Effects of Fe, Si, Cu, and TiB2 Grain Refiner Amounts on the Hot Tearing Susceptibility of 5083, 6061, and 7075 Aluminum Ingots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloy Designations
2.2. HTS Measurement
2.3. Microstructure Analysis
3. Results
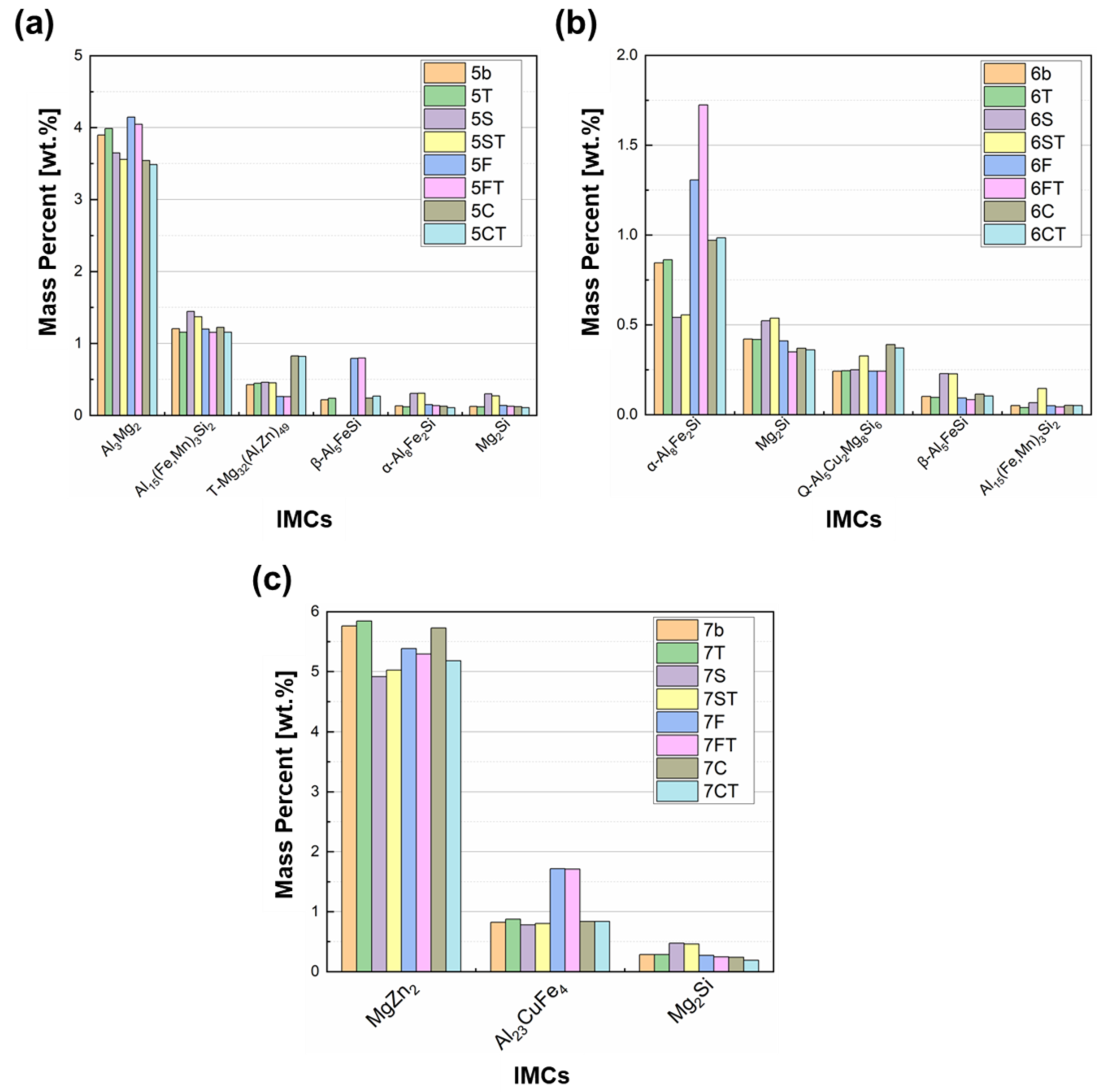
3.1. Influence of Compositional Increments on HTS
3.2. Results of CALPHAD Evaluation
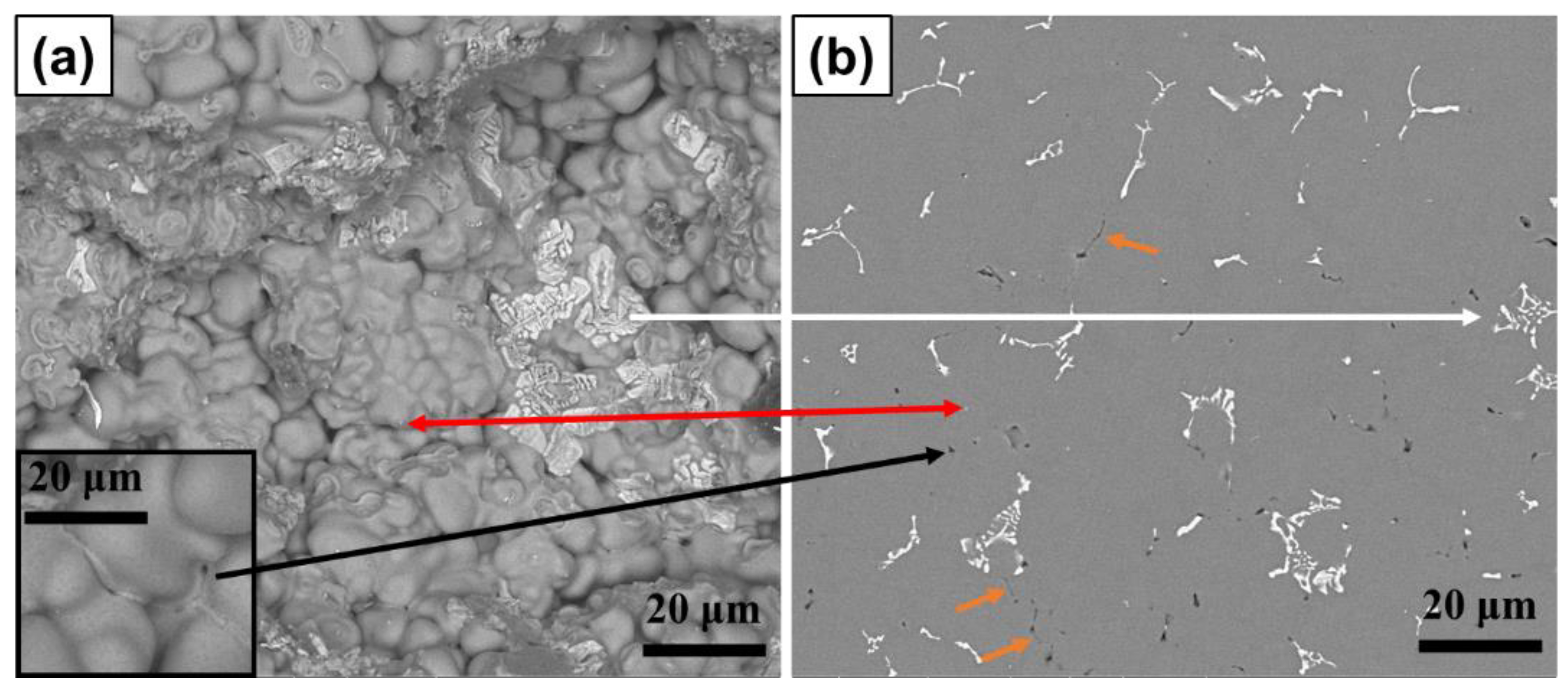
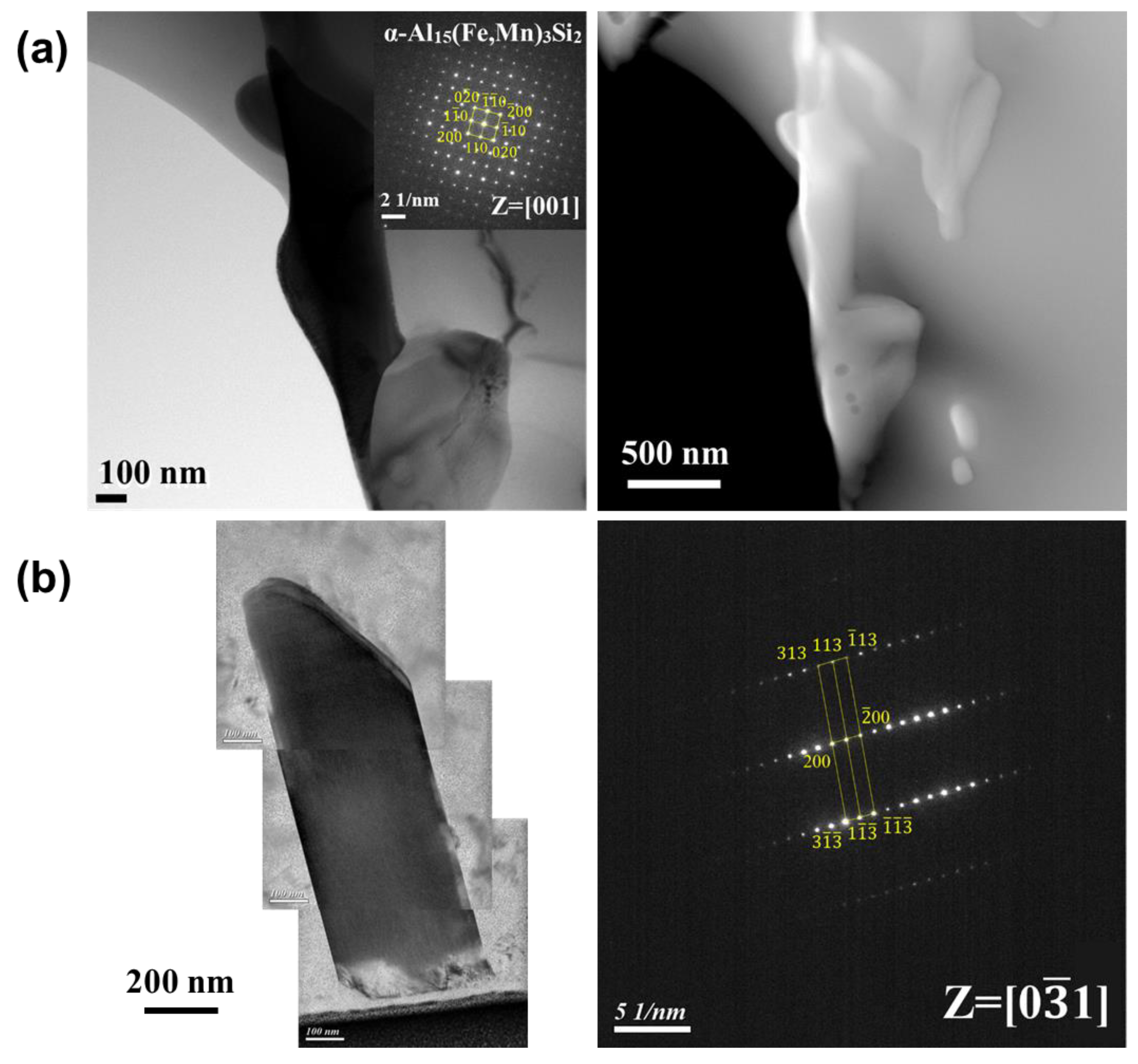
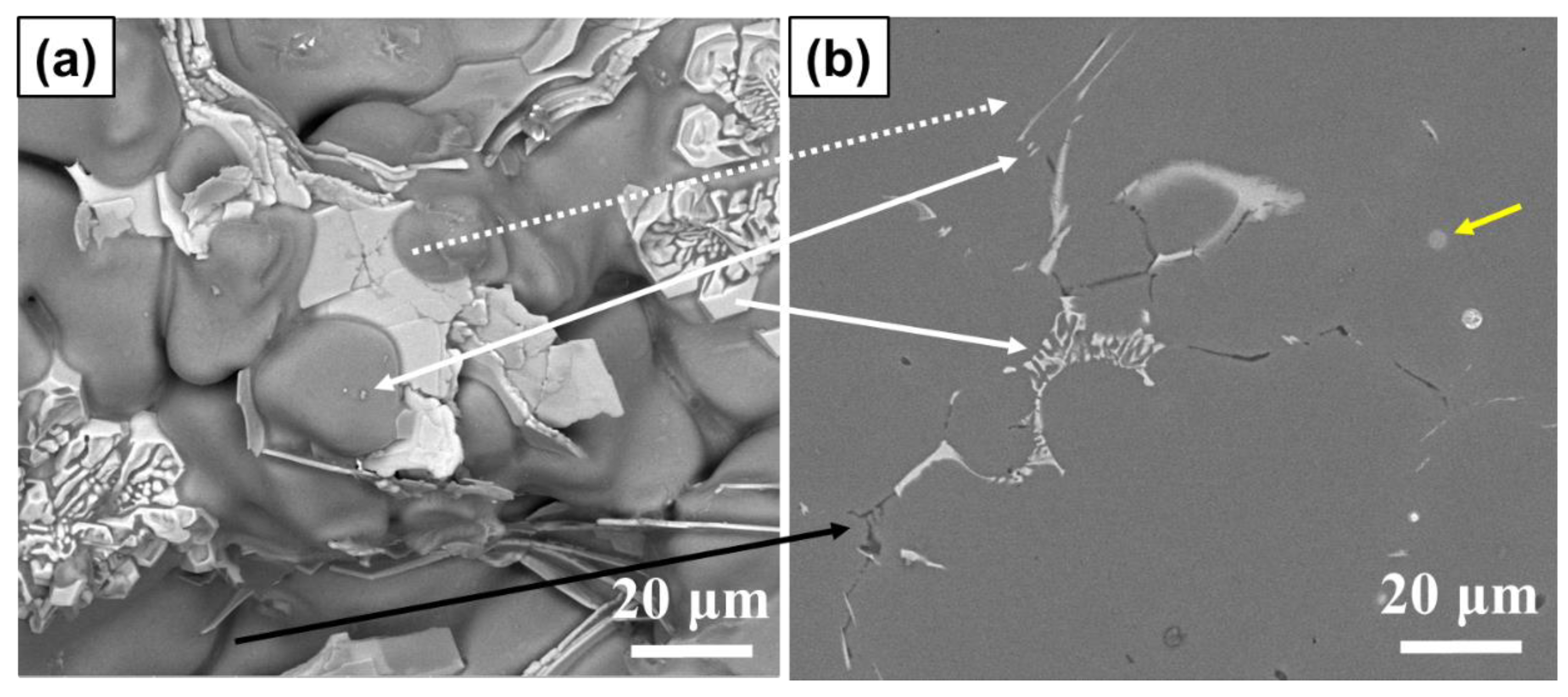
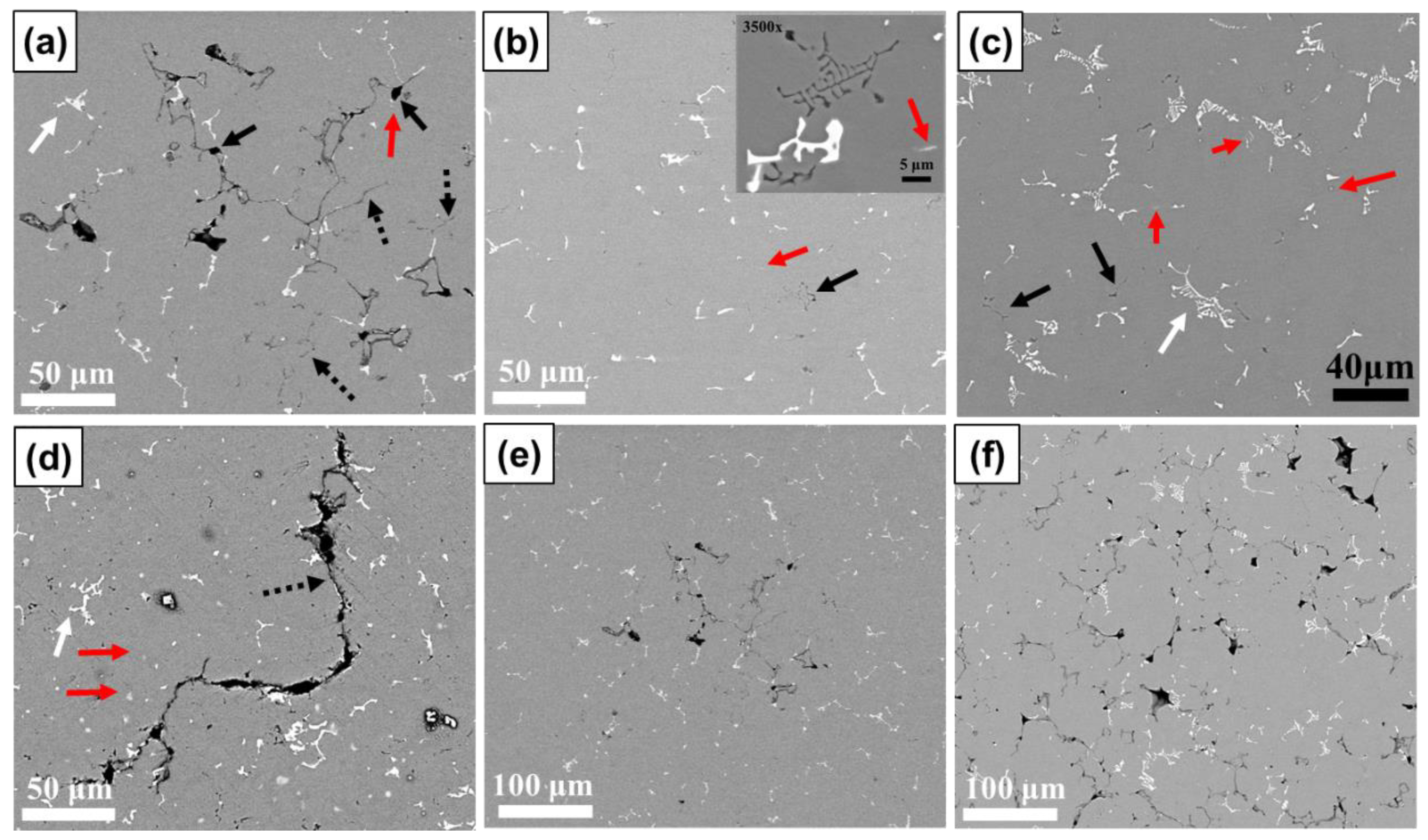
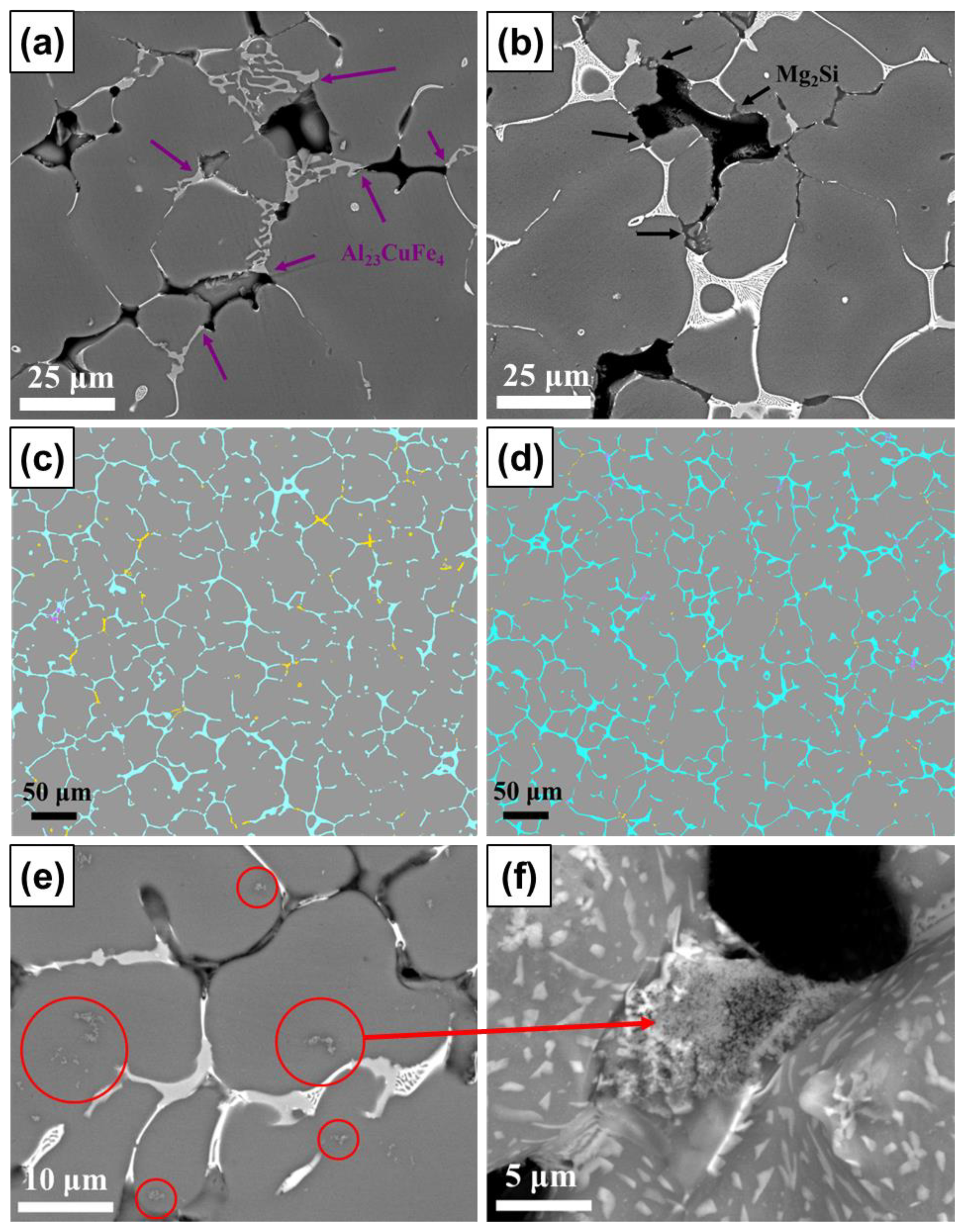
3.3. Phase Identification and IMC Distribution
3.4. Influence of Compositional Variation on Microstructure
4. Discussion
5. Conclusions
- In aluminum alloy 5083, when the Si content is increased to 0.2 wt.% or higher, Mg2Si assumes fishbone morphology, whereas the amount and size of α-Al15(Fe,Mn)3Si2 remain almost unchanged. Increasing the Fe content from 0.3 to 0.5 wt.% greatly increases the amount of α-Al15(Fe,Mn)3Si2, the morphology of which tends to be a fishbone structure prone to stress concentration. When the Cu content is increased from 0.03 to 0.09 wt.%, T-Mg32(Al,Zn)49 greatly hinders feeding. Both T-Mg32(Al,Zn)49 and Al3Mg2 contribute to hot tearing, although through different mechanisms. T-Mg32(Al,Zn)49 leads to decreased feeding capability, whereas Al3Mg2 causes continuous cracking resulting from stress concentration. Increasing the amount of Ti from 0.05 to approximately 0.1 wt.% effectively refines the grains, enhancing the overall feeding capability and reducing the continuous cracking of fishbone-shaped IMCs.
- In aluminum alloy 6061, when the Si content is increased from 0.6 to approximately 0.9 wt.%, the amount of α-Al15(Fe,Mn)3Si2 does not increase, but the amounts of Mg2Si and β-Al5FeSi do. Mg2Si does not affect HTS, whereas β-Al5FeSi positively affects HTS. Increasing the Fe content from 0.3 to 0.5 wt.% dramatically increases the amount of fishbone-shaped α-Al15(Fe,Mn)3Si2 but cannot effectively reduce the amount of harmful plate/needle-like β-Al5FeSi. Increasing the Cu content from 0.3 to 0.5 wt.