Cyclic Oxidation Kinetics and Thermal Stress Evolution of TiAl Alloys at High Temperature
Abstract
:1. Introduction
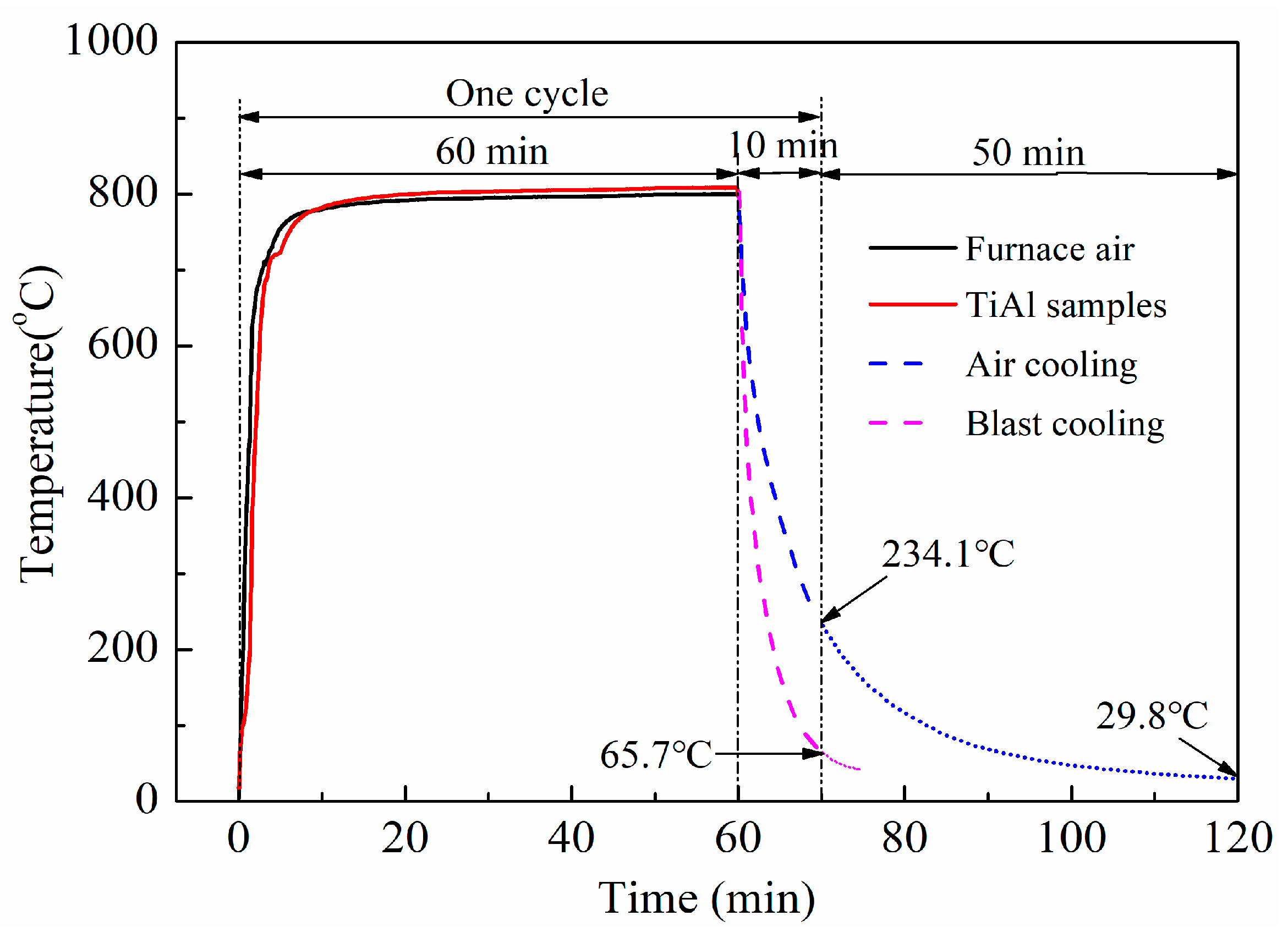
2. Materials and Methods
