Leaching Kinetics of Hemimorphite with 5-Sulfosalicylic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Procedures
2.3. SEM-EDS
3. Results and discussion
3.1. Leaching Reactions
3.2. Effect of Reaction Temperature
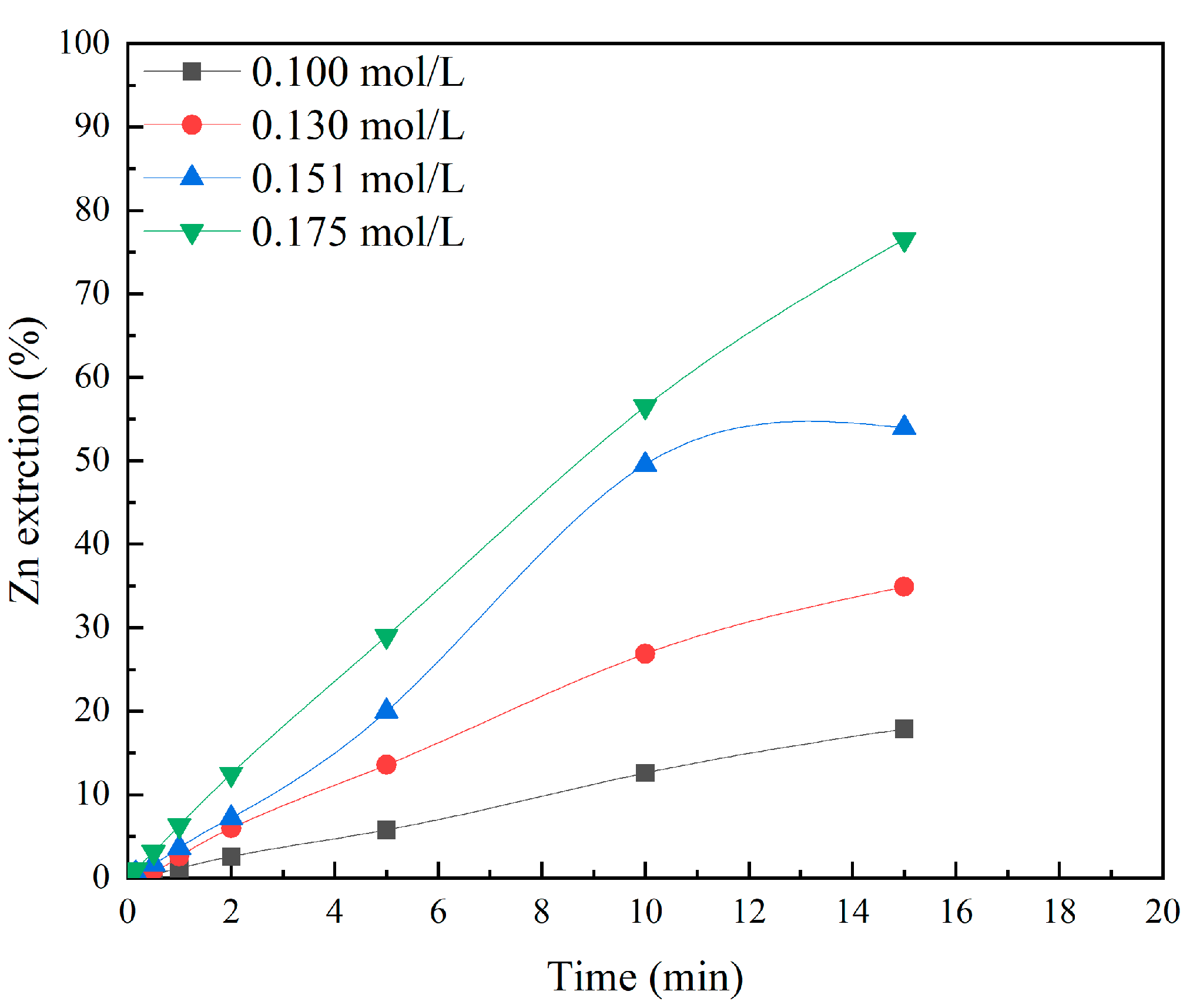
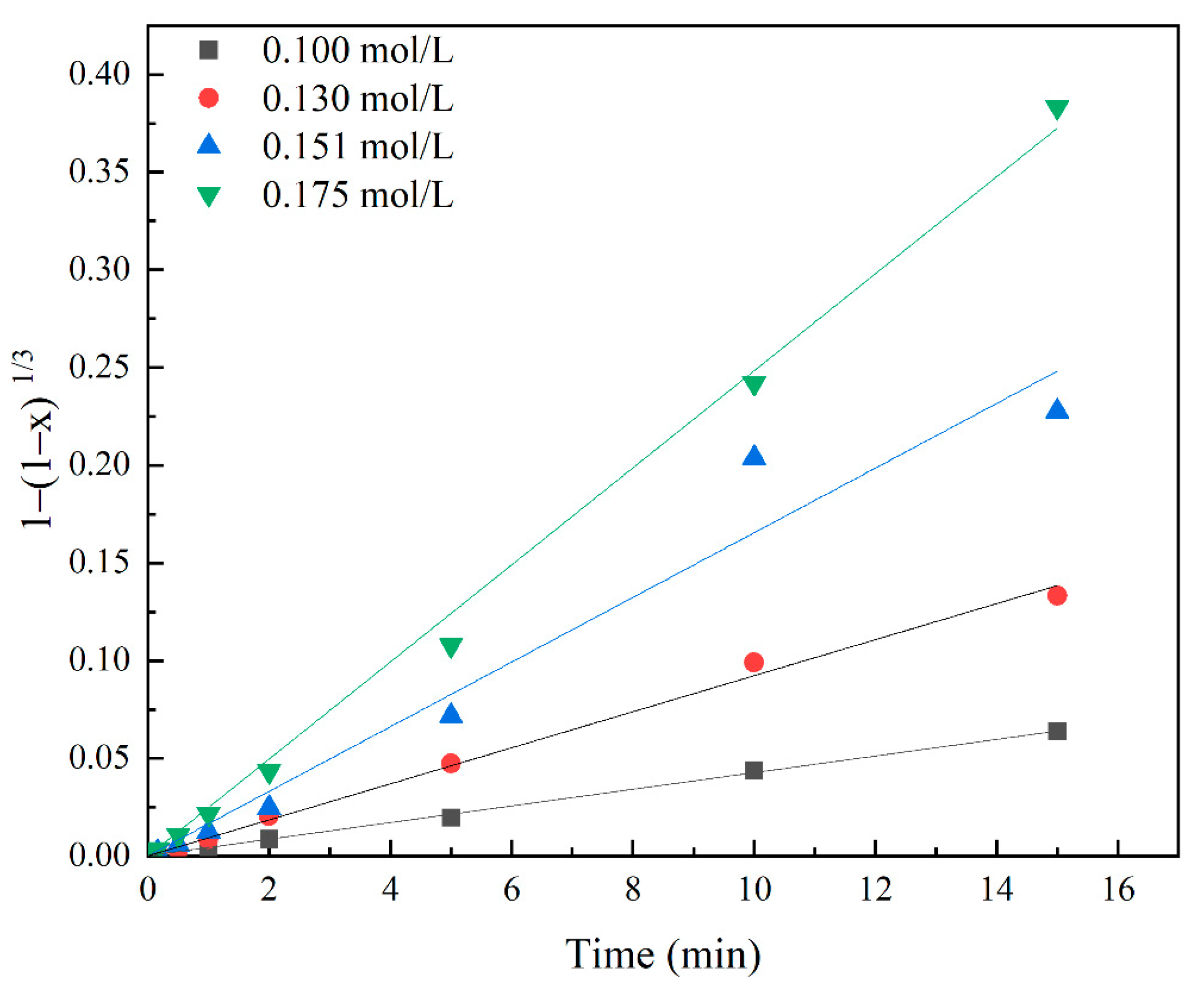
3.3. Effects of 5-Sulfosalicylic Acid Concentration
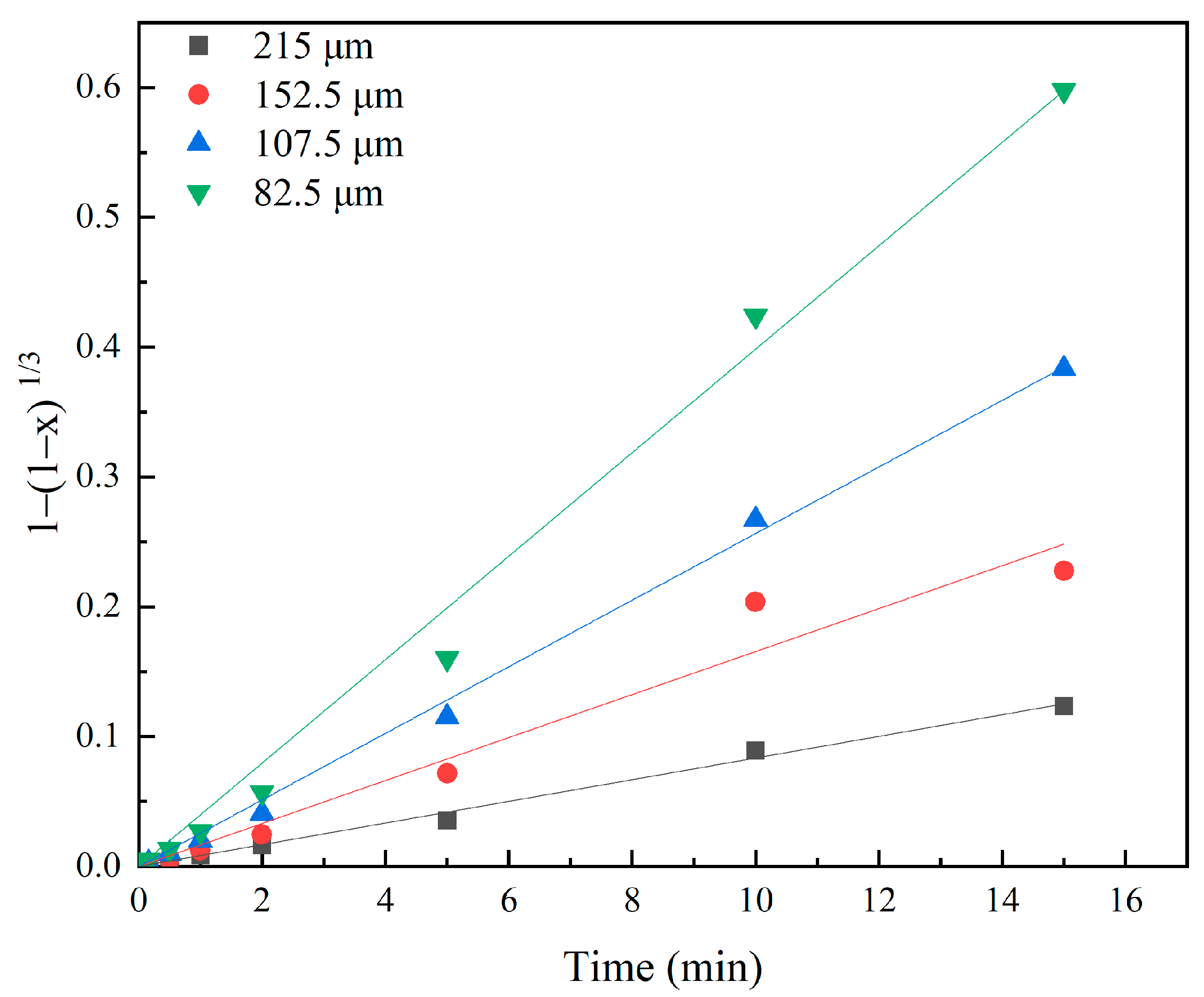
3.4. Effects of Particle Size
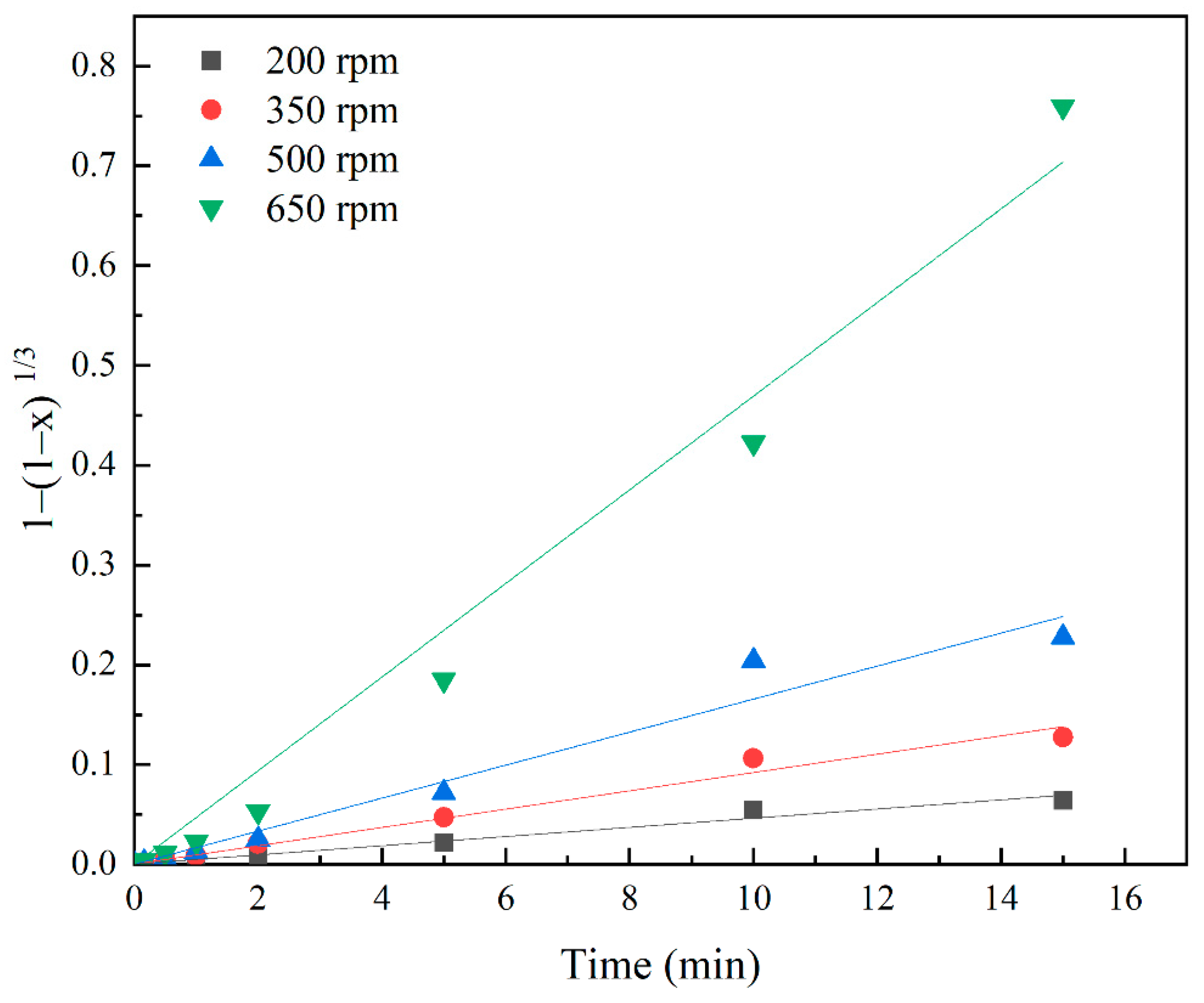
3.5. Effects of Stirring Speed
3.6. Investigation of Kinetics
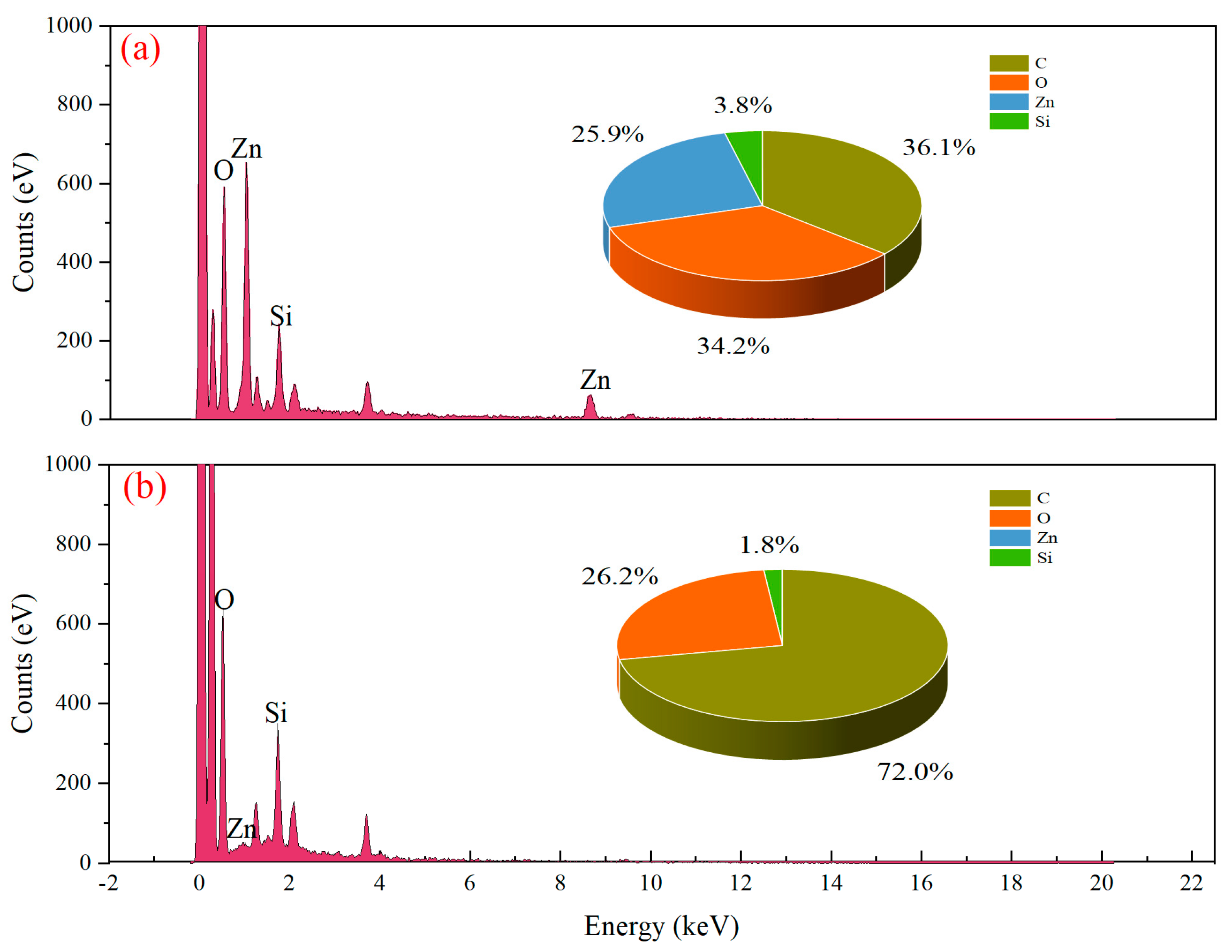
3.7. SEM-EDS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, K.; Lu, Y.; Liu, J.; Cheng, S.; Liu, S.; Cao, Y.; Li, G. Selective flotation separation of hemimorphite from quartz using the biosurfactant sodium N-lauroylsarcosinate as a novel collector. Miner. Eng. 2023, 198, 108073. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Wang, Y.; Li, S.; Zhao, H.; Liu, C.; Sun, Z. Adsorption characteristics of Pb(Ⅱ)ions on sulfidized hemimorphite surface under ammonium sulfate system. J. Min. Sci. Technol. Engl. 2023, 33, 8. [Google Scholar] [CrossRef]
