On the Effect of Exposure Time on Al-Si10-Mg Powder Processed by Selective Laser Melting
Abstract
:1. Introduction
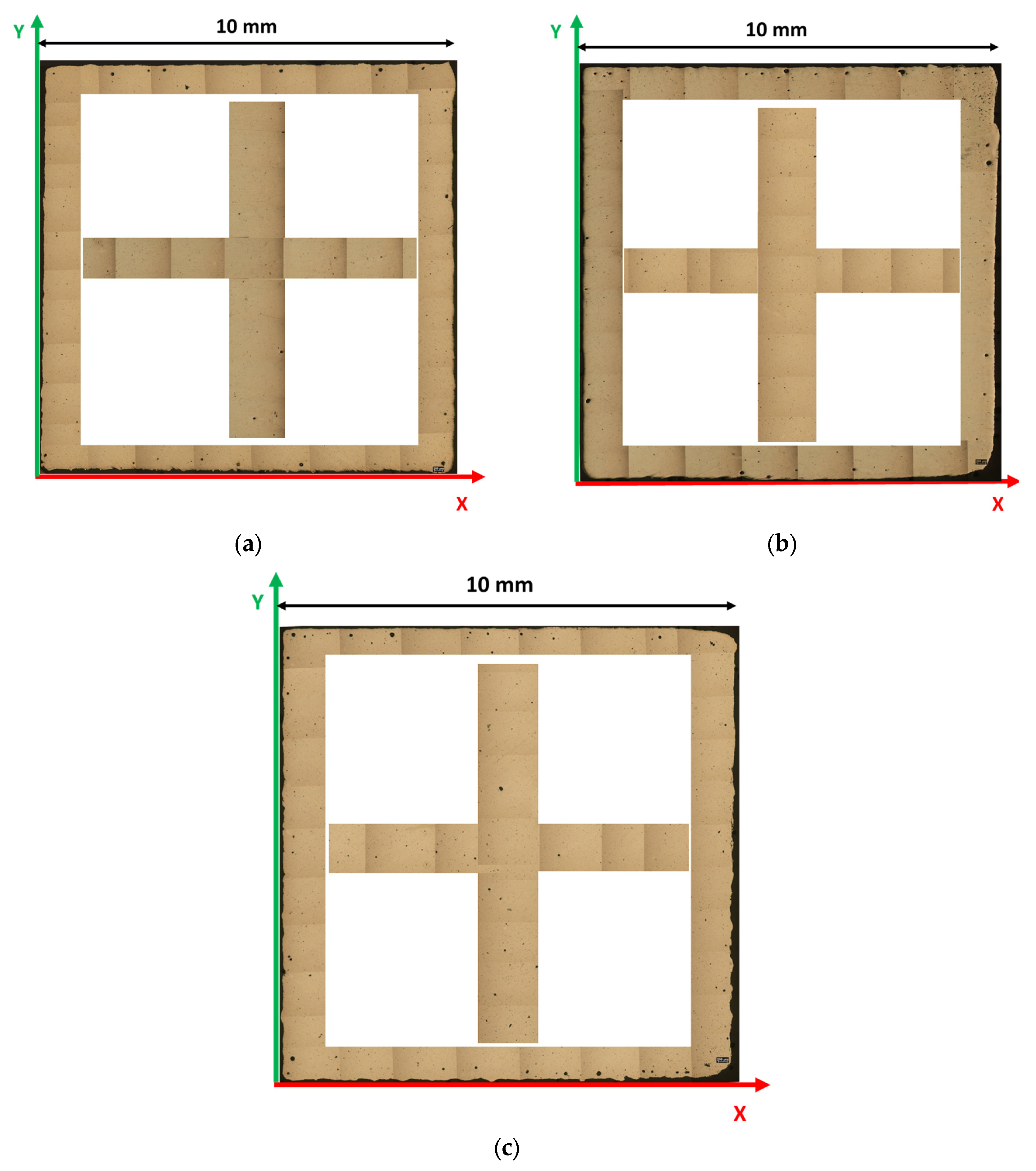
2. Materials and Methods
3. Results and Discussion
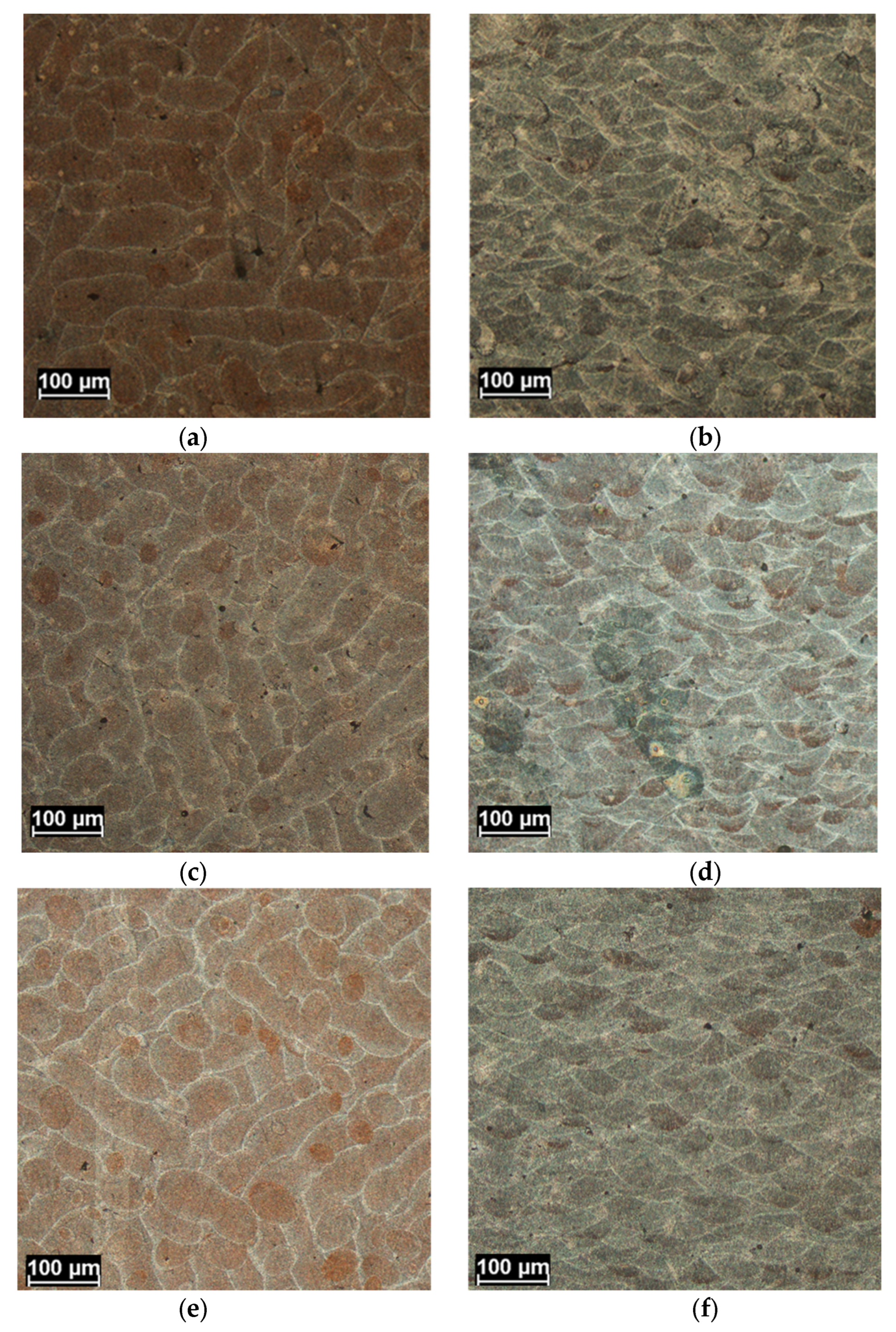
