Structure and Properties of Amorphous Quasi-High-Entropy Fe-Co-Ni-Cr-(Mo,V)-B Alloys with Various Boron Content
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
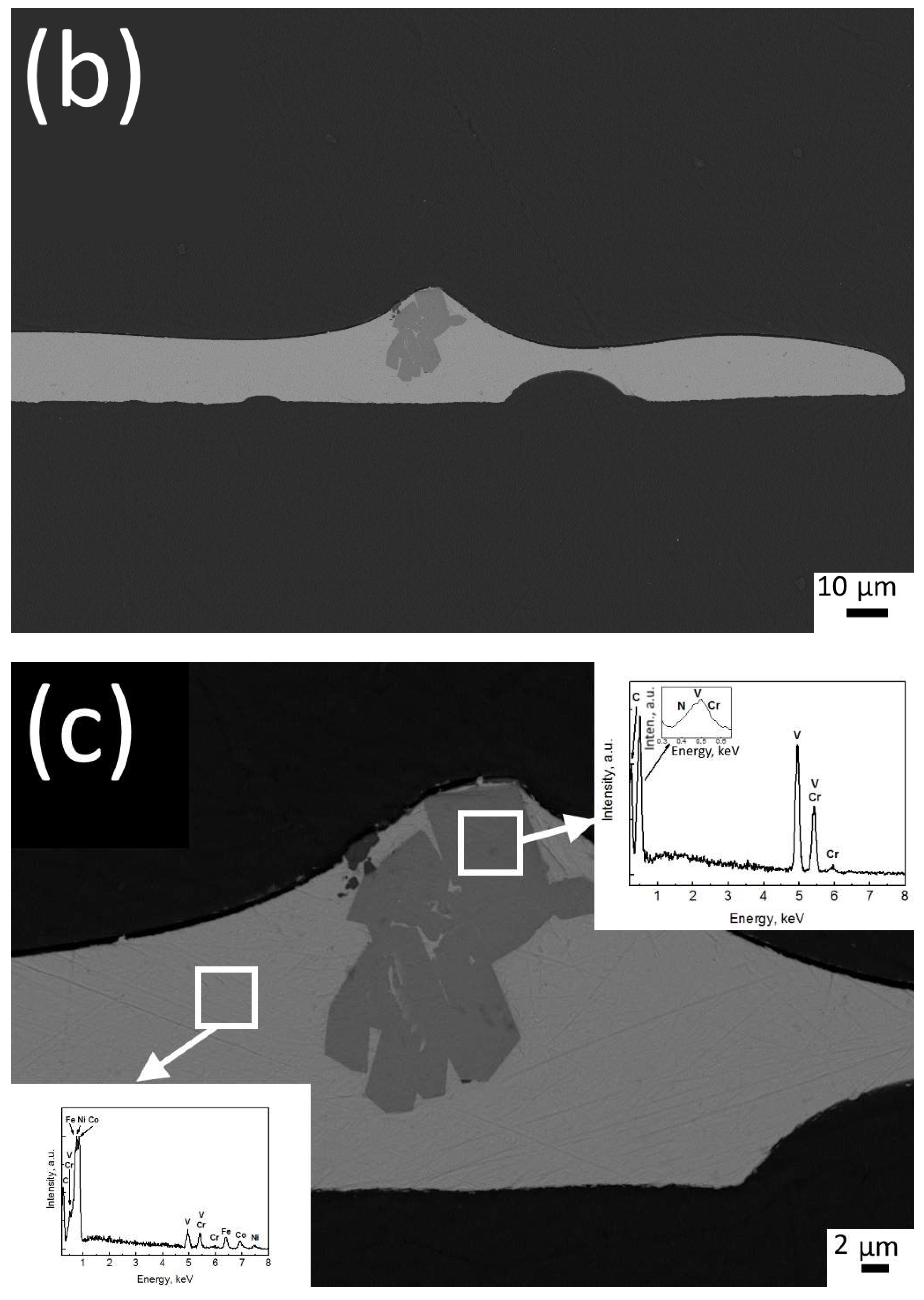
3.1. Structure Investigation
3.2. Calorimetric Studies
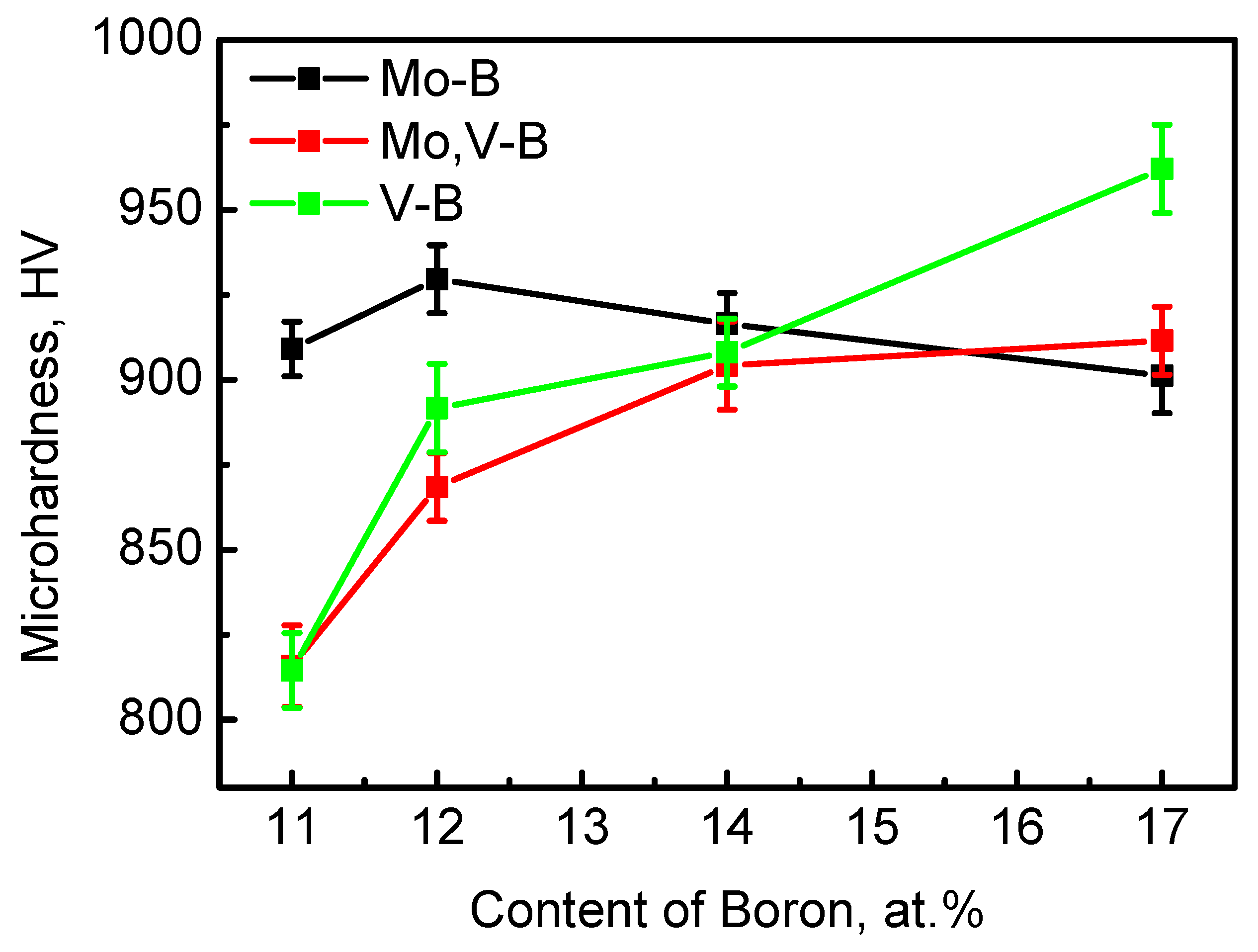
3.3. Microhardness Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klement, W.; Willens, R.H.; Duwez, P. Non-Crystalline Structure in Solidified Gold–Silicon Alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic Glasses. In Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 305–385. [Google Scholar]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315153483. [Google Scholar]
- Terekhov, S.V. Single- and Multistage Crystallization of Amorphous Alloys. Phys. Met. Metallogr. 2020, 121, 664–669. [Google Scholar] [CrossRef]
- Suryanarayana, C. In Situ Mechanical Crystallization of Amorphous Alloys. J. Alloys Compd. 2023, 961, 171032. [Google Scholar] [CrossRef]
- Bazlov, A.I.; Parkhomenko, M.S.; Ubyivovk, E.V.; Zanaeva, E.N.; Bazlova, T.A.; Gunderov, D.V. Severe Plastic Deformation Influence on the Structure Transformation of the Amorphous Zr62.5Cu22.5Al10Fe5 Alloy. Intermetallics 2023, 152, 107777. [Google Scholar] [CrossRef]
- Judy, J.W. Microactuators. In MEMS; Elsevier: Amsterdam, The Netherlands, 2006; pp. 751–803. [Google Scholar]
- Manvi, M.; Mruthyunjaya Swamy, K.B. Microelectronic Materials, Microfabrication Processes, Micromechanical Structural Configuration Based Stiffness Evaluation in MEMS: A Review. Microelectron. Eng. 2022, 263, 111854. [Google Scholar] [CrossRef]
- Sharma, P.; Inoue, A. Metallic Glass. In Handbook of Silicon Based MEMS Materials and Technologies; Elsevier: Amsterdam, The Netherlands, 2010; pp. 447–472. [Google Scholar]
- Tilli, M.; Haapalinna, A. Properties of Silicon. In Handbook of Silicon Based MEMS Materials and Technologies; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–17. [Google Scholar]
- Ashby, M.; Greer, A. Metallic Glasses as Structural Materials. Scr. Mater. 2006, 54, 321–326. [Google Scholar] [CrossRef]