- Xing, D.; Huang, Y.; Lin, C.; Zuo, W.; Deng, R. Strengthening of sulfidization flotation of hemimorphite via fluorine ion modification. Sep. Purif. Technol. 2021, 269, 118769. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.; Wu, D.; Wang, Z.; Chen, H. Surface modification of hemimorphite by using ammonium carbamate and its response to flotation. Appl. Surf. Sci. 2022, 605, 154775. [Google Scholar] [CrossRef]
- Yi, Y.; Li, P.; Zhang, G.; Feng, Q.; Han, G. Stepwise activation of hemimorphite surfaces with lead ions and its contribution to sulfidization flotation. Sep. Purif. Technol. 2022, 299, 121679. [Google Scholar] [CrossRef]
- Zuo, Q.; Yang, J.; Shi, Y.; Wu, D. Use of sodium sulfosalicylate as an activator in hemimorphite sulfidation xanthate flotation. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 641, 128552. [Google Scholar] [CrossRef]
- Zuo, Q.; Yang, J.; Shi, Y.; Wu, D. Activating hemimorphite using a sulfidation-flotation process with sodium sulfosalicylate as the complexing agent. J. Mater. Res. Technol. 2020, 9, 10110–10120. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Wu, D.; Zeng, J. Sodium sulfosalicylate activation mechanism on sulfidation flotation of smithsonite using dodecylamine as a collector. Miner. Eng. 2023, 192, 107987. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, S.; Chen, Y. Leaching of hemimorphite in neutral solution at high temperature. J. Min. Metall. Sect. B Metall. 2020, 56, 203–208. [Google Scholar] [CrossRef]