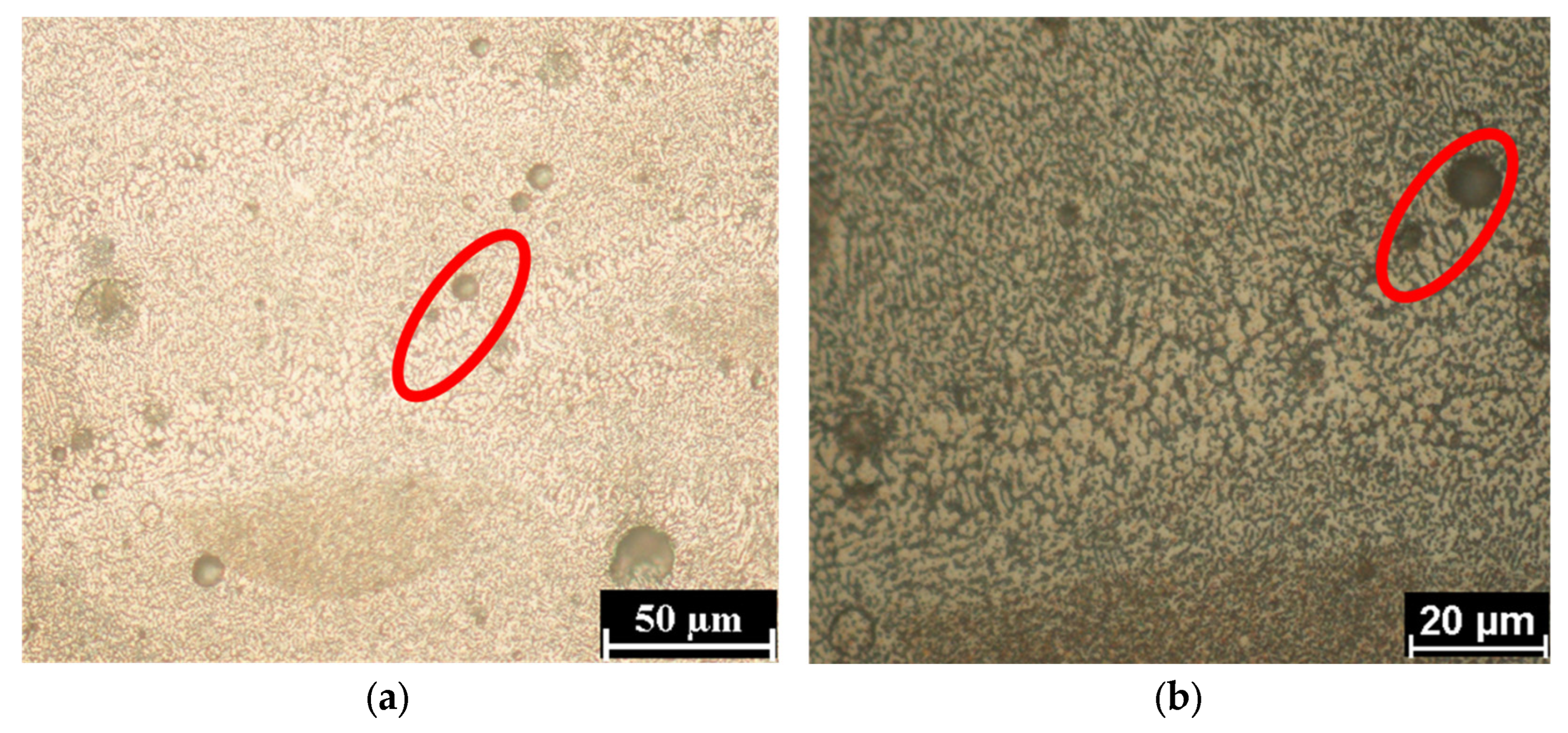
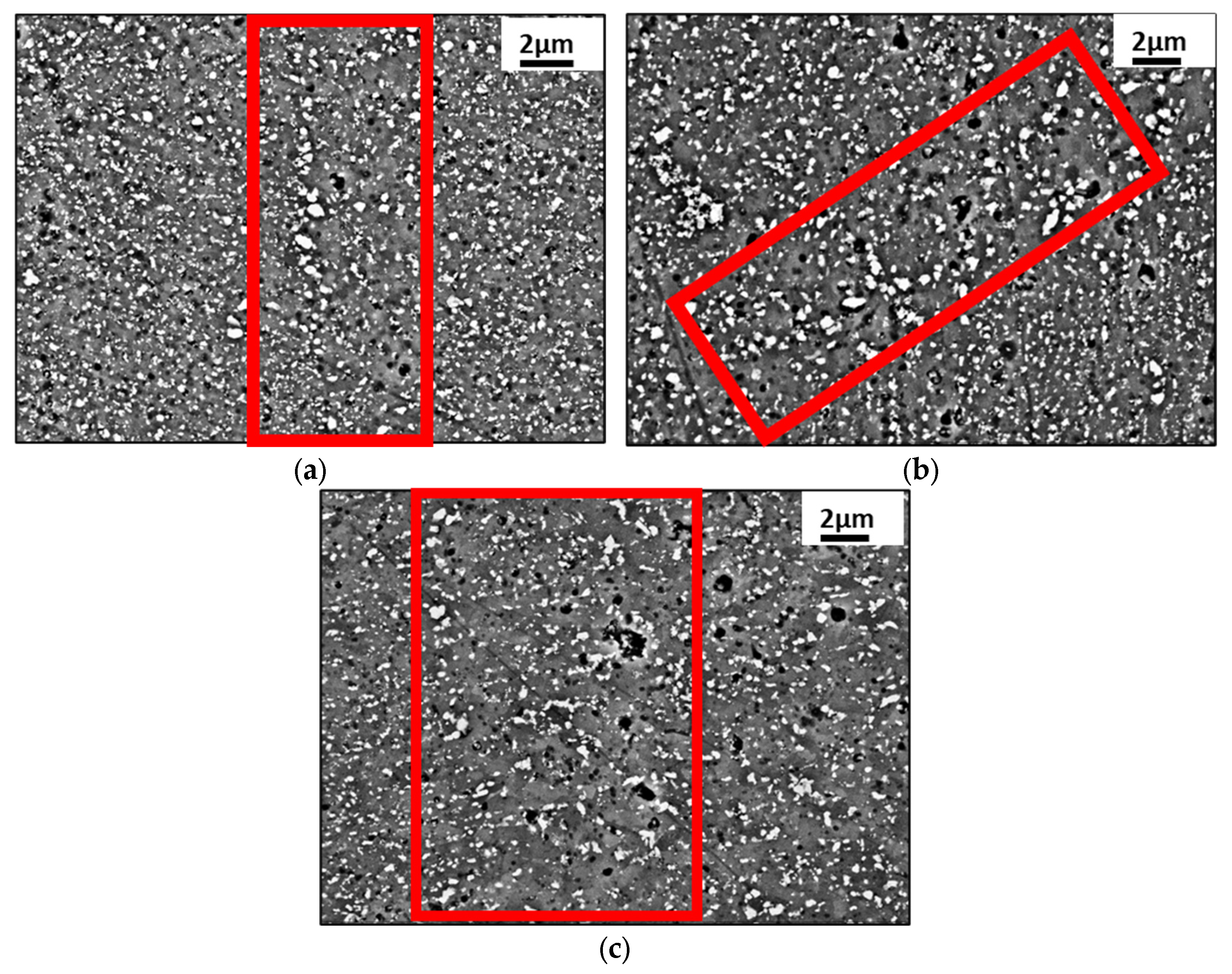
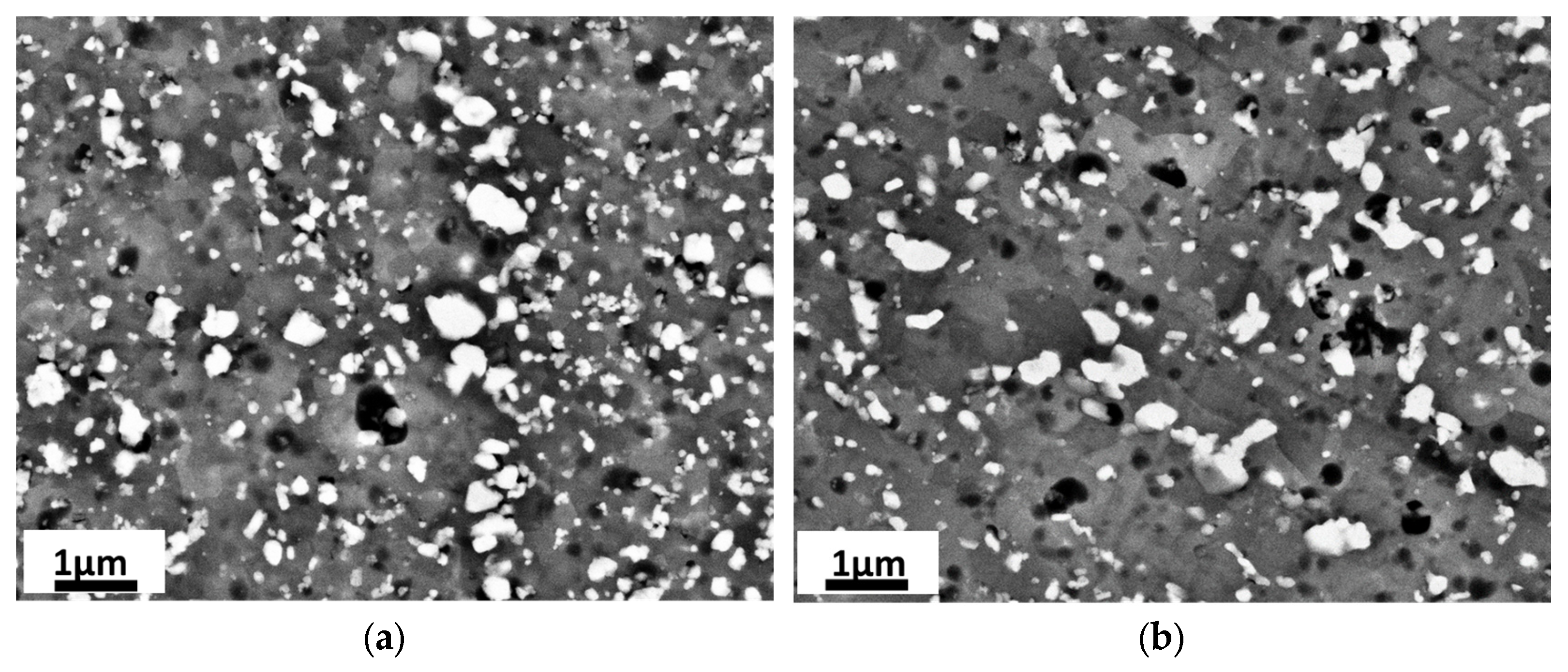
3.1. Microstructure Characterization
- The boundary microstructure is coarser compared to the inner part of the melting pool.