3. Results and Discussion
3.1. Oxidation Kinetics Analysis
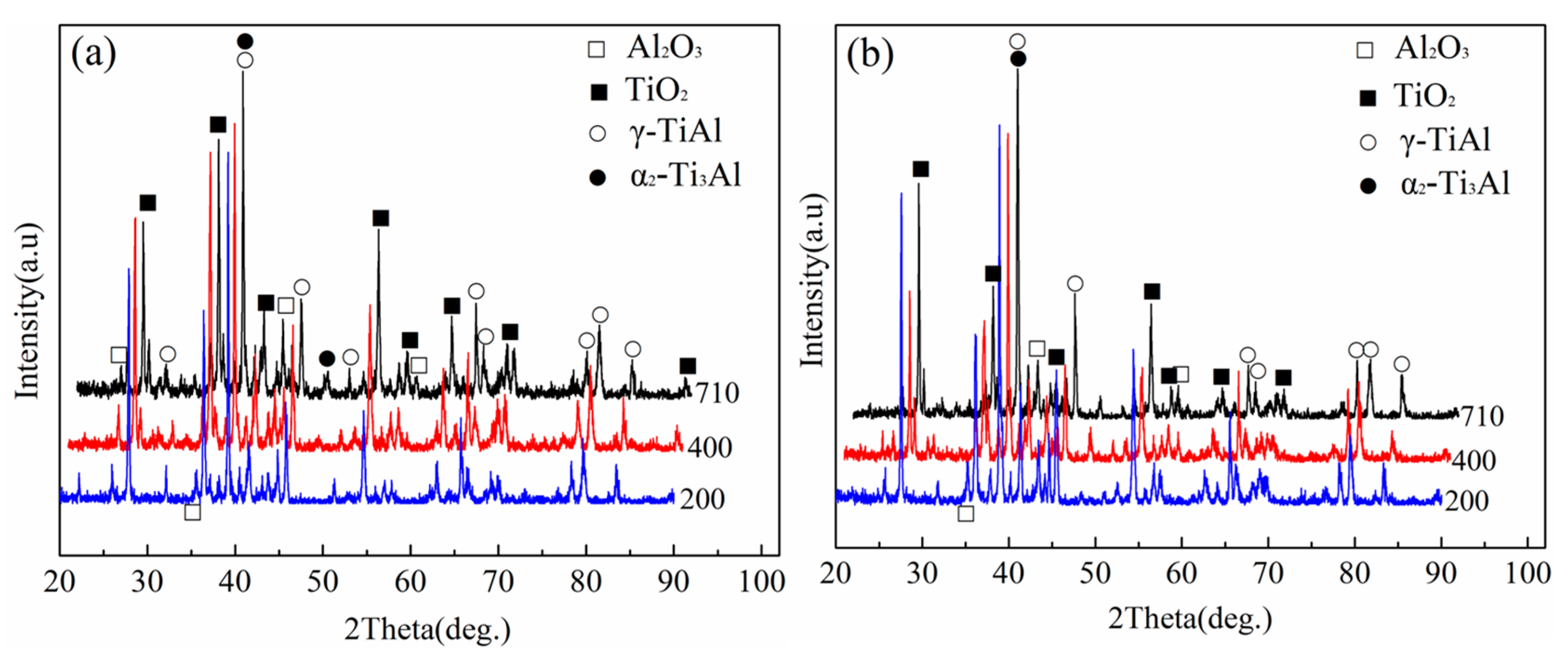
3.2. Phase and Morphology Analysis of Oxidation Surface