- Yang, K.; Sun, C.; Qu, H.; Shuo, L.; Luo, Y.; Zhang, L.; Ma, A. Recovery of Zinc from Oxide-Sulphide Zinc Ore Through Oxidation and Chelation//Materials Engineering—From Ideas to Practice: An EPD Symposium in Honor of Jiann-Yang Hwang; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 161–169. [Google Scholar] [CrossRef]
- Kumaş, C.; Obut, A. Effect of heating on structure and leaching characteristics of a zinc carbonate ore. Physicochem. Probl. Miner. Process. 2021, 57, 23–32. [Google Scholar] [CrossRef]
- Soltani, F.; Darabi, H.; Aram, R.; Ghadiri, M. Leaching and solvent extraction purification of zinc from Mehdiabad complex oxide ore. Sci. Rep. 2021, 11, 1566. [Google Scholar] [CrossRef]
- Seo, S.; Han, K.S.; Lee, S.I.; Kim, M.J. The Recovery of Cu, Co, Zn, and Mn from a Complex Oxide Ore Using an Enhanced Reduction Leaching. Metals 2021, 11, 1636. [Google Scholar] [CrossRef]
- Ehsani, A.; Ehsani, I.; Obut, A. Preparation of different zinc compounds from a smithsonite ore through ammonia leaching and subsequent heat treatment. Physicochem. Probl. Miner. Process. 2021, 57, 96–106. [Google Scholar] [CrossRef]