% reduces the NES; it expands the solidification range, leading to an increase in the contraction rate and an increase in the amount of plate/needle-like β-Al5FeSi. Increasing the Ti content also effectively refines the grains, improving the overall feeding capability and reducing stress concentration and severe blockage of remaining liquid channels, thereby mitigating continuous cracking.
- In aluminum alloy 7075, when the Si content is increased from 0.1 to approximately 0.2 wt.%, an increase in the Mg2Si content hinders the flow of remaining liquid and leads to a decrease in the amount of αAl+Mg(Zn,Cu,Al)2 eutectic. Increasing the Fe content from 0.2 to 0.45 wt.% greatly increases the amount of Al23CuFe4, which also impedes the flow of interdendritic liquid. When the Cu content is increased from 1.7 to approximately 2.3 wt.%, the content of Al23CuFe4 does not increase considerably; instead, the additional Cu tends to promote Mg(Zn,Cu,Al)2 formation, resulting in a substantial increase in the remaining liquid content. When the Ti content is increased from 0.05 to approximately 0.1 wt.%, the grain size decreases, and the overall feeding capability is improved. However, the grain refiner may not be fully dispersed, and this would lead to the formation of numerous TiB2 clusters, which hinder flow in the liquid channel. By contrast, in the 5083 and 6061 aluminum alloys, in which needle-shaped or fishbone-shaped IMCs tend to form, the benefit of reducing the size of needle-shaped or fishbone-shaped IMCs due to the addition of grain refiner outweighs the disadvantage of TiB2 clusters obstructing the flow of remaining liquid. Therefore, Ti increments rather than base material increments are found to lead to lower HTS in 5083 and 6061 aluminum alloys.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, W.F. Structure and Properties of Engineering Alloys; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Campbell, J. Complete Casting Handbook: Metal Casting Processes, Metallurgy, Techniques and Design; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Nadella, R.; Eskin, D.G.; Du, Q.; Katgerman, L. Macrosegregation in direct-chill casting of aluminium alloys. Prog. Mater. Sci. 2008, 53, 421–480. [Google Scholar] [CrossRef]
