A Study of a Cryogenic CuAlMn Shape Memory Alloy
Abstract
:1. Introduction
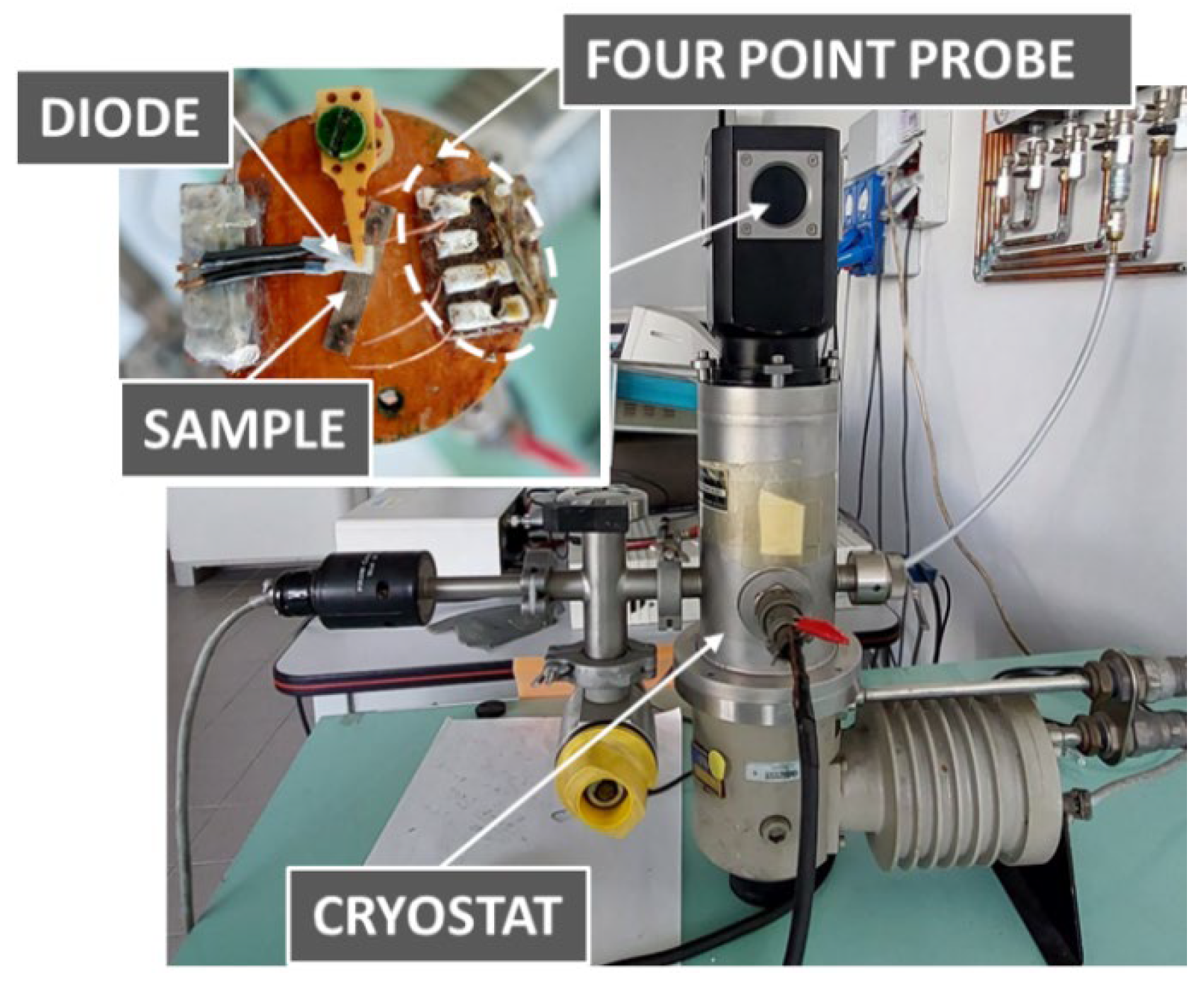
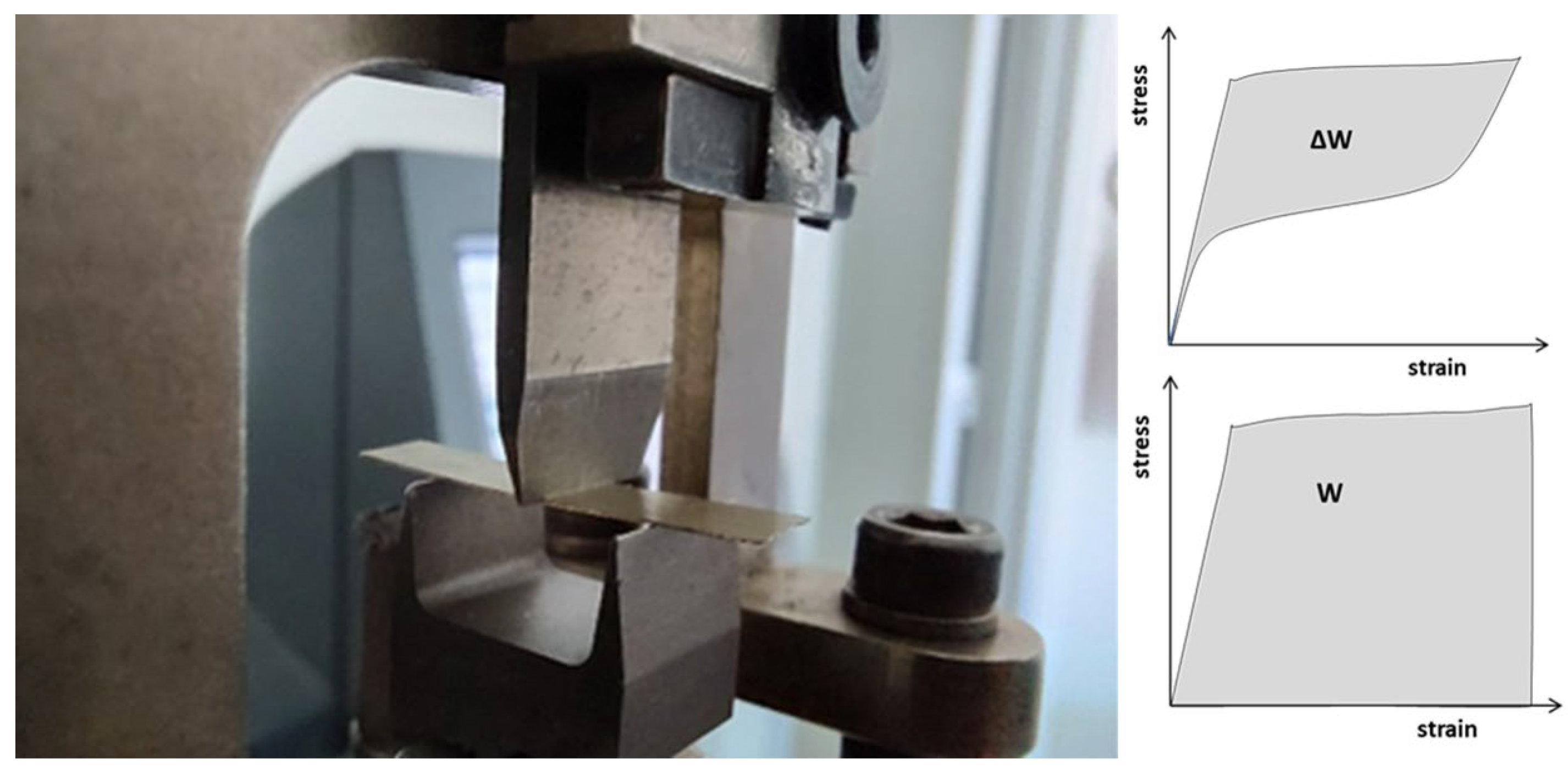
2. Materials and Methods
3. Results
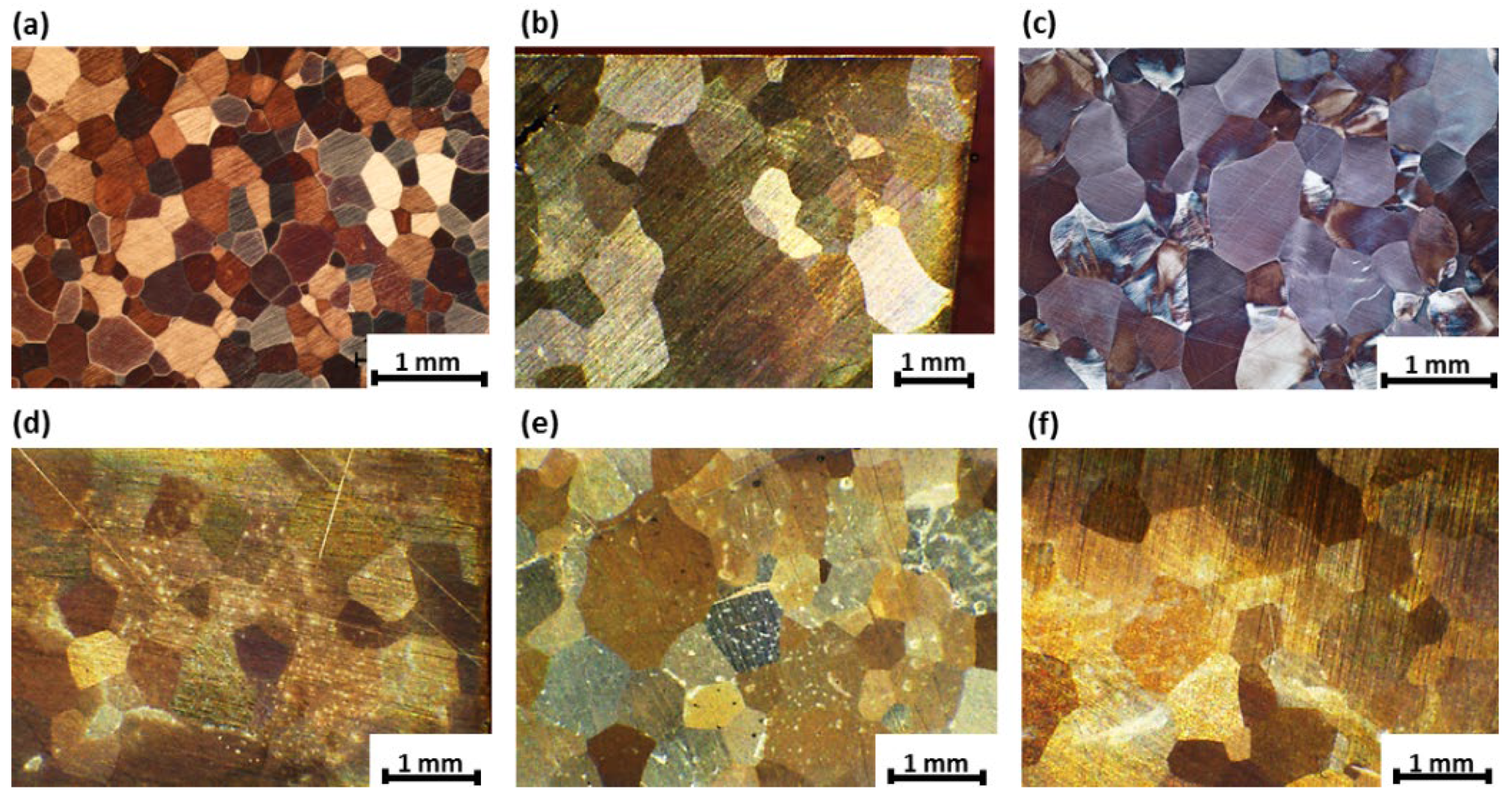
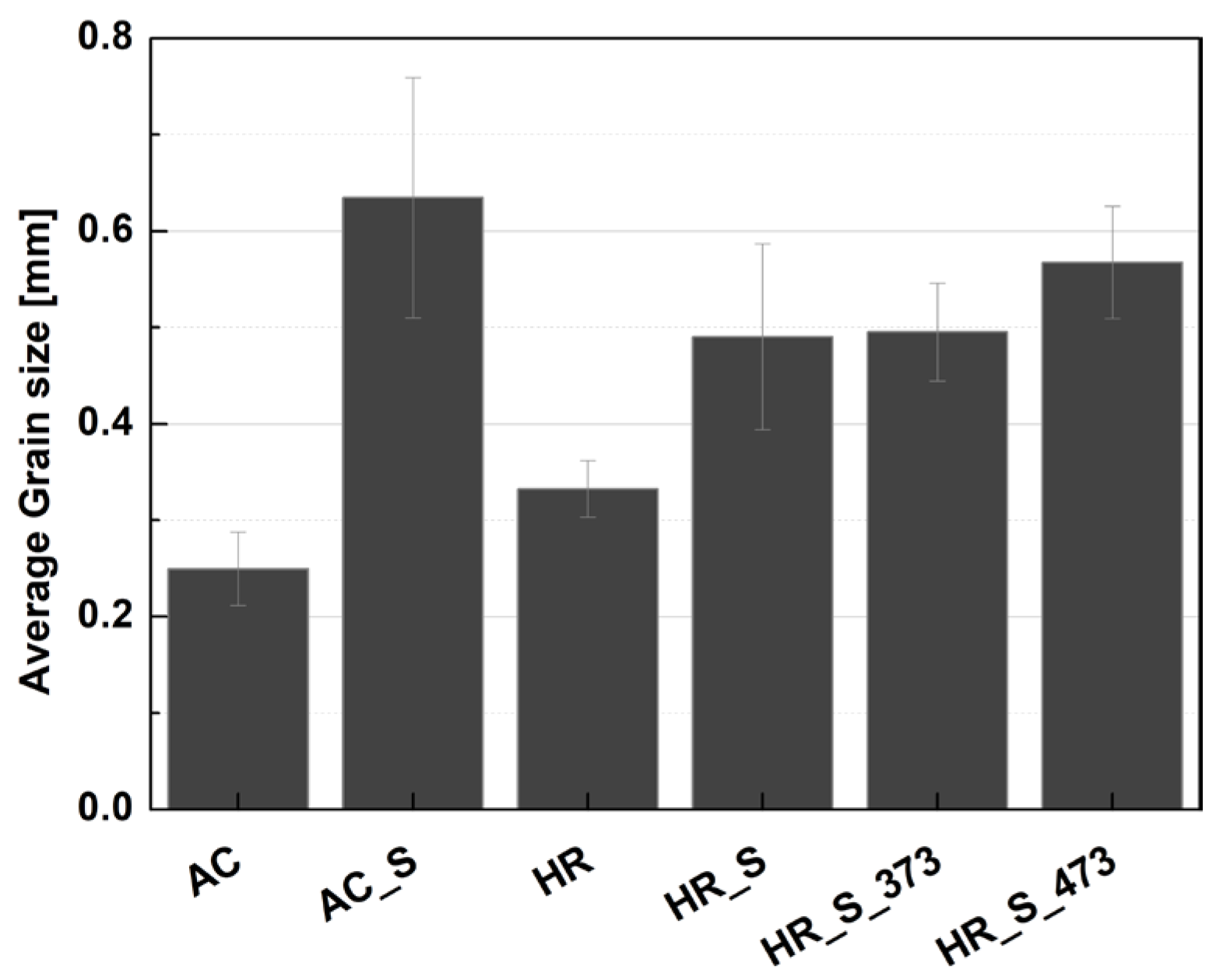
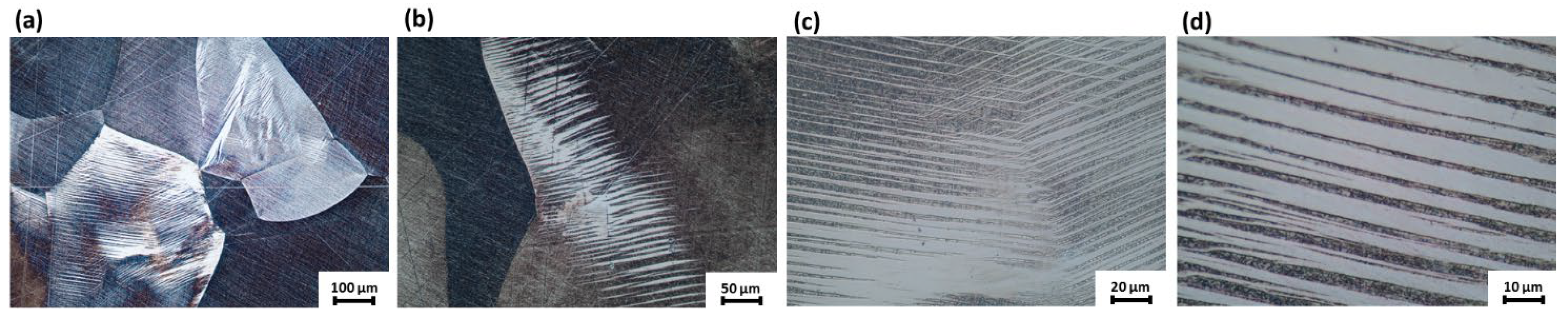
3.1. Microstructure
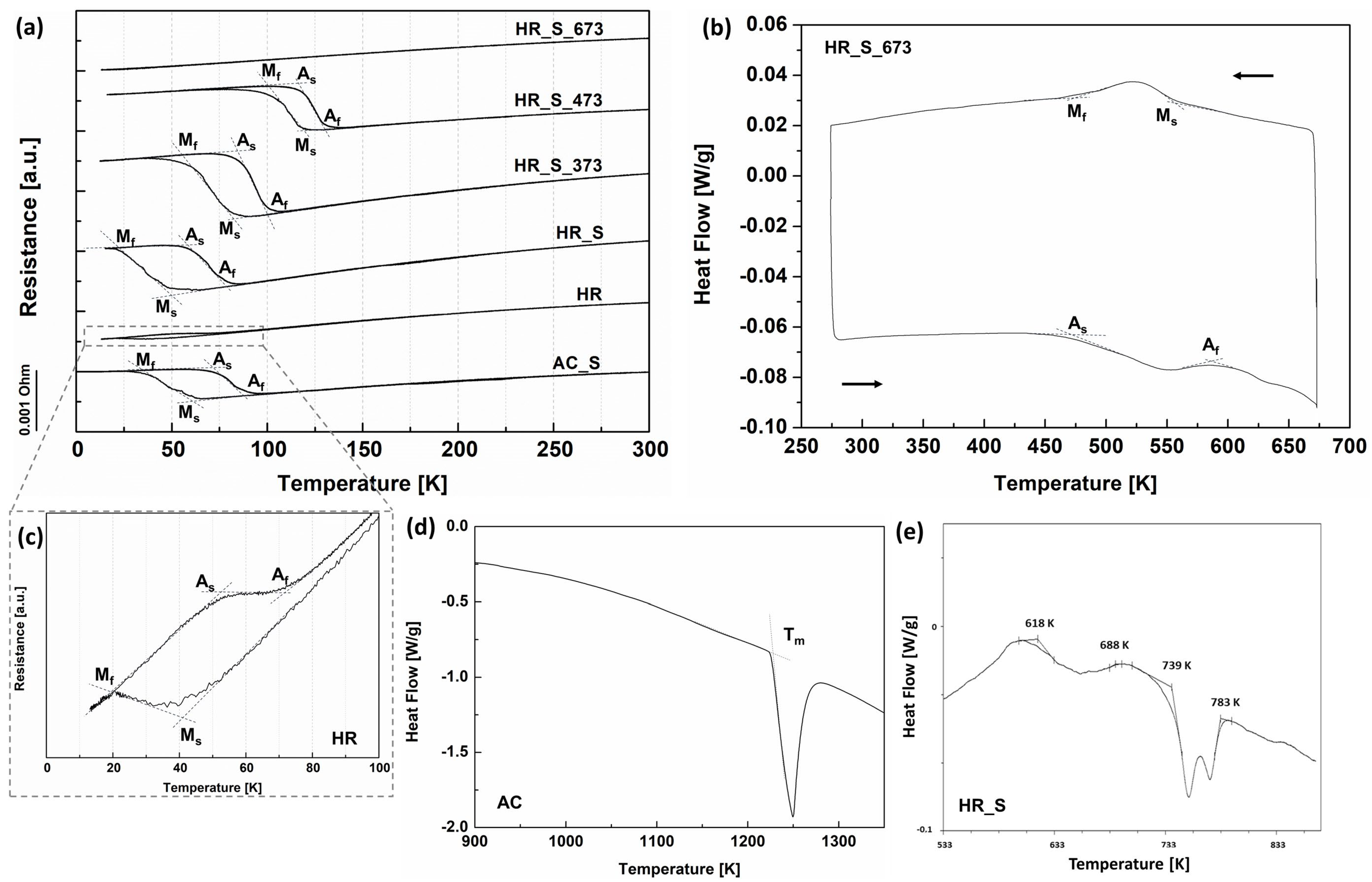
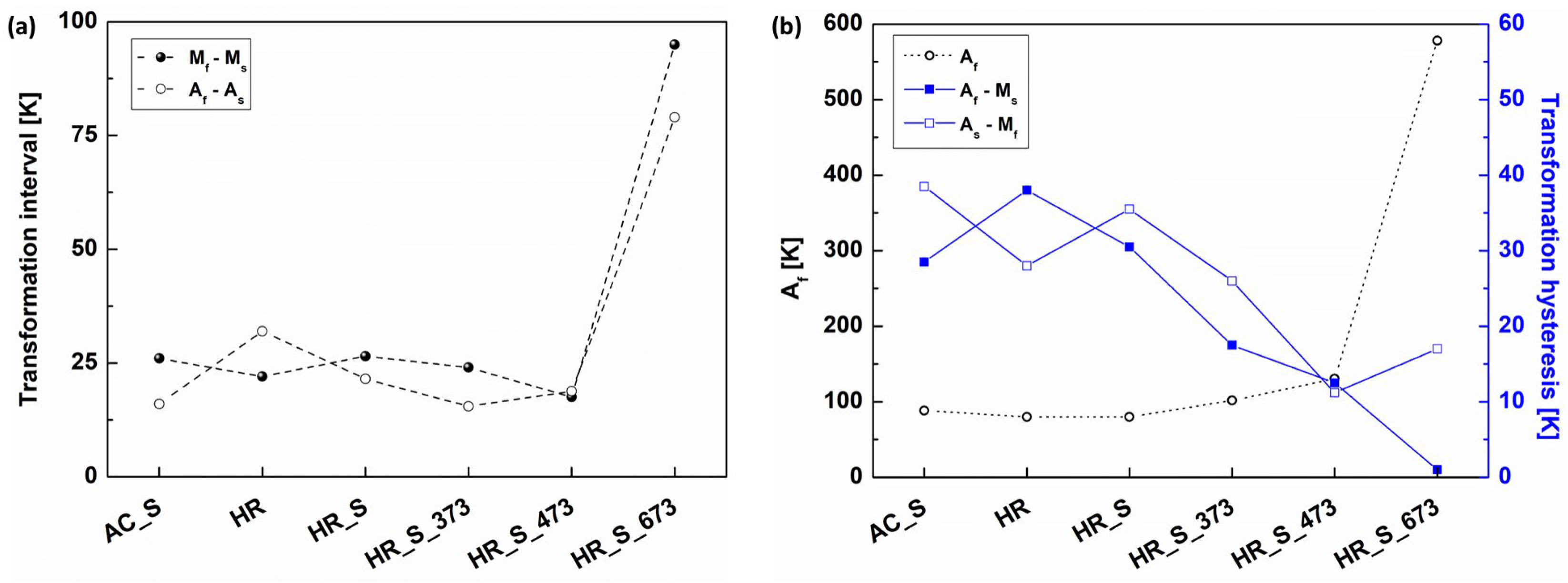
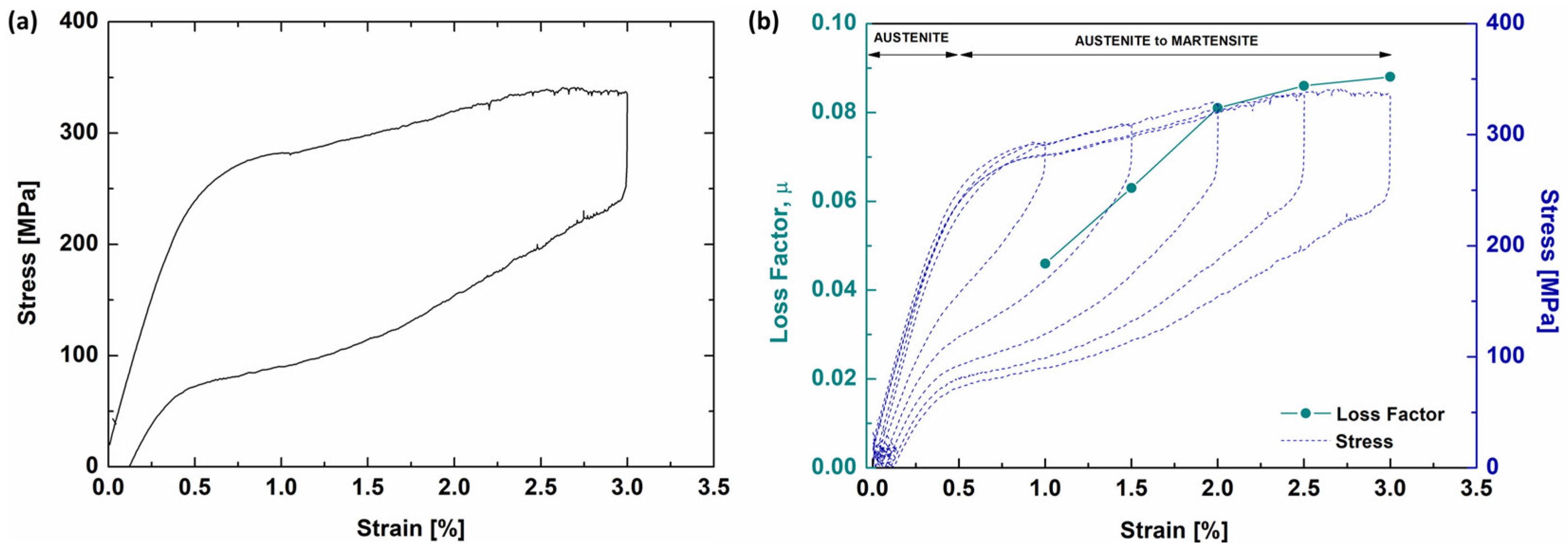
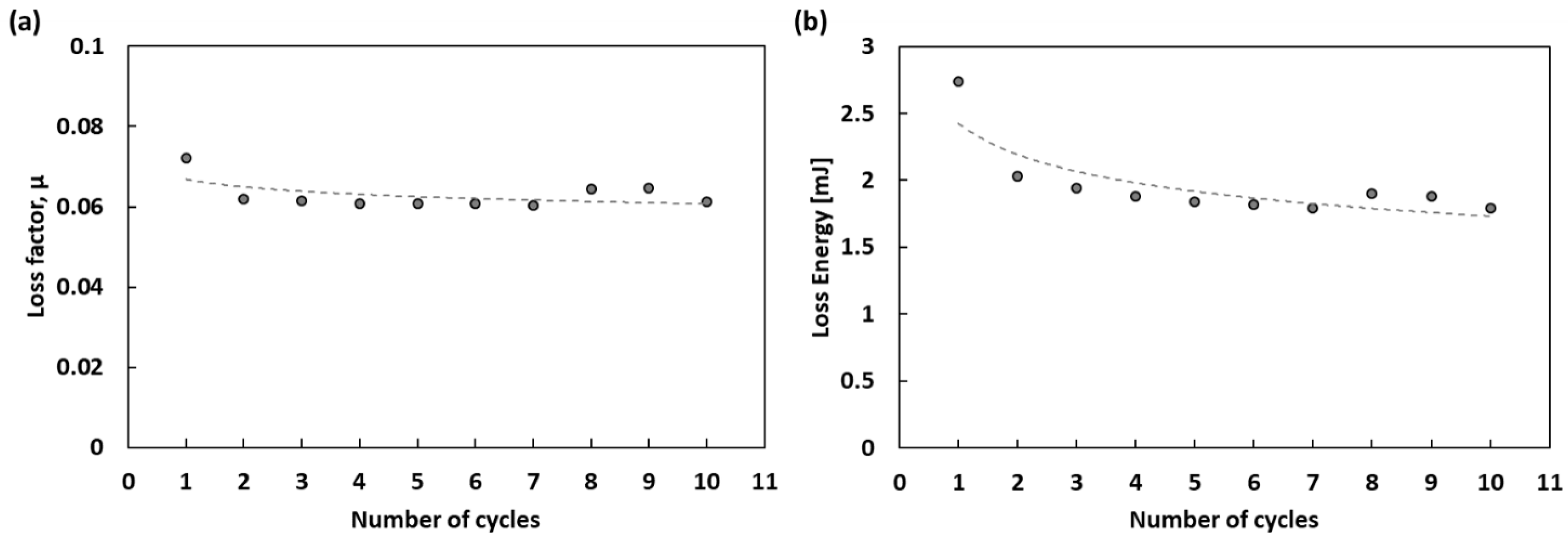
3.2. Thermal and Mechanical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duerig, T.W.; Melton, K.N.; Stockel, D.; Wayman, C.M. Engineering Aspects of Shape Memory Alloys; Elsevier Ltd.: Amsterdam, The Netherlands; Butterworth–Heinemann: Oxford, UK, 1990. [Google Scholar] [CrossRef]
- Concilio, A.; Antonucci, V.; Auricchio, F.; Lecce, L.; Sacco, E. Shape Memory Alloy Engineering for Aerospace, Structural and Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands; Butterworth–Heinemann: Oxford, UK, 2021. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Patel, S.K.; Behera, B.; Swain, B.; Roshan, R.; Sahoo, D.; Behera, A. A review on NiTi alloys for biomedical applications and their biocompatibility. Mater. Today Proc. 2020, 33, 5548–5551. [Google Scholar] [CrossRef]
- Nespoli, A.; Dallolio, V.; Villa, E.; Passaretti, F. A new design of a Nitinol ring-like wire for suturing in deep surgical field. Mater. Sci. Eng. C 2015, 56, 30–36. [Google Scholar] [CrossRef]
- Kim, M.S.; Heo, J.K.; Rodrigue, H.; Lee, H.T.; Pané, S.; Han, M.W.; Ahn, S.H. Shape Memory Alloy (SMA) Actuators: The Role of Material, Form, and Scaling Effects. Adv. Mater. 2023, 35, 2208517. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A. Reconciling viability and cost-effective shape memory alloy options—A review of copper and iron based shape memory metallic systems. Eng. Sci. Technol. Int. J. 2016, 19, 1582–1592. [Google Scholar] [CrossRef]
- Sutou, Y.; Omori, T.; Kainuma, R.; Ishida, K. Ductile Cu–Al–Mn based shape memory alloys: General properties and applications. Mater. Sci. Technol. 2008, 24, 896–901. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Z.H.; Huang, H.-Y.; Xie, J.-X. Microstructure and superelasticity control by rolling and heat treatment in columnar-grained Cu-Al-Mn shape memory alloy. Mater. Sci. Eng. A 2017, 696, 315–322. [Google Scholar] [CrossRef]
- Sasmaz, M.; Bayri, A.; Aydogdu, Y. The Magnetic Behavior and Physical Characterization of Cu–Mn–Al Ferromagnetic Shape Memory Alloy. J. Supercond. Nov. Magn. 2011, 24, 757–762. [Google Scholar] [CrossRef]
- Prado, M.O.; Lovey, F.C.; Civale, L. Magnetic properties of Cu-Mn-Al alloys with shape memory effect. Acta Mater. 1998, 46, 137–147. [Google Scholar] [CrossRef]
- Cesari, E.; Pons, J.; Santamarta, R.; Segui, C.; Chernenko, V.A. Ferromagnetic shape memory alloys: An overview. Arch. Metall. Mater. 2004, 49, 779–789. [Google Scholar]