- Long, H.; Tan, X.; Ni, S.; Ma, A.; Li, S.; Zhu, D. Ammoniacal leaching behavior and regularity of zinc ash. Int. J. Chem. React. Eng. 2022, 5, 87. [Google Scholar] [CrossRef]
- He, F.; Gao, L.; Chen, L.; Rao, B.; Shen, H.; Peng, K.; Gao, G.; Zhang, M. Study on the mechanism and kinetics of sulfuric acid leaching scandium from rich scandium anatase. Physicochem. Probl. Miner. Process. 2022, 58, 146171. [Google Scholar] [CrossRef]
- Koohestani, H.; Khatami, E.S.; Babaei, K. Comparative investigation of leaching of zinc from wastes of the zinc alloy production process. Miner. Process. Extr. Met. 2021, 130, 362–368. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Song, S.; Xue, N.; Zhang, J.; Yang, S.; Liu, C. Experimental and kinetic study of zinc leaching from metallurgical slag by 5-sulfosalicylic acid. Physicochem. Probl. Miner. Process. 2021, 57, 8–20. [Google Scholar] [CrossRef]
- Xin, C.F.; Xia, H.Y.; Zhang, Q.; Zhang, L.B.; Zhang, W. Leaching of zinc and germanium from zinc oxide dust in sulfuric acid-ozone media. Arab. J. Chem. 2021, 14, 103450. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, Y.; Jiang, Y. Adsorption Behavior of a Tri-functionalized Imprinted Resin with High Selectivity for 5-Sulfosalicylic Acid:Batch Experiments and DFT Calculation. J. Hazard. Mater. 2021, 412, 125271. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Ghanbari, F.; Chang, F.-C.; Hu, C.; Lin, K.-Y.A.; Du, Y. Enhanced degradation of 5-sulfosalicylic acid using peroxymonosulfate activated by ordered porous silica-confined Co3O4 prepared via a solvent-free confined space strategy. Sep. Purif. Technol. 2020, 249, 116972. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Chen, Z.; Wang, R.; Xu, Z. Study on aluminum removal through 5-sulfosalicylic acid targeting complexing and D290 resin adsorption. Miner. Eng. 2020, 147, 106175. [Google Scholar] [CrossRef]
- Tunca, E.; Bülbül, M.; Ilkimen, H.; Canlıdinç, R.S.; Yenikaya, C. Investigation of the effects of the proton transfer salts of 2-aminopyridine derivatives with 5-sulfosalicylic acid and their Cu(II) complexes on cancer-related carbonic anhydrases: CA IX and CA XII. Chem. Pap. 2020, 74, 2365–2374. [Google Scholar] [CrossRef]
- Ristić, P.; Todorović, T.R.; Blagojević, V.; Klisurić, O.R.; Marjanović, I.; Holló, B.B.; Vulić, P.; Gulea, M.; Donnard, M.; Monge, M.; et al. 1D and 2D Silver-Based Coordination Polymers with Thiomorpholine-4-carbonitrile and Aromatic Polyoxoacids as Coligands: Structure, Photocatalysis, Photoluminescence, and TD-DFT Study. Cryst. Growth Des. 2020, 20, 4461–4478. [Google Scholar] [CrossRef]