- The morphology is less elongated (more equiaxed) in the boundary compared to the inner part, where it appears mainly cellular.
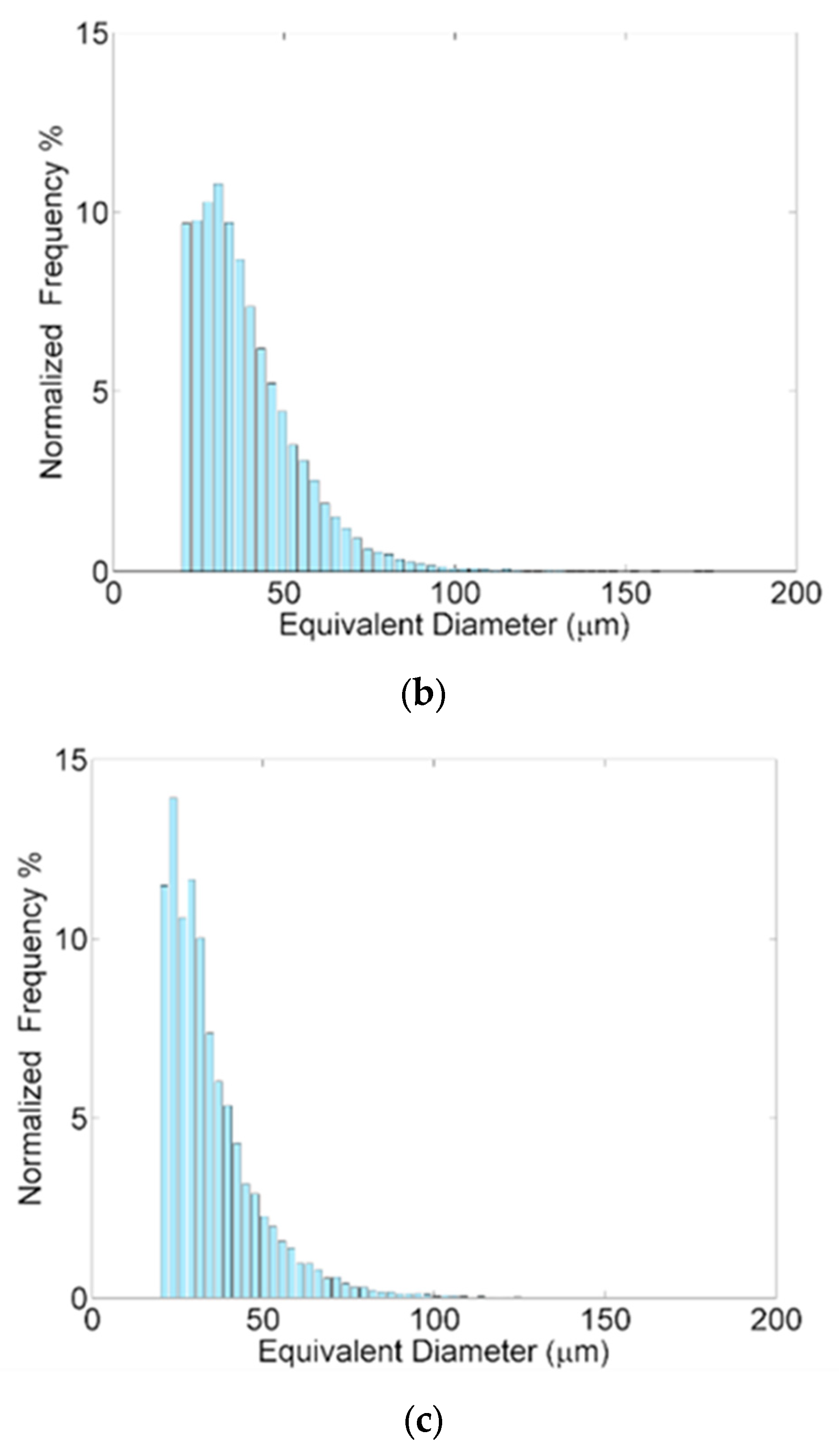
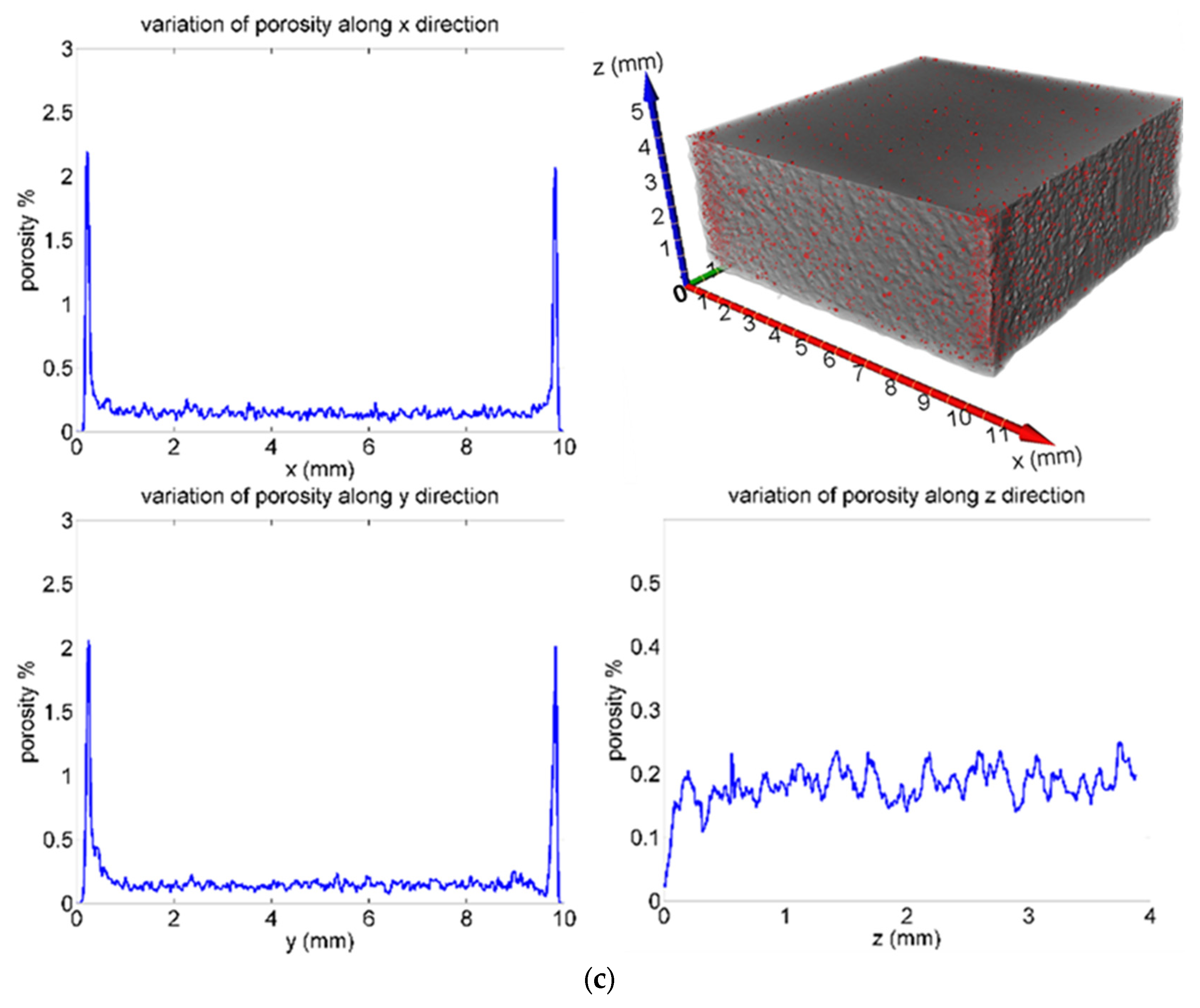
3.2. Porosity and Hardness
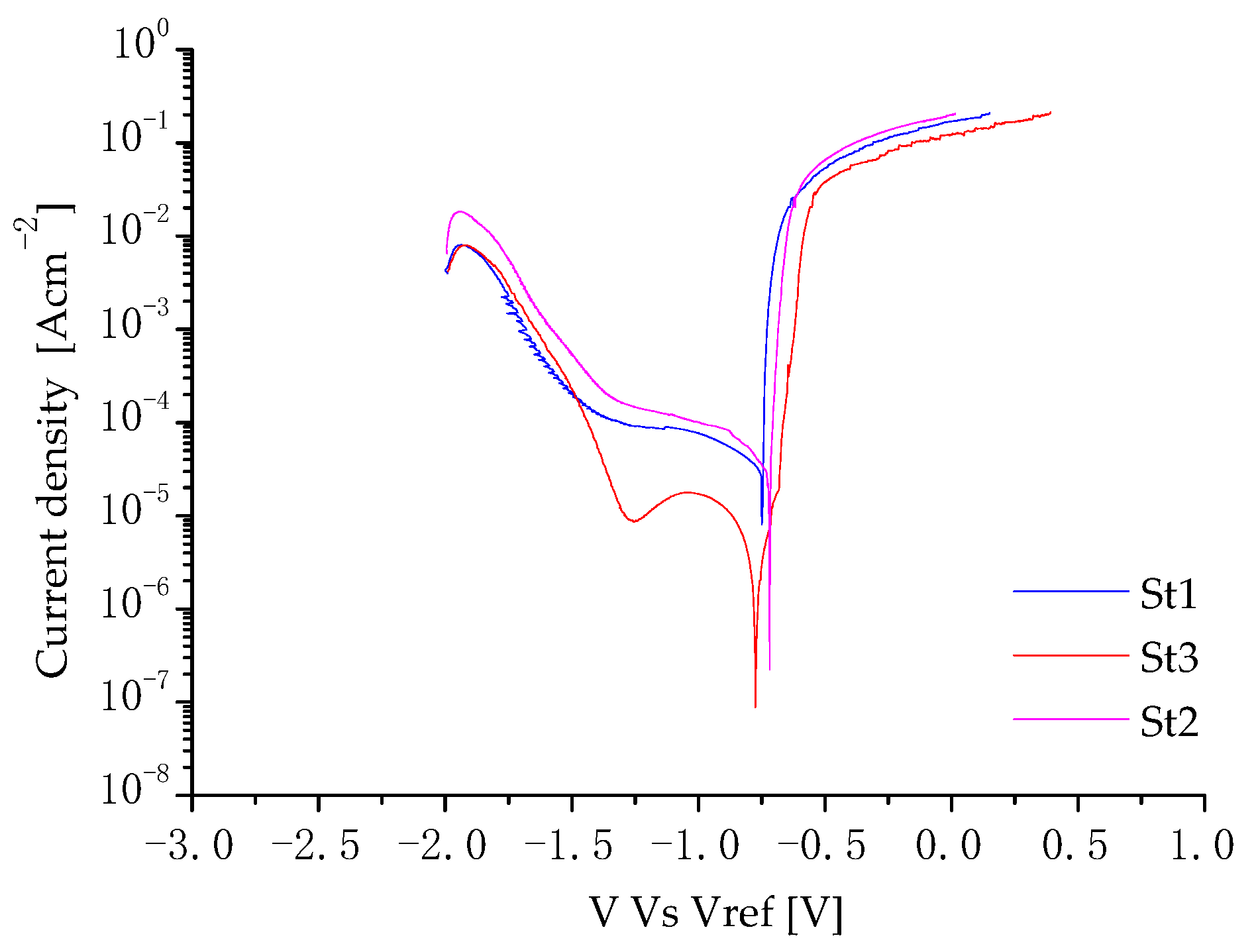
3.3. Corrosion Behavior
4. Conclusions
- The melt pool size increases with exposure time. Specifically, at the highest exposure time the melt pool showed an increase of 19% in width and 48% in depth with respect to the lowest exposure time.