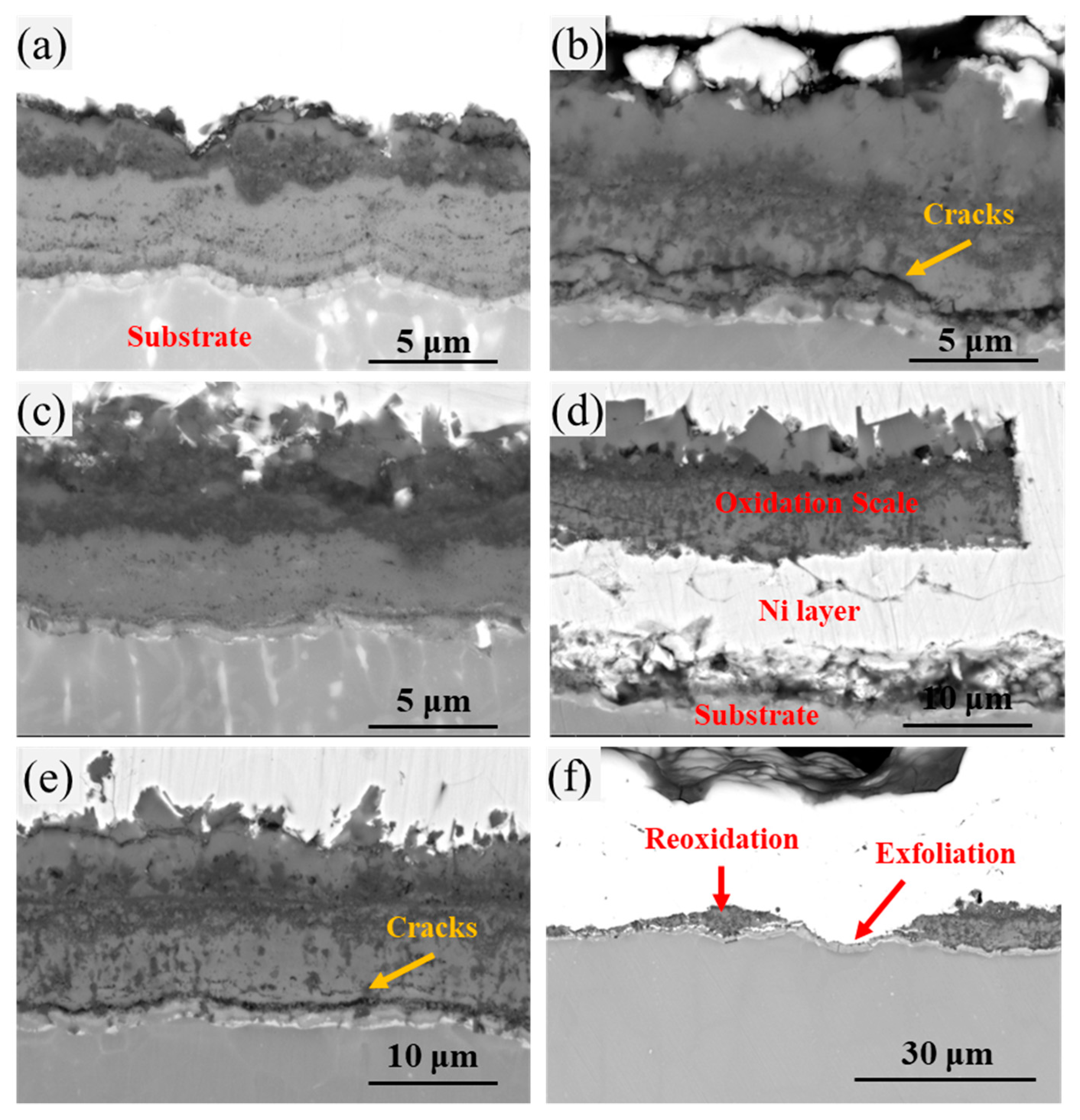
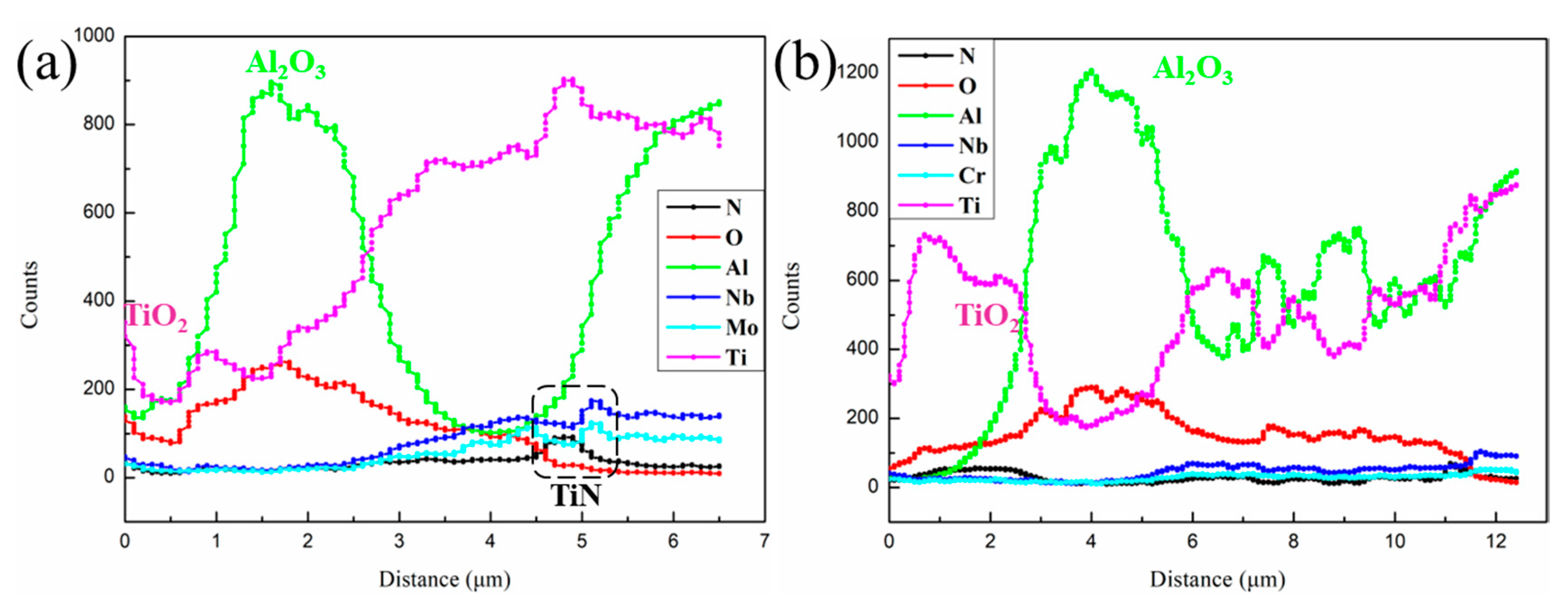
3.3. Cross-Sectional Morphology of Oxide Scale
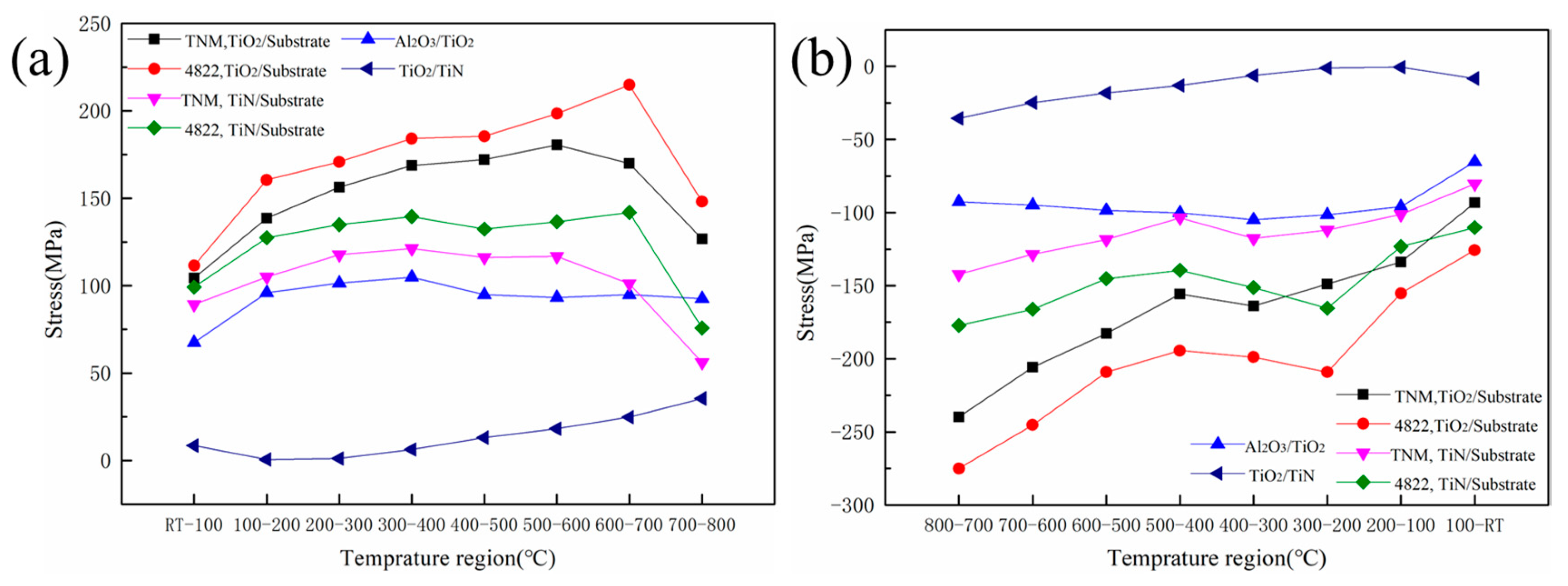