- Eskin, D.G.; Suyitno; Katgerman, L. Mechanical properties in the semi-solid state and hot tearing of aluminium alloys. Prog. Mater. Sci. 2004, 49, 629–711. [Google Scholar] [CrossRef]
- Dantzig, J.A.; Rappaz, M. Solidification; EPFL Press: London, UK, 2009. [Google Scholar]
- Dahle, A.K.; StJohn, D.H. Rheological behaviour of the mushy zone and its effect on the formation of casting defects during solidification. Acta Mater. 1998, 47, 31–41. [Google Scholar] [CrossRef]
- Eskin, D.G. Physical Metallurgy of Direct Chill Casting of Aluminum Alloys; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Meylan, B.; Terzi, S.; Gourlay, C.M.; Dahle, A.K. Dilatancy and rheology at 0–60% solid during equiaxed solidification. Acta Mater. 2011, 59, 3091–3101. [Google Scholar] [CrossRef]
- Zhang, L. Ultrasonic Processing of Aluminum Alloys; Delft University of Technology: Delft, The Netherlands, 2013. [Google Scholar]
- Eskin, D.G.; Katgerman, L.; Suyitno; Mooney, J.F. Contraction of aluminum alloys during and after solidification. Metall. Mater. Trans. A 2004, 35, 1325–1335. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Katgerman, L.; Du, Q.; Zhang, J.; Zhuang, L. Recent advances in hot tearing during casting of aluminium alloys. Prog. Mater. Sci. 2021, 117, 100741. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.R.; Zhao, Z.Y.; Li, H.X.; Katgerman, L.; Zhang, J.S.; Zhuang, L.Z. Effect of Main Elements (Zn, Mg, and Cu) on Hot Tearing Susceptibility During Direct-Chill Casting of 7xxx Aluminum Alloys. Metall. Mater. Trans. A 2019, 50, 3603–3616. [Google Scholar] [CrossRef]
- Pereira, L.H.; Asato, G.H.; Otani, L.B.; Jorge, A.M., Jr.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Changing the solidification sequence and the morphology of iron-containing intermetallic phases in AA6061 aluminum alloy processed by spray forming. Mater. Charact. 2018, 145, 507–515. [Google Scholar] [CrossRef]