- Azuma, D.; Ito, N.; Ohta, M. Recent Progress in Fe-Based Amorphous and Nanocrystalline Soft Magnetic Materials. J. Magn. Magn. Mater. 2020, 501, 166373. [Google Scholar] [CrossRef]
- Sai Ram, B.; Paul, A.K.; Kulkarni, S.V. Soft Magnetic Materials and Their Applications in Transformers. J. Magn. Magn. Mater. 2021, 537, 168210. [Google Scholar] [CrossRef]
- Li, F.C.; Liu, T.; Zhang, J.Y.; Shuang, S.; Wang, Q.; Wang, A.D.; Wang, J.G.; Yang, Y. Amorphous–Nanocrystalline Alloys: Fabrication, Properties, and Applications. Mater. Today Adv. 2019, 4, 100027. [Google Scholar] [CrossRef]
- Herzer, G. Modern Soft Magnets: Amorphous and Nanocrystalline Materials. Acta Mater. 2013, 61, 718–734. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, X.; Lu, W.; Liang, D.-D.; Wen, Z.; Wang, Z.; Xiang, H.; Shen, J. Design of Cu-Zr-Al and Cu-Zr-Al-Sn Bulk Amorphous Alloys with High Glass-Forming Ability. J. Non-Cryst. Solids 2019, 521, 119531. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Chen, Y.B.; Wei, J.; Wang, X.; Qiu, Z.G.; Zeng, D.C. Enhanced Ms of Fe-Rich Fe-B-Cu Amorphous/Nanocrystalline Alloys Achieved by Annealing Treatments. J. Alloys Compd. 2023, 939, 168621. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Iron-Based Bulk Metallic Glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent Development and Application Products of Bulk Glassy Alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Z.-W.; Jiang, J.-Z. High Entropy Metallic Glasses: Glass Formation, Crystallization and Properties. J. Alloys Compd. 2021, 866, 158852. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Li, Q.; Shen, J.; Liu, H.; Zhao, Y.; Yang, W. Fe-Based Bulk Metallic Glass with Unprecedented Plasticity at Room Temperature. Intermetallics 2021, 139, 107377. [Google Scholar] [CrossRef]
- Luborsky, F.; Reeve, J.; Davies, H.; Liebermann, H. Effect of Fe-B-Si Composition on Maximum Thickness for Casting Amorphous Metals. IEEE Trans. Magn. 1982, 18, 1385–1387. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Zhang, T.; Inoue, A. Bulk Glass Formation of Ti-Zr-Hf-Cu-M (M=Fe, Co, Ni) Alloys. Mater. Trans. 2002, 43, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Li, Y.; Takeuchi, A.; Xie, G.; Miao, H.; Zhang, W. Soft Magnetic Fe25Co25Ni25(B, Si)25 High Entropy Bulk Metallic Glasses. Intermetallics 2015, 66, 8–12. [Google Scholar] [CrossRef]
