of metallurgical-grade silicon using zirconium as an impurity getter. Sep. Purif. Technol. 2017, 173, 364–371. [Google Scholar] [CrossRef]
- Xi, F.; Li, S.; Ma, W.; Ding, Z.; Lei, Y.; Chen, Z.; Wu, J. Removal of impurities from metallurgical grade silicon with metal assisted chemical leaching. Hydrometallurgy 2018, 178, 250–255. [Google Scholar] [CrossRef]
- Xi, F.; Li, S.; Ma, W.; He, Z.; Geng, C.; Chen, Z.; Xie, K. Simple and high-effective purification of metallurgical-grade silicon through Cu-catalyzed chemical leaching. Jom 2018, 70, 2041–2047. [Google Scholar] [CrossRef]
- Xi, F.; Cui, H.; Li, Y.; Ding, Z.; Li, S.; Ma, W.; Wu, J. Novel enhancing impurities purification from silicon powder through metal-catalyzed chemical corrosion. Powder Technol. 2019, 352, 53–61. [Google Scholar] [CrossRef]
- Lai, H.; Huang, L.; Gan, C.; Xing, P.; Li, J.; Luo, X. Enhanced acid leaching of metallurgical grade silicon in hydrofluoric acid containing hydrogen peroxide as oxidizing agent. Hydrometallurgy 2016, 164, 103–110. [Google Scholar] [CrossRef]
- Xi, F.; Zhang, Z.; Wan, X.; Li, S.; Ma, W.; Chen, X.; Wang, L. High-performance porous silicon/nanosilver anodes from industrial low-grade silicon for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 49080–49089. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Sun, Y.; Li, X.; Wang, J.; Chen, S.; Schweizer, S.; Wehrspohn, R.B. Conversion of bulk metallurgical silicon into photocatalytic nanoparticles by copper-assisted chemical etching. ACS Sustain. Chem. Eng. 2016, 4, 6590–6599. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; De Boor, J.; Gösele, U. Metal-assisted chemical etching of silicon: A review: In memory of Prof. Ulrich Gösele. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef] [PubMed]



| Impurities | Fe | Cu | Al | Cl | Ti | Ca |
|---|---|---|---|---|---|---|
| Content | 3.703 | 3.039 | 1.28 | 0.714 | 0.501 | 0.462 |
| HCl Conc., M | Al | Cu | Fe |
|---|---|---|---|
| 0.5 | 0.930 | 1.770 | 1.660 |
| 1.0 | 0.900 | 1.380 | 1.481 |
| 2.0 | 0.860 | 1.338 | 1.463 |
| 4.0 | 0.775 | 1.310 | 1.410 |
| HF Conc., M | Al | Cu | Fe |
|---|---|---|---|
| 0.5 | 1.050 | 2.620 | 0.784 |
| 1.0 | 0.850 | 1.680 | 0.758 |
| 2.0 | 0.758 | 1.420 | 0.630 |
| 4.0 | 0.640 | 1.131 | 0.548 |
| Sample | Fe | Al | Cu |
|---|---|---|---|
| HF + HCl | 0.591 | 0.542 | 1.262 |
| HF + H2O2 | 0.122 | 0.651 | 0.261 |
| Sample | Fe | Al | Cu |
|---|---|---|---|
| CuACE | 0.095 | 0.460 | 1.489 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Li, Z.; Xi, F.; Li, S.; Ma, W.; Wu, J.; Wei, K. Purification of Organosilicon Waste Silicon Powder with Hydrometallurgy. Metals 2023, 13, 950. https://doi.org/10.3390/met13050950
Zhao L, Li Z, Xi F, Li S, Ma W, Wu J, Wei K. Purification of Organosilicon Waste Silicon Powder with Hydrometallurgy. Metals. 2023; 13(5):950. https://doi.org/10.3390/met13050950
Chicago/Turabian StyleZhao, Liping, Zuyu Li, Fengshuo Xi, Shaoyuan Li, Wenhui Ma, Jijun Wu, and Kuixian Wei. 2023. "Purification of Organosilicon Waste Silicon Powder with Hydrometallurgy" Metals 13, no. 5: 950. https://doi.org/10.3390/met13050950