- Wang, J.S. In-Situ Observation and Mathematical Modelling of the Nucleation and Growth of Intermetallics and Micropores during the Solidification of Aluminium Alloys; Imperial College: London, UK, 2009. [Google Scholar]
- Samuel, F.H.; Samuel, A.M.; Doty, H.W.; Valtierra, S. Decomposition of Fe-intermetallics in Sr-modified cast 6XXX type aluminum alloys for automotive skin. Metall. Mater. Trans. A 2001, 32, 2061–2075. [Google Scholar] [CrossRef]
- Abou Khatwa, M.K.; Malakhov, D.V. On the thermodynamic stability of intermetallic phases in the AA6111 aluminum alloy. Calphad 2006, 30, 159–170. [Google Scholar] [CrossRef]
- Dash, M.; Makhlouf, M. Effect of key alloying elements on the feeding characteristics of aluminum–silicon casting alloys. J. Light Met. 2001, 1, 251–265. [Google Scholar] [CrossRef]
- Caceres, C.; Djurdjevic, M.; Stockwell, T.; Sokolowski, J. The effect of Cu content on the level of microporosity in Al-Si-Cu-Mg casting alloys. Scr. Mater. 1999, 40, 631–637. [Google Scholar] [CrossRef]
- Easton, M.A.; Wang, H.; Grandfield, J.; Davidson, C.J.; StJohn, D.H.; Sweet, L.D.; Couper, M.J. Observation and Prediction of the Hot Tear Susceptibility of Ternary Al-Si-Mg Alloys. Metall. Mater. Trans. A 2012, 43, 3227–3238. [Google Scholar] [CrossRef]
- Razaz, G.; Carlberg, T. Hot tearing susceptibility of AA3000 aluminum alloy containing Cu, Ti, and Zr. Metall. Mater. Trans. A 2019, 50, 3842–3854. [Google Scholar] [CrossRef]
- Cao, G.; Kou, S. Hot cracking of binary Mg–Al alloy castings. Mater. Sci. Eng. A 2006, 417, 230–238. [Google Scholar] [CrossRef]
- Wang, K.; Fu, P.; Peng, L.; Wang, Y.; Ding, W. A simplified hot-tearing criterion for shape castings based on temperature-field simulation. Metall. Mater. Trans. A 2019, 50, 5271–5280. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Seung, H.S. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Wahlmann, B.; Leidel, D.; Markl, M.; Körner, C. Numerical alloy development for additive manufacturing towards reduced cracking susceptibility. Crystals 2021, 11, 902. [Google Scholar] [CrossRef]