- Ding, J.; Inoue, A.; Han, Y.; Kong, F.L.; Zhu, S.L.; Wang, Z.; Shalaan, E.; Al-Marzouki, F. High Entropy Effect on Structure and Properties of (Fe,Co,Ni,Cr)-B Amorphous Alloys. J. Alloys Compd. 2017, 696, 345–352. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Qi, T. New Soft Magnetic Fe25Co25Ni25(P,C,B)25 High Entropy Bulk Metallic Glasses with Large Supercooled Liquid Region. J. Alloys Compd. 2017, 693, 25–31. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.H.; Li, X.; Wang, J.; Wang, B.; Wang, K.M. Effect of Co Content on Structural Stability and Soft Magnetic Properties for (Fe1−xCox)86Hf7B6Cu1 Amorphous Alloy. Phys. Met. Metallogr. 2020, 121, 123–127. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase Stability in High Entropy Alloys: Formation of Solid-Solution Phase or Amorphous Phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrov, V.D.; Aleksandrova, O.V.; Shchebetovskaya, N.V. Nucleation of Substitutional Solid Solutions during the Solidification of Binary Liquid Solutions. Russ. Metall. 2013, 2013, 192–197. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Xie, L.; Zhang, G.; Mu, B.; Chang, C.; Li, H.; Ma, X. Development of Novel Fe-Based Bulk Metallic Glasses with Excellent Wear and Corrosion Resistance by Adjusting the Cr and Mo Contents. Intermetallics 2023, 153, 107801. [Google Scholar] [CrossRef]
- Wang, F.; Inoue, A.; Kong, F.L.; Zhu, S.L.; Shalaan, E.; Al-Marzouki, F.; Botta, W.J.; Kiminami, C.S.; Ivanov, Y.P.; Greer, A.L. Formation, Stability and Ultrahigh Strength of Novel Nanostructured Alloys by Partial Crystallization of High-Entropy (Fe0.25Co0.25Ni0.25Cr0.125Mo0.125)86–89B11–14 Amorphous Pha. Acta Mater. 2019, 170, 50–61. [Google Scholar] [CrossRef]
- Wang, F.; Inoue, A.; Kong, F.L.; Han, Y.; Zhu, S.L.; Shalaan, E.; Al-Marouki, F. Formation, Thermal Stability and Mechanical Properties of High Entropy (Fe,Co,Ni,Cr,Mo)-B Amorphous Alloys. J. Alloys Compd. 2018, 732, 637–645. [Google Scholar] [CrossRef]