- Lázpita, P.; Villa, E.; Villa, F.; Chernenko, V.A. Temperature Dependent Stress-Strain behavior and Martensite Stabilization in Magnetic Shape Memory Ni51.1Fe16.4Ga26.3Co6.2. Metals 2021, 11, 920. [Google Scholar] [CrossRef]
- Villa, E.; Villa, F.; Rodriguez Crespo, B.; Lazpita, P.; Salazar, D.; Chernenko, V.A.; Hosoda, H. Shape memory and elastocaloric properties of melt-spun NiMn-based Heusler alloys. J. Alloys Compd. 2023, 965, 171437. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Anaele, J.U.; Okotete, E.A. Martensite aging phenomena in Cu-based alloys: Effects on structural transformation, mechanical and shape memory properties: A critical review. Sci. Afr. 2021, 12, e00760. [Google Scholar] [CrossRef]
- Mallik, U.S.; Sampath, V. Effect of composition and ageing on damping characteristics of Cu–Al–Mn shape memory alloys. Mater. Sci. Eng. A 2008, 478, 48–55. [Google Scholar] [CrossRef]
- Kozubuski, R.; Soltys, J. Precipitation from metastable β-phase in the Heusler alloy Cu2.00Al1.00Mn1.00. J. Mater. Sci. 1983, 18, 1689–1697. [Google Scholar] [CrossRef]
- Gordo, P.; Frederico, T.; Melicio, R.; Amorim, A. Implementation of a Cryogenic Facility for Space Debris Analysis. Appl. Sci. 2021, 11, 948. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, A. Cryogenic technology for infrared detection in space. Sci. Rep. 2022, 12, 2349. [Google Scholar] [CrossRef]
- Nespoli, A.; Ninarello, D.; Fanciulli, C. A Review on Shape Memory Alloys with Martensitic Transition at Cryogenic Temperatures. Metals 2023, 13, 1311. [Google Scholar] [CrossRef]
- Umale, T.; Salas, D.; Tomes, B.; Arroyave, R.; Karaman, I. The effects of wide range of compositional changes on the martensitic transformation characteristics of NiTiHf shape memory alloys. Scr. Mater. 2019, 161, 78–83. [Google Scholar] [CrossRef]