- Jung, J.G.; Cho, Y.H.; Lee, J.M.; Kim, H.W.; Euh, K. Designing the composition and processing route of aluminum alloys using CALPHAD: Case studies. CALPHAD 2019, 64, 236–247. [Google Scholar] [CrossRef]
- Cinkilic, E.; Moodispaw, M.; Zhang, J.; Miao, J.; Luo, A.A. A New Recycled Al–Si–Mg Alloy for Sustainable Structural Die Casting Applications. Metall. Mater. Trans. A 2022, 53, 2861–2873. [Google Scholar] [CrossRef]
- Yan, L.Z.; Zhang, Y.A.; Li, X.W.; Li, Z.H.; Wang, F.; Liu, H.W.; Xiong, B.Q. Microstructural evolution of Al–0.66Mg–0.85Si alloy during homogenization. Trans. Nonferrous Met. Soc. China 2014, 24, 939–945. [Google Scholar] [CrossRef]
- Sweet, L.; Zhu, S.M.; Gao, S.X.; Taylor, J.A.; Easton, M.A. The Effect of Iron Content on the Iron-Containing Intermetallic Phases in a Cast 6060 Aluminum Alloy. Metall. Mater. Trans. A 2011, 42, 1737–1749. [Google Scholar] [CrossRef]
- Gao, M.; Feng, C.R.; Wei, R.P. An analytical electron microscopy study of constituent particles in commercial 7075-T6 and 2024-T3 alloys. Metall. Mater. Trans. A 1998, 29, 1145–1151. [Google Scholar] [CrossRef]
- Gao, W.; Ning, J.; Gu, X.; Chen, L.; Liang, H.; Li, W.; Lewandowski, J.J. Precipitation Behavior and Corrosion Properties of Stirred Zone in FSWed AA5083 Al-Mg Alloy after Sensitization. Metals 2023, 13, 1618. [Google Scholar] [CrossRef]
- Ceschini, L.; Boromei, I.; Morri, A.; Seifeddine, S.; Svensson, I.L. Microstructure, tensile and fatigue properties of the Al–10% Si–2% Cu alloy with different Fe and Mn content cast under controlled conditions. J. Mater. Process. Technol. 2009, 209, 5669–5679. [Google Scholar] [CrossRef]
- Takai, R.; Endo, N.; Hirohara, R.; Tsunoda, T.; Yoshida, M. Experimental and numerical analysis of grain refinement effect on hot tearing susceptibility for Al–Mg alloys. Int. J. Adv. Manuf. Technol. 2019, 100, 1867–1880. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.; Pan, Y.; Li, H.; Zhou, L.; Zhang, J.; Zhuang, L. Liquation Cracking Tendency of Novel Al-Mg-Zn Alloys with a Zn/Mg Ratio below 1.0 during Fusion Welding. Metals 2020, 10, 222. [Google Scholar] [CrossRef]