- Islam, S.; Ansary, R.H.; Farooque, A.; Asraf, A. Synthesis and Characterization of Schiff Base Complexes of Cu(II), Co(II) and Cd(II) Derived from Ethylenediamine and Benzaldehyde Derivatives. Asian J. Appl. Chem. Res. 2020, 6, 34–46. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. Electrospray ionization stochastic dynamic mass spectrometric 3D structural analysis of ZnII–ion containing complexes in solution. Inorg. Nano-Metal Chem. 2021, 52, 1407–1429. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, F.; Zhang, J.; Ma, G.; Zhao, Y.; Zhang, J.; Chen, G. Catalytic oxidation of polymer used in oilfield by supported Co(II) complex within a high pH range. Comptes Rendus Chim. 2022, 24, 61–68. [Google Scholar] [CrossRef]
- Liu, S.; Ding, E.; Ning, P.; Xie, G.; Yang, N. Vanadium extraction from roasted vanadium-bearing steel slag via pressure acid leaching. J. Environ. Chem. Eng. 2021, 9, 105195. [Google Scholar] [CrossRef]
- Shi, Y.; Zuo, Q.; Liu, D.; Wu, D. Dissolution kinetics of malachite in trichloroacetic acid solution. J. Iran. Chem. Soc. 2022, 19, 2581–2590. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, G.; Xiang, P.; Tao, Y.; Tang, Y.; Hu, Y. Effect of activator on kinetics of direct acid leaching of vanadium from clay vanadium ore. Sep. Purif. Technol. 