- Zak, G.; Kneissl, A.C.; Zatulskij, G. Shape memory effect in cryogenic Cu-Al-Mn alloys. Scripta Mater. 1996, 34, 363–367. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.Y.; Su, Y.J. Tuning the operation temperature window of the elastocaloric effect in Cu-Al-Mn shape memory alloys by composition design. J. Alloys Compd. 2020, 828, 154265. [Google Scholar] [CrossRef]
- Lei, Y.; Qin, X.; Wan, F.; Liu, P.; Chen, L.; Wang, J. In-situ observation of martensitic transformation in Cu-Al-Mn cryogenic shape memory alloys. Fusion Eng. Des. 2017, 125, 603–607. [Google Scholar] [CrossRef]
- Bian, Z.; Song, J.; Liu, P.; Wan, F.; Lei, Y.; Wang, Q.; Yang, S.; Zhan, Q.; Chen, L.; Wang, J. In Situ Observation of Thermoelastic Martensitic Transformation of Cu-Al-Mn Cryogenic Shape Memory Alloy with Compressive Stress. Materials 2022, 15, 3794. [Google Scholar] [CrossRef] [PubMed]
- Trehern, W.; Ozcan, H.; Franco, B.; Hite, N.; Malone, N.; Loveall, B.; Morrison, T.D.; Benafan, O.; Karaman, I. Exploring thermomechanical functionality of CuAlMn as an extreme low temperature shape memory alloys. Mater. Lett. 2022, 308, 131246. [Google Scholar] [CrossRef]