- Puncreobutr, C.; Lee, P.; Kareh, K.; Connolley, T.; Fife, J.; Phillion, A. Influence of Fe-rich intermetallics on solidification defects in Al–Si–Cu alloys. Acta Mater. 2014, 68, 42–51. [Google Scholar] [CrossRef]
- Ganjehfard, K.; Taghiabadi, R.; Noghani, M.T.; Ghoncheh, M.H. Tensile properties and hot tearing susceptibility of cast Al-Cu alloys containing excess Fe and Si. Int. J. Miner. Metall. 2021, 28, 718–728. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.L.; Liu, J.C.; Li, H.X.; Du, Q.; Zhang, J.S.; Zhuang, L.Z. The Influences of Grain Size and Morphology on the Hot Tearing Susceptibility, Contraction, and Load Behaviors of AA7050 Alloy Inoculated with Al-5Ti-1B Master Alloy. Metall. Mater. Trans. A 2016, 47, 4024–4037. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Cui, H.; Zhuang, L.; Zhang, J. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al–Mg alloys. J. Alloys Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Nadim, A.; Taghiabadi, R.; Razaghian, A. Effect of Mn modification on the Tribological properties of in situ Al-15Mg2Si composites containing Fe as an impurity. J. Tribol. 2018, 140, 061610. [Google Scholar] [CrossRef]
- Nagaumi, H.; Suzuki, S.; Okane, T.; Umeda, T. Effect of Iron Content on Hot Tearing of High-Strength Al-Mg-Si Alloy. Mater. Trans. 2006, 47, 2821–2827. [Google Scholar] [CrossRef]
- Sweet, L.; Easton, M.A.; Taylor, J.A.; Grandfield, J.F.; Davidson, C.J.; Lu, L.; Couper, M.J.; StJohn, D.H. Hot Tear Susceptibility of Al-Mg-Si-Fe Alloys with Varying Iron Contents. Metall. Mater. Trans. A 2013, 44, 5396–5407. [Google Scholar] [CrossRef]






| 5083 | Mg | Si | Fe | Cu | Cr | Mn | Zn | Ti | Al |
| 5b | 4.72 | 0.12 | 0.30 | 0.05 | 0.07 | 0.68 | 0.12 | 0.05 | Bal. |
| 5T | 4.79 | 0.12 | 0.30 | 0.05 | 0.07 | 0.66 | 0.12 | 0.09 | |
| 5S | 4.73 | 0.23 | 0.32 | 0.05 | 0.08 | 0.68 | 0.13 | 0.07 | |