3.4. Stress-Induced Cracking Mechanism in the Cyclic Oxidation Process
4. Conclusions
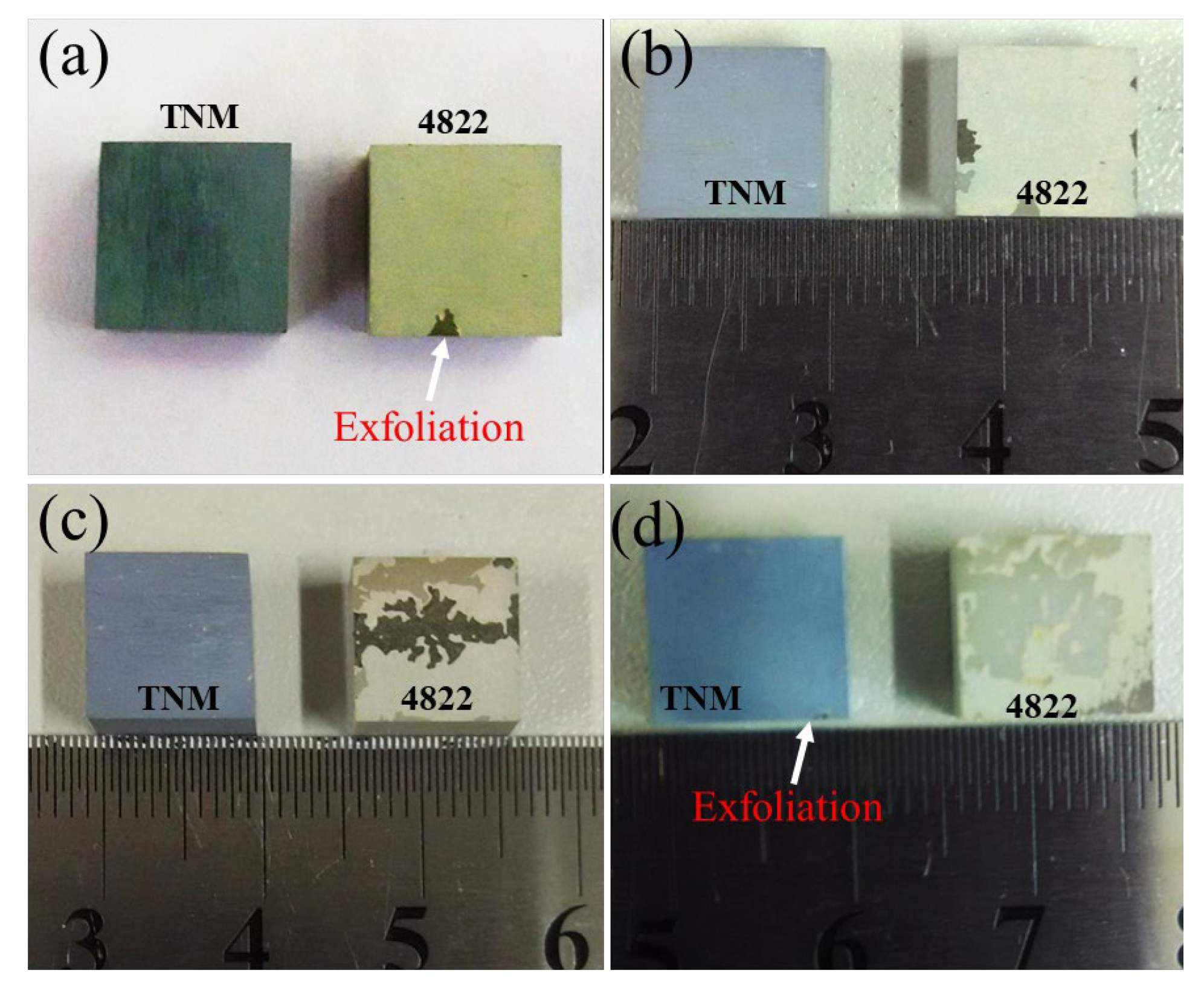
- In the flowing air atmosphere, the oxidation weight gain curves of the two TiAl alloys at 800 °C show a parabolic type. The oxide scale is mainly composed of TiO2 and Al2O3, and the TNM alloy shows better anti-oxidation peeling performance.