2022, 281, 119937. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H.; Ilyas, N. Biotechnological recycling of hazardous waste PCBs using Sulfobacillus thermosulfidooxidans through pretreatment of toxicant metals: Process optimization and kinetic studies. Chemosphere 2022, 286, 131978. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H. Mobilization of platinum and palladium from exhausted catalytic converters using bio-cyanide and an ionic-liquid as mass transport carriers. Green Chem. 2022, 24, 5204–5218. [Google Scholar] [CrossRef]







| Composition | Zn | SiO2 | Al2O3 | CaO | Fe |
|---|---|---|---|---|---|
| Content (%) | 53.04 | 23.69 | 0.018 | 0.014 | 0.017 |
| Parameter | Value |
|---|---|
| Concentration (mol/L) | 0.100, 0.130, 0.151 *, 0.175 |
| Temperature [K (°C)] | 293 (20), 303 (30) *, 313 (40),323 (50) |
| Stirring speed (rpm) | 200, 350, 500 *, 600 |
| Average particle size (μm) | 215, 152.5 *,107.5, 82.5 |
| Parameter | Surface Chemical Reaction | Diffusion through the Product Layer | ||
|---|---|---|---|---|
| 1 − (1 − x)1/3 | 1−3(1 − x)2/3 + 2(1 − x) | |||
| kr (min−1) | R2 | kd (min−1) | R2 | |
| Temperature [K (°C)] | ||||
| 293 (20) | 0.0065 | 0.985 | 0.0015 | 0.975 |
| 303 (30) | 0.0165 | 0.978 | 0.0088 | 0.946 |