| 5ST | 4.63 | 0.22 | 0.31 | 0.05 | 0.08 | 0.67 | 0.13 | 0.13 | |
| 5F | 4.81 | 0.13 | 0.52 | 0.03 | 0.07 | 0.52 | 0.12 | 0.06 | |
| 5FT | 4.73 | 0.13 | 0.51 | 0.03 | 0.07 | 0.51 | 0.12 | 0.10 | |
| 5C | 4.74 | 0.12 | 0.31 | 0.09 | 0.08 | 0.68 | 0.14 | 0.08 | |
| 5CT | 4.68 | 0.12 | 0.31 | 0.09 | 0.08 | 0.67 | 0.13 | 0.12 | |
| 6061 | Mg | Si | Fe | Cu | Cr | Mn | Zn | Ti | Al |
| 6b | 0.90 | 0.65 | 0.33 | 0.31 | 0.07 | 0.03 | 0.05 | 0.05 | Bal. |
| 6T | 0.90 | 0.65 | 0.33 | 0.31 | 0.07 | 0.03 | 0.05 | 0.09 | |
| 6S | 0.96 | 0.94 | 0.26 | 0.32 | 0.07 | 0.04 | 0.05 | 0.04 | |
| 6ST | 0.98 | 0.97 | 0.27 | 0.33 | 0.07 | 0.04 | 0.05 | 0.08 | |
| 6F | 0.89 | 0.66 | 0.48 | 0.31 | 0.07 | 0.04 | 0.05 | 0.04 | |
| 6FT | 0.82 | 0.65 | 0.62 | 0.31 | 0.07 | 0.03 | 0.05 | 0.11 | |
| 6C | 0.90 | 0.69 | 0.37 | 0.50 | 0.07 | 0.03 | 0.05 | 0.04 | |
| 6CT | 0.88 | 0.68 | 0.38 | 0.48 | 0.07 | 0.03 | 0.05 | 0.09 | |
| 7075 | Mg | Si | Fe | Cu | Cr | Mn | Zn | Ti | Al |
| 7b | 2.84 | 0.13 | 0.22 | 1.72 | 0.21 | 0.07 | 6.01 | 0.04 | Bal. |
| 7T | 2.87 | 0.13 | 0.23 | 1.75 | 0.20 | 0.07 | 6.04 | 0.07 | |
| 7S | 2.57 | 0.22 | 0.22 | 1.70 | 0.27 | 0.07 | 5.76 | 0.04 | |
| 7ST | 2.59 | 0.22 | 0.22 | 1.79 | 0.21 | 0.07 | 5.77 | 0.08 | |
| 7F | 2.57 | 0.12 | 0.44 | 1.69 | 0.21 | 0.07 | 5.88 | 0.03 | |
| 7FT | 2.52 | 0.12 | 0.44 | 1.75 | 0.21 | 0.07 | 5.72 | 0.07 | |
| 7C | 2.57 | 0.11 | 0.22 | 2.29 | 0.20 | 0.07 | 5.84 | 0.04 | |
| 7CT | 2.35 | 0.10 | 0.22 | 2.30 | 0.22 | 0.07 | 5.46 | 0.10 |
| Crack Width Rating | Hot Tearing Location Rating | Rod Length Rating [mm] | |||
|---|---|---|---|---|---|
| Hairline | 1 | Closed to the sprue | 1 | 150.5 | 1 |
| Light | 2 | At ball end | 1.5 | 112.5 | 2 |
| Severe | 3 | In the middle of the rod | 2 | 74.5 | 3 |
| Complete separation | 4 | - | - | 36.5 | 4 |
| 5083 | Grain Size (µm) | 6061 | Grain Size (µm) | 7075 | Grain Size (µm) |
|---|---|---|---|---|---|
| 5b | 78.18 ± 12.68 | 6b | 64.78 ± 9.13 | 7b | 54.07 ± 8.33 |
| 5T | 71.18 ± 9.64 | 6T | 51.5 ± 7.67 | 7T | 50.43 ± 7.16 |