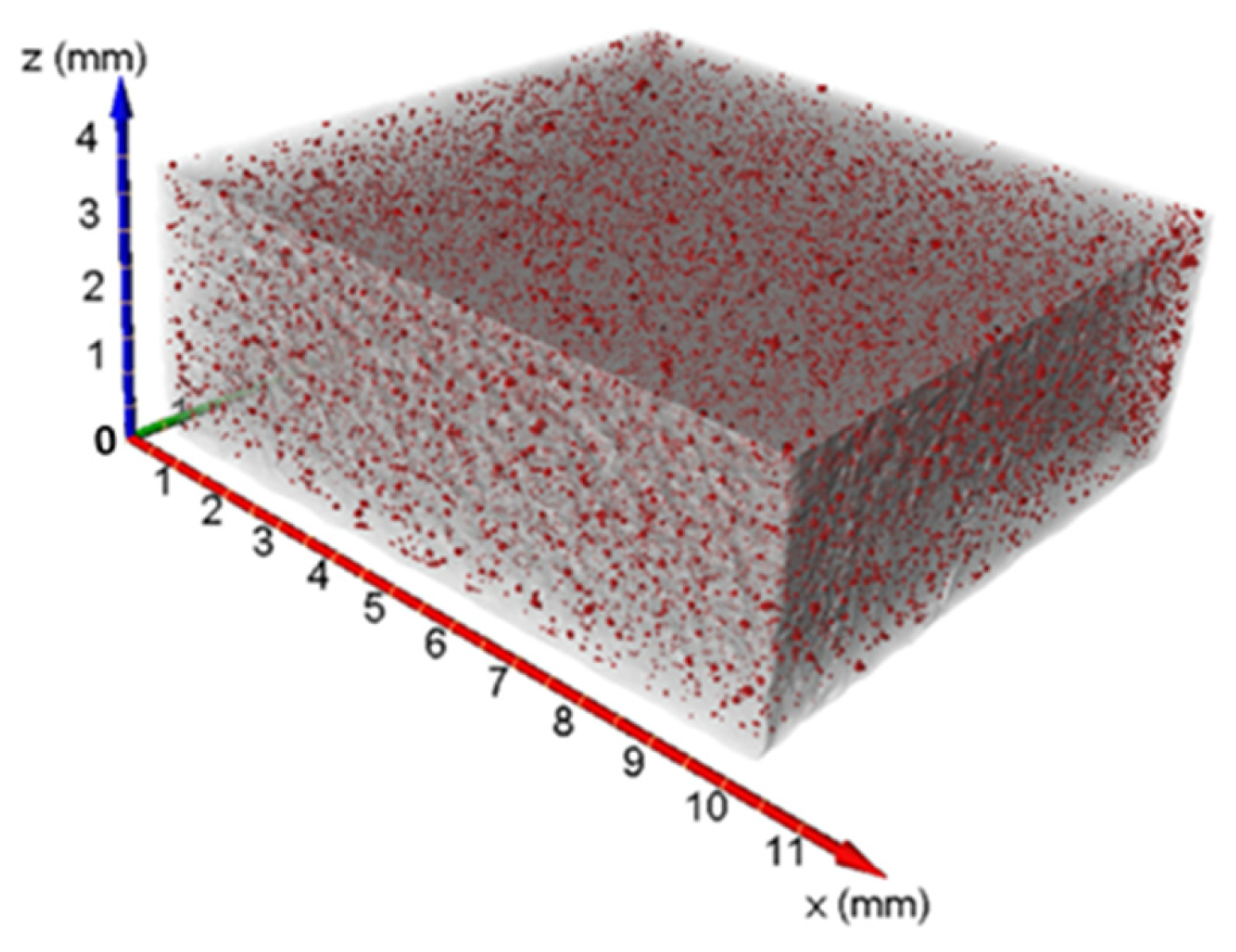
- The number, the size and therefore the average volume of the voids decrease with increasing exposure time. Particularly, the volume percentage of the voids decreased by 50% at the highest exposure time with respect to the lowest exposure time.
- Due to the opposite effect of exposure time on voids occurrence and microstructure scale, the hardness values do not change with exposure time.