- The Nb and Mo elements hinder the dissolution of oxygen atoms in the TiAl alloy substrate and promote the formation of continuous dense Al2O3. In addition, the Nb and Mo elements are enriched at the substrate/oxide interface, which hinders the external diffusion of Ti and Al atoms and the internal diffusion of oxygen atoms, thus improving the oxidation resistance of the TNM alloy.
- It is found that the thermal stress of the TNM alloy during heating and cooling is less than that of the 4822 alloy. It is also found that the thermal stress between the oxide scale and the substrate is greater than that inside the oxide scale, which explains the reason why the oxide scale peeling mainly occurs at the interface between the oxide scale and the substrate.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Liang, Y.; Li, C.; Lin, J. Microstructure and Mechanical Properties of TiAl Matrix Composites Reinforced by Carbides. Metals 2022, 12, 790. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, G.; Guo, W.; Tian, S.; Lin, H.; Zeng, S. Deformation Behavior of Ti2AlNb—Based Alloy during Dynamic Compression. Adv. Eng. Mater. 2018, 20, 1800281. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, S.L. Advances in gamma alloy materials–processes–application technology: Successes, dilemmas, and future. JOM 2018, 70, 553–560. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Clemens, H.; Boeck, B.; Wallgram, W.; Schmoelzer, T.; Droessler, L.M.; Zickler, G.A.; Leitner, H.; Otto, A. Experimental Studies and Thermodynamic Simulations of Phase Transformations in Ti-(41-45) Al-4Nb-1Mo-0.1 B Alloys; MRS Online Proceedings Library (OPL): Warrendale, PA, USA, 2008; p. 1128. [Google Scholar]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of novel β-solidifying TiAl alloys with adjustable β/B2-phase fraction and excellent hot-workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Wallgram, W.; Clemens, H.; Kremmer, S.; Otto, A.; Güther, V. Hot-Die Forging of a β-Stabilized γ-TiAl Based Alloy; MRS Online Proceedings Library (OPL): Warrendale, PA, USA, 2008; p. 1128. [Google Scholar]
- Bik, M.; Galetz, M.; Mengis, L.; White, E.; Wieczorek, W.; Łyszczarz, K.; Mroczka, K.; Marchewka, J.; Sitarz, M. Oxidation behaviour of uncoated and PDC-SiAlOC glass-coated TiAl at 750° C in dry and humid air. Appl. Surf. Sci. 2023, 632, 157601. [Google Scholar] [CrossRef]
- Yoshihara, M.; Miura, K. Effects of Nb addition on oxidation behavior of TiAl. Intermetallics 1995, 3, 357–363. [Google Scholar] [CrossRef]
- Lee, D. Effect of Cr, Nb, Mn, V, W and Si on high temperature oxidation of TiAl alloys. Met. Mater. Int. 2005, 11, 141–147. [Google Scholar] [CrossRef]
- Garip, Y. Investigation of isothermal oxidation performance of TiAl alloys sintered by different processing methods. Intermetallics 2020, 127, 106985. [Google Scholar] [CrossRef]
- Su, Z.; Song, X.; Duan, Z.; Chen, H.; Huang, H.; Liu, Y.; Han, Y. High Temperature Oxidation Behaviors of Powder Metallurgical γ-TiAl Based Alloys: Effects of Surface Defects on Morphology of the Oxide Scale. Metals 2022, 12, 1743. [Google Scholar] [CrossRef]
- Yang, G.; Bai, W.; Han, S.; Wang, Y.; Cheng, L.; Zuo, J.; Kim, S.W. An enhanced oxidation resistance in Ti-40Al-8Nb alloys with submicron (ω0+ γ) microstructure: A comparative study. Corros. Sci. 2023, 213, 110989. [Google Scholar] [CrossRef]