| 313 (40) | 0.0347 | 0.994 | 0.0321 | 0.931 |
| 323 (50) | 0.0469 | 0.983 | 0.0480 | 0.921 |
| Concentration (mol/L) | ||||
| 0.1 | 0.0043 | 0.999 | 0.0007 | 0.939 |
| 0.13 | 0.0092 | 0.997 | 0.0029 | 0.962 |
| 0.151 | 0.0165 | 0.979 | 0.0088 | 0.946 |
| 0.175 | 0.0248 | 0.998 | 0.0185 | 0.931 |
| Particle size (μm) | ||||
| 215 | 0.0083 | 0.997 | 0.0025 | 0.944 |
| 152.5 | 0.0165 | 0.979 | 0.0088 | 0.946 |
| 107.5 | 0.0256 | 0.998 | 0.0194 | 0.951 |
| 82.5 | 0.0398 | 0.995 | 0.0391 | 0.954 |
| Stirring speed (rpm) | ||||
| 200 | 0.0046 | 0.987 | 0.0008 | 0.957 |
| 350 | 0.0092 | 0.989 | 0.0029 | 0.966 |
| 500 | 0.0165 | 0.979 | 0.0088 | 0.946 |
| 600 | 0.0469 | 0.987 | 0.0482 | 0.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wen, S.; Cao, J.; Wu, D.; Wang, Y. Leaching Kinetics of Hemimorphite with 5-Sulfosalicylic Acid. Metals 2023, 13, 1249. https://doi.org/10.3390/met13071249
Li Y, Wen S, Cao J, Wu D, Wang Y. Leaching Kinetics of Hemimorphite with 5-Sulfosalicylic Acid. Metals. 2023; 13(7):1249. https://doi.org/10.3390/met13071249
Chicago/Turabian StyleLi, Yaohong, Shuming Wen, Jing Cao, Dandan Wu, and Yijie Wang. 2023. "Leaching Kinetics of Hemimorphite with 5-Sulfosalicylic Acid" Metals 13, no. 7: 1249. https://doi.org/10.3390/met13071249