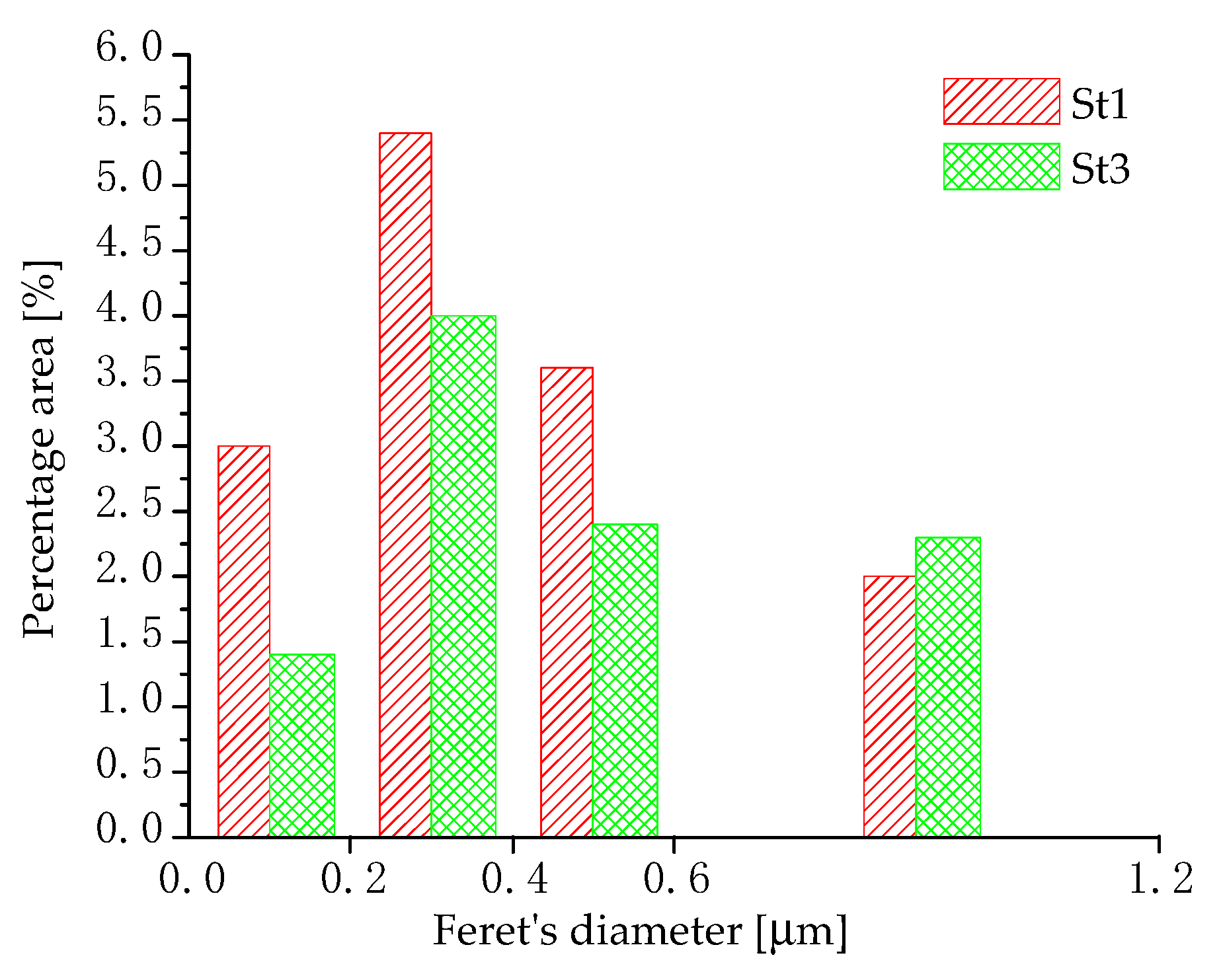
- After the stress relief heat treatment (300 °C-2 h), the lowest density and coarsest size of silicon particles have been found in the sample processed at the highest exposure time.
- The corrosion resistance increases with exposure time, despite the coarser microstructure, showing that corrosion behavior in the analyzed samples is much more sensitive to the voids occurrence than to the microstructural features.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polmear, I.J. Light Alloys-Metallurgy of the Light Metals, 3rd ed.; Arnold: London, UK, 1995; pp. 169–180. [Google Scholar]
- Smith, W.F. Structure and Properties of Engineering Alloys, 2nd ed.; McGraw-Hill: London, UK, 1993; pp. 218–223. [Google Scholar]
- Aboulkhair, N.T.; Simonelli, M.; Parry, L.; Ashcroft, I.; Tuck, C. 3D printing of Aluminium alloys: Additive Manufacturing of Aluminium alloys using selective laser melting. Prog. Mater. Sci. 2019, 106, 100578. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Maa, H.Y.; Wang, J.C.; Qin, P.; Liu, Y.J.; Chen, L.Y.; Wang, L.Q.; Zhang, L.C. Advances in additively manufactured titanium alloys by powder bed fusion and directed energy deposition: Microstructure, defects, and mechanical behavior. J. Mater. Sci. Technol. 2024, 183, 32–62. [Google Scholar] [CrossRef]
- Pan, S.H.; Yao, G.C.; Cui, Y.N.; Meng, F.-S.; Luo, C.; Zheng, T.-Q.; Singh, G. Additive manufacturing of tungsten, tungsten-based alloys, and tungsten matrix composites. Tungsten 2023, 5, 1–31. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, R.; Liu, Y.; Zhang, L. Understanding melt pool characteristics in laser powder bed fusion: An overview of single- and multi-track melt pools for process optimization. Adv. Powder Mater. 2023, 2, 100137. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Maskery, I.; Tuck, C.; Ashcroft, I.; Everitt, N.M. On the formation of AlSi10mg single tracks and layers in selective laser melting: Microstructure and nano-mechanical properties. J. Mater. Process. Technol. 2016, 230, 88–98. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Krakhmalev, I.P.; Yadroitsava, S.J.; Smurov, I. Energy input effect on morphology and microstructure of selective laser melting single track from metallic powder. J. Mater. Process. Technol. 2013, 213, 606–613. [Google Scholar] [CrossRef]
- Lam, L.P.; Zhang, D.Q.; Liu, Z.H.; Chua, C.K. Phase analysis and microstructure characterisation of AlSi10Mg parts produced by selective laser melting. Virtual Phys. Prototyp. 2015, 10, 207–215. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, Z.; Jiang, Y.; Wang, G.W.; Yang, Y.; Zhang, L.C. Gradient in microstructure and mechanical property of selective laser melted AlSi10Mg. J. Alloys Compd. 2018, 735, 1414–1421. [Google Scholar] [CrossRef]
- Leo, P.; Del Prete, A.; Primo, T.; Nacucchi, M. Al-Si10-Mg Manufactured by Selective Laser Melting: Microstructure Sensitivity to Close Values of the Heat Input. Metals 2023, 13, 590. [Google Scholar] [CrossRef]
- Maskery, I.; Aboulkhair, N.T.; Corfield, M.R.; Tuck, C.; Clare, A.T.; Leach, R.K.; Wildman, R.D.; Ashcroft, I.A.; Hague, R.J.M. Quantification and characterisation of porosity in selectively laser melted Al–Si10–Mg using X-ray computed tomography. Mater. Charact. 2016, 111, 193–204. [Google Scholar] [CrossRef]