- Swadźba, R.; Laska, N.; Bauer, P.P.; Krztoń, H. Effect of pre-oxidation on cyclic oxidation resistance of γ-TiAl at 900° C. Corros. Sci. 2020, 177, 108985. [Google Scholar] [CrossRef]
- Crespo-Villegas, J.; Cavarroc, M.; Knittel, S.; Martinu, L.; Klemberg-Sapieha, J.E. Protective TixSiy coatings for enhanced oxidation resistance of the ɣ-TiAl alloy at 900° C. Surf. Coat. Technol. 2022, 430, 127963. [Google Scholar] [CrossRef]
- Yang, L.; Gao, F.; Zhou, Z.; Jia, Y.; Du, Y.; Wang, J.; Qiao, Y.; Zhu, S.; Wang, F. Oxidation behavior of the AlN coatings on the TiAl alloy at 900° C. Corros. Sci. 2023, 211, 110891. [Google Scholar] [CrossRef]
- Swadźba, R.; Swadźba, L.; Mendala, B.; Bauer, P.P.; Laska, N.; Schulz, U. Microstructure and cyclic oxidation resistance of Si-aluminide coatings on γ-TiAl at 850° C. Surf. Coat. Technol. 2020, 403, 126361. [Google Scholar] [CrossRef]
- Donchev, A.; Gleeson, B.; Schütze, M. Thermodynamic considerations of the beneficial effect of halogens on the oxidation resistance of TiAl-based alloys. Intermetallics 2003, 11, 387–398. [Google Scholar] [CrossRef]
- Magnan, J.; Weatherly, G.C.; Cheynet, M.C. The nitriding behavior of Ti-Al alloys at 1000° C. Metall. Mater. Trans. A 1999, 30, 19–29. [Google Scholar] [CrossRef]
- Narayana, P.L.; Kim, J.H.; Yun, D.W.; Kim, S.E.; Reddy, N.S.; Yeom, J.T.; Seo, D.; Hong, J.K. High temperature isothermal oxidation behavior of electron beam melted multi-phase γ-TiAl alloy. Intermetallics 2022, 141, 107424. [Google Scholar] [CrossRef]
- Rahmel, A.; Schütze, M.; Quadakkers, W.J. Fundamentals of TiAl oxidation—A critical review. Mater. Corros. 1995, 46, 271–285. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Lu, W.; Chen, C.L.; Wang, F.H.; Lin, J.P.; Chen, G.L.; He, L.L. Phase transformation in the nitride layer during the oxidation of TiAl-based alloys. Scr. Mater. 2007, 56, 773–776. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Sun, J.; Tu, B.; Wang, F. Effect of nitridation on the oxidation behavior of TiAl-based intermetallic alloys. Intermetallics 2001, 9, 697–703. [Google Scholar] [CrossRef]
- Tian, S.; He, A.; Liu, J. Oxidation resistance of TiAl alloy improved by hot-pack rolling and cyclic heat treatment. Mater. Charact. 2021, 178, 111196. [Google Scholar]
- Zhao, P.X.; Li, X.B.; Xing, W.W.; Bo, C.H.; Kui, L.I. Cyclic oxidation behavior of Nb/Mn/Si alloying beta-gamma TiAl alloys. Trans. Nonferrous Met. Soc. China 2023, 33, 128–140. [Google Scholar] [CrossRef]
- Lin, X.J.; Huang, H.J.; Yuan, X.G.; Wang, Y.X.; Zheng, B.W.; Zuo, X.J.; Zhou, G. High-temperature oxidation behavior of a cast Ti-47.5 Al-2.5 V-1.0 Cr-0.2 Zr alloy. China Foundry 2022, 19, 443–454. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Q.; Gao, S.; Shang, J. Synthesis and characterization of niobium-doped TiO2 nanotube arrays by anodization of Ti–20Nb alloys. J. Mater. Sci. Technol. 2012, 28, 865–870. [Google Scholar] [CrossRef]
- Anada, H.; Shida, Y. Effect of Mo addition on the oxidation behavior of TiAl intermetallic compound. Mater. Trans. JIM 1995, 36, 533–539. [Google Scholar] [CrossRef]
- Jiang, S.S.; Zhang, K.F. Study on controlling thermal expansion coefficient of ZrO2–TiO2 ceramic die for superplastic blow-forming high accuracy Ti-6Al-4V component. Mater. Des. 2009, 30, 3904–3907. [Google Scholar] [CrossRef]