| 5S | 68.25 ± 8.70 | 6S | 64.61 ± 8.55 | 7S | 38.99 ± 3.94 |
| 5ST | 67.93 ± 8.63 | 6ST | 59.59 ± 6.00 | 7ST | 31.07 ± 4.70 |
| 5F | 74.45 ± 8.82 | 6F | 55.67 ± 8.51 | 7F | 40.87 ± 4.89 |
| 5FT | 67.94 ± 9.46 | 6FT | 50.28 ± 8.51 | 7FT | 37.74 ± 4.16 |
| 5C | 79.9 ± 10.67 | 6C | 82.61 ± 18.01 | 7C | 55.78 ± 7.63 |
| 5CT | 73.02 ± 10.43 | 6CT | 63.43 ± 10.80 | 7CT | 54.7 ± 9.80 |
| IMCs | 5083 | 6061 | 7075 | Color | Morphology | HTS Value | |
|---|---|---|---|---|---|---|---|
| Mg(Zn,Cu,Al)2 | √ | white | fishbone-shaped or small circular dots or irregular | ↓ | |||
| α-Al15(Fe,Mn)3Si2 | √ | √ | white | fishbone-shaped or small circular dots | ↑ (5) | –(6) | |
| β-Al5FeSi | √ | white | plate or needle-like | ↑ | |||
| Al23CuFe4 | √ | gray | irregular | ↑ | |||
| Q-Al15Cu2Mg8Si6 | √ | gray/white | spherical | – | |||
| Mg2Si | √ | √ | √ | black | irregular or small circular dots | ↑ (5, 7) | –(6) |
| Al3Mg2 | √ | gray/black | irregular (thinner than Mg2Si) | ↑ (lower effect) | |||
| T-Mg32(Al,Zn)49 | √ | gray | small circular dots (about 3~5μm) | ↑ (higher effect) | |||
| TiB2 | √ | √ | √ | gray | irregular particle | – (↑ if clustering) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.-Y.; Huang, H.-C.; Tseng, C.-Y.; Chen, M.-C.; Lee, S.-L.; Lin, C.-C.; Su, T.-C. Effects of Fe, Si, Cu, and TiB2 Grain Refiner Amounts on the Hot Tearing Susceptibility of 5083, 6061, and 7075 Aluminum Ingots. Metals 2024, 14, 15. https://doi.org/10.3390/met14010015
Liang K-Y, Huang H-C, Tseng C-Y, Chen M-C, Lee S-L, Lin C-C, Su T-C. Effects of Fe, Si, Cu, and TiB2 Grain Refiner Amounts on the Hot Tearing Susceptibility of 5083, 6061, and 7075 Aluminum Ingots. Metals. 2024; 14(1):15. https://doi.org/10.3390/met14010015
Chicago/Turabian StyleLiang, Kai-Yu, Hao-Chuan Huang, Ching-Yao Tseng, Mien-Chung Chen, Sheng-Long Lee, Chi-Cheng Lin, and Te-Cheng Su. 2024. "Effects of Fe, Si, Cu, and TiB2 Grain Refiner Amounts on the Hot Tearing Susceptibility of 5083, 6061, and 7075 Aluminum Ingots" Metals 14, no. 1: 15. https://doi.org/10.3390/met14010015