- Patel, D.; Pandey, A. Powder bed fusion of aluminium alloys: A review of experimental explorations—Microstructure, mechanical properties, and recent advances. Mater. Today Proc. 2023, 82, 168–177. [Google Scholar] [CrossRef]
- Miller, K. The short crack problem. Fatigue Fract. Eng. Mater. Struct. 1982, 5, 223–232. [Google Scholar] [CrossRef]
- Schijve, J. Fatigue of Structures and Materials, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 13–21. [Google Scholar]
- Takahashi, K.; Murakami, Y. Quantitative evaluation of effect of surface roughness on fatigue strength. In Engineering against Fatigue; Benyon., J., Brown, M., Smith, R., Lindley, T., Tomkins, B., Eds.; Balkema Publishers: London, UK, 1999; pp. 693–703. [Google Scholar]
- Suraratchai, M.; Limido, J.; Mabru, C.; Chieragatti, R. Modelling the influence of machined surface roughness on the fatigue life of aluminium alloy. Int. J. Fatigue 2008, 30, 2119–2126. [Google Scholar] [CrossRef]
- Leon, A.; Aghion, E. Effect of surface roughness on corrosion performance of AlSi10Mg alloy produced by Selective Laser Melting (SLM). Mater. Charact. 2017, 131, 188–194. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Everitt, N.M.; Ashcroft, I.; Tuck, C. Reducing porosity in AlSi10Mg parts processed by selective laser melting. Addit. Manuf. 2014, 1, 77–86. [Google Scholar] [CrossRef]
- Haboudou, A.; Peyre, P.; Vannes, A.B.; Peix, G. Reduction of porosity content generated during Nd: YAG laser welding of A356 and AA5083 aluminium alloys. Mater. Sci. Eng. A 2003, 363, 40–52. [Google Scholar] [CrossRef]
- Messler, R.W., Jr. Principles of Welding: Processes, Physics, Chemistry, and Metallurgy; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Roehling, J.D.; Coughlin, D.R.; Gibbs, J.W.; Baldwin, J.K.; Mertens, J.C.E.; Campbell, G.H.; Clarke, A.J.; McKeown, J.T. Rapid solidification growth mode transitions in Al-Si alloys by dynamic transmission electron microscopy. Acta Mater. 2017, 131, 22–30. [Google Scholar] [CrossRef]
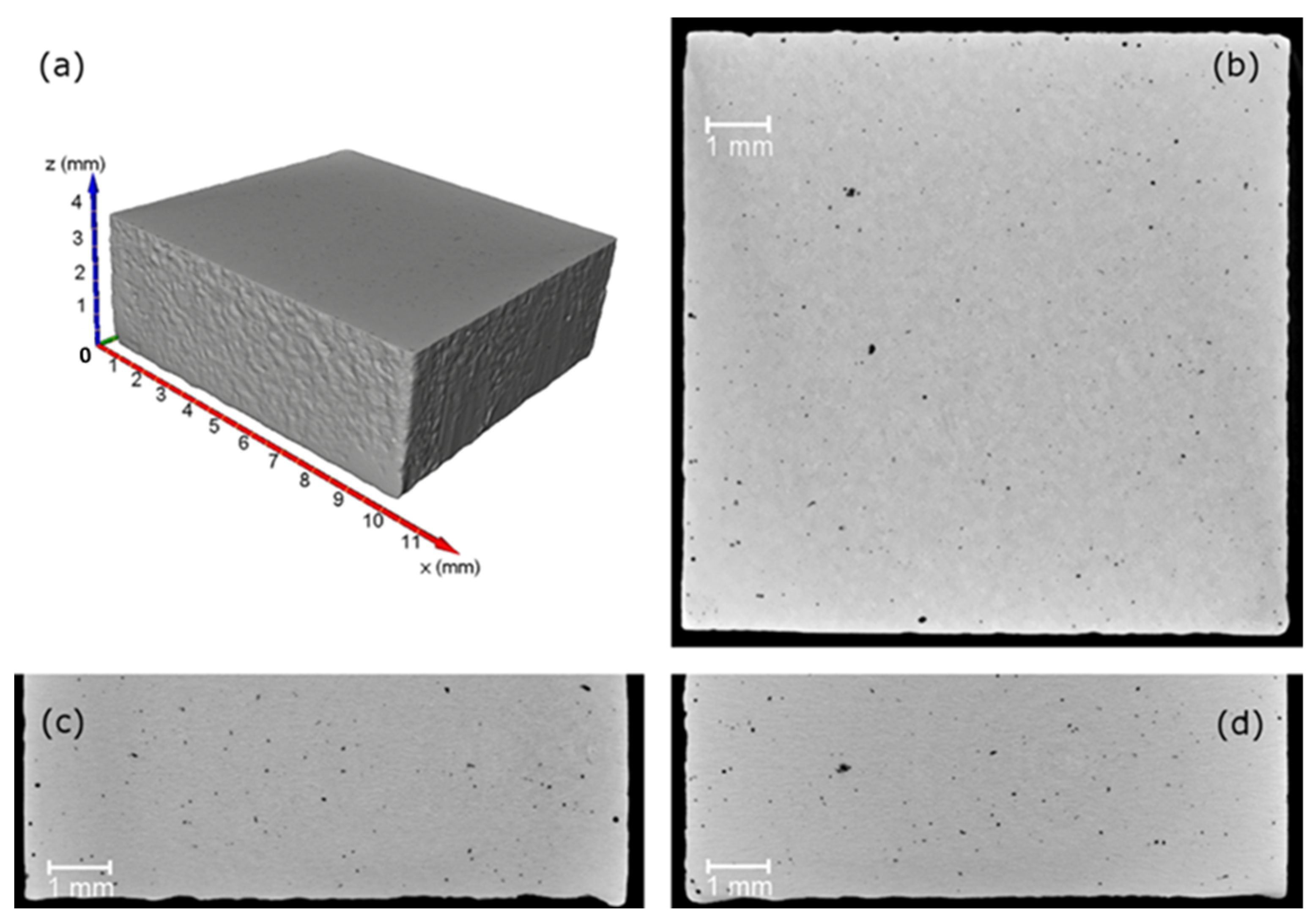
- Du Plessis, A.; Yadroitsev, I.; Yadroitsava, I.; Le Roux, S.G. X-Ray microcomputed tomography in additive manufacturing: A review of the current technology and applications. 3D Print. Addit. Manuf. 2018, 5, 227–247. [Google Scholar] [CrossRef]
- Bernsen, J. Dynamic thresholding of grey-level images. In Proceedings of the Eighth International Conference on Pattern Recognition, Paris, France, 27–31 October 1986; pp. 1251–1255. [Google Scholar]
- Sankur, B. Survey over image thresholding techniques and quantitative performance evaluation. J. Electron. Imaging 2004, 13, 146. [Google Scholar] [CrossRef]
- Kim, F.H.; Moylan, S.P.; Garboczi, E.J.; Slotwinski, J.A. Investigation of pore structure in cobalt chrome additively manufactured parts using X-ray computed tomography and three-dimensional image analysis. Addit. Manuf. 2017, 17, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, A.; Sperling, P.; Beerlink, A.; Tshabalala, L.; Hoosain, S.; Mathe, N.; le Roux, S.G. Standard method for microCT-based additive manufacturing quality control 1: Porosity analysis. MethodsX 2018, 5, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- ISO 6507-1; Metallic Materials—Vickers Hardness Test—Part 1: Test Method. ISO: Geneva, Switzerland, 2018.