- Fang, S.; Wang, C.; Li, C.L.; Luan, J.H.; Jiao, Z.B.; Liu, C.T.; Hsueh, C.H. Microstructures and Mechanical Properties of CoCrFeMnNiVx High Entropy Alloy Films. J. Alloys Compd. 2020, 820, 153388. [Google Scholar] [CrossRef]
- Lee, D.N.; Kim, K.; Lee, Y.; Choi, C.-H. Factors Determining Crystal Orientation of Dendritic Growth during Solidification. Mater. Chem. Phys. 1997, 47, 154–158. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Todoroki, H.; Shiga, N.; Ishii, T. Solubility of Nitrogen in Fe-Cr-Ni-Mo Stainless Steel under a 1 Atm N2 Gas Atmosphere. ISIJ Int. 2012, 52, 1601–1606. [Google Scholar] [CrossRef] [Green Version]
- Kowanda, C.; Speidel, M. Solubility of Nitrogen in Liquid Nickel and Binary Ni–Xi Alloys (Xi=Cr, Mo,W, Mn, Fe, Co) under Elevated Pressure. Scr. Mater. 2003, 48, 1073–1078. [Google Scholar] [CrossRef]
- Singh, R. Production of Steel. In Applied Welding Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–52. [Google Scholar]
- Alexis, V.; Frédéric, R.; Jean, Q.; Eric, A. Determination of Gray Cast Iron Age Strengthening by Nondestructive Methods: Effect of Alloying Elements. J. Mater. Eng. Perform. 2019, 28, 4026–4033. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Klemm-Toole, J.; Clarke, A.J.; Findley, K.O. Improving the Fatigue Performance of Vanadium and Silicon Alloyed Medium Carbon Steels after Nitriding through Increased Core Fatigue Strength and Compressive Residual Stress. Mater. Sci. Eng. A 2021, 810, 141008. [Google Scholar] [CrossRef]
- Xie, Y.; Miyamoto, G.; Furuhara, T. High-Throughput Investigation of Cr-N Cluster Formation in Fe-35Ni-Cr System during Low-Temperature Nitriding. Acta Mater. 2023, 253, 118921. [Google Scholar] [CrossRef]
- Rudy, E.; Benesovsky, F. Untersuchungen in Den Systemen: Hafnium—Bor—Stickstof Und Zirkonium—Bor—Stickstoff. Monatshefte Chem. Verwandte Teile Anderer Wiss. 1961, 92, 415–441. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Chu, D.; Xie, S.; Sun, H.; Ferry, M.; Li, S. Composition Dependence of the Microstructure and Soft Magnetic Properties of Fe-Based Amorphous/Nanocrystalline Alloys: A Review Study. J. Non-Cryst. Solids 2014, 391, 61–82. [Google Scholar] [CrossRef]