- Yao, H.; Ouyang, L.; Ching, W. Ab Initio Calculation of Elastic Constants of Ceramic Crystals. J. Am. Ceram. Soc. 2007, 90, 3194–3204. [Google Scholar] [CrossRef]




| Materials | TNM | 4822 | Al2O3 | TiO2 | TiN | ||
|---|---|---|---|---|---|---|---|
| Temperature | Heating | Cooling | Heating | Cooling | — | — | — |
| RT | — | — | — | — | — | — | — |
| 100 °C | 11.47 | 11.22 | 11.69 | 12.27 | 6.50 | 8.20 | 7.80 |
| 200 °C | 11.54 | 11.43 | 12.03 | 11.91 | 6.70 | 8.43 | 8.45 |
| 300 °C | 12.16 | 12.00 | 12.49 | 13.35 | 6.83 | 8.66 | 8.70 |
| 400 °C | 12.67 | 12.56 | 13.02 | 13.35 | 7.00 | 8.89 | 9.10 |
| 500 °C | 12.97 | 12.60 | 13.27 | 13.47 | 7.40 | 9.11 | 9.55 |
| 600 °C | 13.39 | 13.44 | 13.79 | 14.03 | 7.66 | 9.34 | 9.95 |
| 700 °C | 13.38 | 14.18 | 14.39 | 15.06 | 7.86 | 9.57 | 10.40 |
| 800 °C | 12.63 | 15.16 | 13.11 | 15.95 | 8.12 | 9.79 | 10.98 |
| 900 °C | 11.22 | 16.35 | 15.34 | 17.36 | 8.33 | — | — |
| 1000 °C | 11.25 | 19.19 | 18.80 | 18.89 | 8.50 | — | — |
| RT to 800 | 12.57 | 12.89 | 12.94 | 13.73 | — | — | — |
| Temperature (°C) | TiO2/Substrate | Al2O3/TiO2 | TiN/Substrate | TiO2/TiN | |||
|---|---|---|---|---|---|---|---|
| TNM | 4822 | TNM/4822 | TNM | 4822 | TNM/4822 | ||
| Heating stage | RT–100 | 104.39 | 111.49 | 67.44 | 89.20 | 99.18 | 8.54 |
| 100–200 | 138.73 | 160.54 | 95.98 | 104.95 | 127.43 | 0.60 | |
| 200–300 | 156.39 | 170.78 | 101.53 | 117.71 | 134.90 | 1.19 | |
| 300–400 | 168.72 | 184.15 | 104.86 | 121.33 | 139.51 | 6.27 | |
| 400–500 | 172.20 | 185.47 | 94.87 | 116.16 | 132.38 | 13.14 | |
| 500–600 | 180.55 | 198.39 | 93.21 | 116.74 | 136.63 | 18.22 | |
| 600–700 | 169.84 | 214.87 | 94.87 | 101.11 | 141.95 | 24.79 | |
| 700–800 | 126.79 | 148.00 | 92.66 | 56.11 | 75.75 | 35.55 | |
| RT–800 | 1217.62 | 1373.70 | 745.43 | 823.31 | 987.74 | 108.32 | |
| Cooling stage | 100–RT | −93.31 | −125.70 | −65.27 | −80.45 | −110.19 | −8.27 |
| 200–100 | −133.86 | −155.19 | −95.98 | −101.24 | −123.16 | −0.60 | |
| 300–200 | −148.85 | −209.12 | −101.53 | −111.97 | −165.50 | −1.19 | |
| 400–300 | −163.85 | −198.86 | −104.86 | −117.62 | −151.25 | −6.27 | |
| 500–400 | −155.80 | −194.39 | −100.13 | −103.68 | −139.49 | −13.14 | |
| 600–500 | −182.76 | −209.09 | −98.38 | −118.43 | −145.17 | −18.22 | |
| 700–600 | −205.73 | −245.19 | −94.87 | −128.44 | −166.15 | −24.79 | |
| 800–700 | −239.78 | −275.04 | −92.66 | −142.14 | −177.16 | −35.55 | |
| 800–RT | −1323.96 | −1612.58 | −753.69 | −903.96 | −1178.08 | −280.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Zhang, T.; Zeng, S.; Zhang, Y.; Song, D.; Chen, Y.; Kang, Q.; Jiang, H. Cyclic Oxidation Kinetics and Thermal Stress Evolution of TiAl Alloys at High Temperature. Metals 2024, 14, 28. https://doi.org/10.3390/met14010028
Tian S, Zhang T, Zeng S, Zhang Y, Song D, Chen Y, Kang Q, Jiang H. Cyclic Oxidation Kinetics and Thermal Stress Evolution of TiAl Alloys at High Temperature. Metals. 2024; 14(1):28. https://doi.org/10.3390/met14010028
Chicago/Turabian StyleTian, Shiwei, Tengkun Zhang, Shangwu Zeng, Yefei Zhang, Dejun Song, Yulai Chen, Qiang Kang, and Haitao Jiang. 2024. "Cyclic Oxidation Kinetics and Thermal Stress Evolution of TiAl Alloys at High Temperature" Metals 14, no. 1: 28. https://doi.org/10.3390/met14010028