- Liu, X.; Zhao, C.; Zhou, X.; Shena, Z.; Liu, W. Microstructure of selective laser melted AlSi10Mg alloy. Mater. Des. 2019, 168, 107677. [Google Scholar] [CrossRef]
- Fiocchi, J.; Tuissi, A.; Bassani, P.; Biffi, C.A. Low temperature annealing dedicated to AlSi10Mg selective laser melting products. J. Alloys Compd. 2017, 217, 3402–3409. [Google Scholar] [CrossRef]
- Wei, L.; Shuai, L.; Jie, L. Effect of heat treatment on AlSi10Mg alloy fabricated by selective laser melting: Microstructure evolution, mechanical properties and fracture mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar]
- Li, X.P.; Wang, X.J.; Saunders, M. A selective laser melting and solution heat treatment refined Al–12Si alloy with a controllable ultrafine eutectic microstructure and 25% tensile ductility. Acta Mater. 2015, 95, 74–82. [Google Scholar] [CrossRef]
- Ogris, E.; Wahlen, A.; Luchinger, H.; Uggowitzer, P.J. On the silicon spheroidization in Al–Si alloys. J. Light Met. 2002, 2, 263–269. [Google Scholar] [CrossRef]
- Patakham, U.; Palasay, A.; Wila, P.; Tongsri, R. MPB characteristics and Si morphologies on mechanical properties and fracture behavior of SLM AlSi10Mg. Mater. Sci. Eng. A 2021, 821, 141602. [Google Scholar] [CrossRef]
- Wanga, L.Z.; Wanga, S.; Hongb, X. Pulsed SLM-manufactured AlSi10Mg alloy: Mechanical properties and microstructural effects of designed laser energy densities. J. Manuf. Process. 2018, 35, 492–499. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdelaziz, M.; Samuel, A.; Doty, H.; Samuel, F. Spheroidization and Coarsening of Eutectic Si Particles in Al-Si-Based Alloys. Adv. Mater. Sci. Eng. 2021, 2021, 6678280. [Google Scholar] [CrossRef]
- Rafieazad, M.; Mohammadi, M.; Gerlich, A.; Nasiri, A. Enhancing the corrosion properties of additively manufactured AlSi10Mg using friction stir processing. Corros. Sci. 2021, 178, 109073. [Google Scholar] [CrossRef]
- Rafieazad, M.; Chatterjee, A.; Nasiri, A.M. Effects of Recycled Powder on Solidification Defects, Microstructure, and Corrosion Properties of DMLS Fabricated AlSi10Mg. J. Miner. Met. Mater. Soc. 2019, 71, 3241–3252. [Google Scholar] [CrossRef]
- Rafieazad, M.; Mohammad, M.; Nasiri, A.M. On microstructure and early stage corrosion performance of heat treated direct metal laser sintered AlSi10Mg. Addit. Manuf. 2019, 28, 107–119. [Google Scholar] [CrossRef]
- Lu, W.Q.; Liu, Y.J.; Wu, X.; Liu, X.C.; Wang, J.C. Corrosion and passivation behavior of Ti-6Al-4V surfaces treated with high-energy pulsed laser: A comparative study of cast and 3D-printed specimens in a NaCl solution. Surf. Coat. Technol. 2023, 470, 129849. [Google Scholar] [CrossRef]
- Gatto, A.; Cappelletti, C.; Defanti, S.; Fabbri, F. The Corrosion Behaviour of Additively Manufactured AlSi10Mg Parts Compared to Traditional Al Alloys. Metals 2023, 13, 913. [Google Scholar] [CrossRef]
- Örnek, C. Additive manufacturing—A general corrosion perspective. Corros. Eng. Sci. 2018, 537, 531–535. [Google Scholar] [CrossRef]
- Leon, A.; Shirizly, A.; Aghion, E. Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals 2016, 6, 148. [Google Scholar] [CrossRef]



| Si | Mg | Fe | Mn | Ti | Cu | Zn | Pb | Sn | Ni | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 10.20 | 0.34 | 0.17 | 0.01 | 0.34 | 0.01 | 0.01 | <0.01 | <0.01 | <0.01 | bal. |
| Samples | Power (W) | Exposure Time (µs) | TOFF (μs) | Point Distance (µm) | Hatch Distance (µm) | Layer Thickness (µm) | VED (J/mm3) | v (mm/s) | E Line (J/mm) |
|---|---|---|---|---|---|---|---|---|---|
| St1 | 375 | 40 | 20 | 120 | 90 | 30 | 69 | 2000 | 0.19 |
| St2 | 375 | 50 | 20 | 140 | 90 | 30 | 69 | 2000 | 0.19 |
| St3 | 375 | 60 | 20 | 160 | 90 | 30 | 69 | 2000 | 0.19 |
| Target | Voltage (KV) | Current (μA) | Integration Time (ms) | Voxel Size (μm) | Number of Radiographies |
|---|---|---|---|---|---|
| Tungsten | 100 | 100 | 500 | 8 | 2000 |
| Sample | Edge Size | Volume (mm3) |
|---|---|---|
| St1 | ≈ 10 × 10 × 3.7 mm3 | 360.17 mm3 |
| St2 | ≈ 10 × 10 × 5.6 mm3 | 560.23 mm3 |
| St3 | ≈10 × 10 × 3.85 mm3 | 383.40 mm3 |
| Sample | Width of Melt Pool L Section (XZ) (No Edge) | Depth of Melt Pool L Section (XZ) (No Edge) | Track Width T Section (XY) (No Edge) |
|---|---|---|---|
| St1 | 132 ± 17 | 50 ± 6 | 87 ± 6 |
| St2 | 142 ± 20 | 76 ± 5 | 87 ± 3 |
| St3 | 157 ± 12 | 74 ± 8 | 92 ± 1 |
| Sample | Si Particles Density (Total Number/µm2) |
|---|---|
| St1 | 11.1 |
| St3 | 8.6 |
| Sample | Pores Number | Porosity % | Equivalent Diameter (µm) | Volume (mm3) | Sphericity |
|---|---|---|---|---|---|
| St1 | 28,627 | 0.372 | Average: 38.50 range: 19.85–181.25 | Average: 4.69 × 10−5 range: 4.10 × 10−6–3.12 × 10−3 | Average: 0.864 range: 0.351–1.0 |
| St2 | 38,490 | 0.327 | Average: 38.85 range: 19.85–176.05 | Average: 4.75 × 10−5 range: 4.10 × 10−6–2.86 × 10−3 | Average: 0.906 range: 0.297–1.0 |
| St3 | 19,605 | 0.181 | Average: 34.84 range: 19.85–152.22 | Average: 3.53 × 10−5 range: 4.10 × 10−6–1.85 × 10−3 | Average: 0.906 range: 0.331–1.0 |
| Sample | HV 0.1/15 L Section (XZ) (No Edge) | HV 0.1/15 T Section (XY) (No Edge) |
|---|---|---|
| St1 | 71 ± 1 | 71 ± 1 |
| St2 | 70 ± 1 | 71 ± 1 |
| St3 | 70 ± 0 | 70 ± 0 |
| Sample | OCP [V] |
|---|---|
| St1-T | −0.772 ± 0.001 |
| St2-T | −0.772 ± 0.001 |
| St3-T | −0.761 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leo, P.; Renna, G.; Soni, N.; De Pascalis, F.; Primo, T.; Del Prete, A. On the Effect of Exposure Time on Al-Si10-Mg Powder Processed by Selective Laser Melting. Metals 2024, 14, 76. https://doi.org/10.3390/met14010076
Leo P, Renna G, Soni N, De Pascalis F, Primo T, Del Prete A. On the Effect of Exposure Time on Al-Si10-Mg Powder Processed by Selective Laser Melting. Metals. 2024; 14(1):76. https://doi.org/10.3390/met14010076
Chicago/Turabian StyleLeo, Paola, Gilda Renna, Neetesh Soni, Fabio De Pascalis, Teresa Primo, and Antonio Del Prete. 2024. "On the Effect of Exposure Time on Al-Si10-Mg Powder Processed by Selective Laser Melting" Metals 14, no. 1: 76. https://doi.org/10.3390/met14010076