- Makino, A.; Men, H.; Kubota, T.; Yubuta, K.; Inoue, A. FeSiBPCu Nanocrystalline Soft Magnetic Alloys with High Bs of 1.9 Tesla Produced by Crystallizing Hetero-Amorphous Phase. Mater. Trans. 2009, 50, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Yue, S.; Zhang, H.; Cheng, R.; Wang, A.; Dong, Y.; He, A.; Ni, H.; Liu, C.-T. Magnetic and Thermal Stabilities of FeSiB Eutectic Amorphous Alloys: Compositional Effects. J. Alloys Compd. 2019, 776, 833–838. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear Bands in Metallic Glasses. Mater. Sci. Eng. R Rep. 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, H.; Liaw, P.K. Metallic Glass Matrix Composites. Mater. Sci. Eng. R Rep. 2016, 100, 1–69. [Google Scholar] [CrossRef] [Green Version]
- Glezer, A.M.; Shurygina, N.A.; Zaichenko, S.G.; Permyakova, I.E. Interaction of Deformation Shear Bands with Nanoparticles in Amorphous-Nanocrystalline Alloys. Russ. Metall. 2013, 2013, 235–244. [Google Scholar] [CrossRef]
- Sharma, A.; Zadorozhnyy, V. Review of the Recent Development in Metallic Glass and Its Composites. Metals 2021, 11, 1933. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazlov, A.; Strochko, I.; Ubyivovk, E.; Parkhomenko, M.; Magomedova, D.; Zanaeva, E. Structure and Properties of Amorphous Quasi-High-Entropy Fe-Co-Ni-Cr-(Mo,V)-B Alloys with Various Boron Content. Metals 2023, 13, 1464. https://doi.org/10.3390/met13081464
Bazlov A, Strochko I, Ubyivovk E, Parkhomenko M, Magomedova D, Zanaeva E. Structure and Properties of Amorphous Quasi-High-Entropy Fe-Co-Ni-Cr-(Mo,V)-B Alloys with Various Boron Content. Metals. 2023; 13(8):1464. https://doi.org/10.3390/met13081464
Chicago/Turabian StyleBazlov, Andrey, Ilia Strochko, Evgeny Ubyivovk, Mark Parkhomenko, Daria Magomedova, and Erzhena Zanaeva. 2023. "Structure and Properties of Amorphous Quasi-High-Entropy Fe-Co-Ni-Cr-(Mo,V)-B Alloys with Various Boron Content" Metals 13, no. 8: 1464. https://doi.org/10.3390/met13081464