- Nespoli, A.; Bassani, E.; Della Torre, D.; Donnini, R.; Villa, E.; Passaretti, F. An experimental study on pseudoelasticity of a NiTi-based damper for civil applications. Smart Mater. Struct. 2017, 26, 105041. [Google Scholar] [CrossRef]
- Sutou, Y.; Koeda, N.; Omori, T.; Kainuma, R.; Ishida, K. Effects of ageing on bainitic and thermally induced martensitic transformations in ductile Cu–Al–Mn-based shape memory alloys. Acta Mater. 2009, 57, 5748–5758. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Chatterjee, S.; Chattopadhyay, S.; Giri, S.; Majumdar, S. Nature of the glassy magnetic state in the Cu2.84Mn0.44Al0.72 shape memory alloy. Europhys. Lett. 2013, 104, 47014. [Google Scholar] [CrossRef]
- Niitsu, K.; Kimura, Y.; Omori, T.; Kainuma, R. Cryogenic superelasticity with large elastocaloric effect. NPG Asia Mater. 2018, 10, e457. [Google Scholar] [CrossRef]
- Liu, J.-L.; Huang, H.-Y.; Xie, J.-X. Effects of aging treatment on the microstructure and superelasticity of columnar-grained Cu71Al18Mn11 shape memory alloy. Int. J. Miner. Metal. Mater. 2016, 23, 1157. [Google Scholar] [CrossRef]
- Kožuh, S.; Gojić, M.; Ivanić, I.; Holjevac Grgurić, T.; Kosec, B.; Anžel, I. The Effect of Heat Treatment on the Microstructure and Mechanical Properties of Cu-Al-Mn Shape Memory Alloy. Kem. Ind. 2018, 67, 11–17. [Google Scholar] [CrossRef]
- Aksu Canbay, C.; Karagoz, Z.; Yakuphanoglua, F. Controlling of Transformation Temperatures of Cu-Al-Mn Shape Memory Alloys by Chemical Composition. Acta Phys. Pol. A 2014, 125, 1163–1166. [Google Scholar] [CrossRef]
- Nespoli, A.; Bettini, P.; Villa, E.; Sala, G.; Passaretti, F.; Grande, A.M. A study on damping property of NiTi elements produced by selective laser-beam melting. Adv. Eng. Mater. 2021, 23, 2001246. [Google Scholar] [CrossRef]


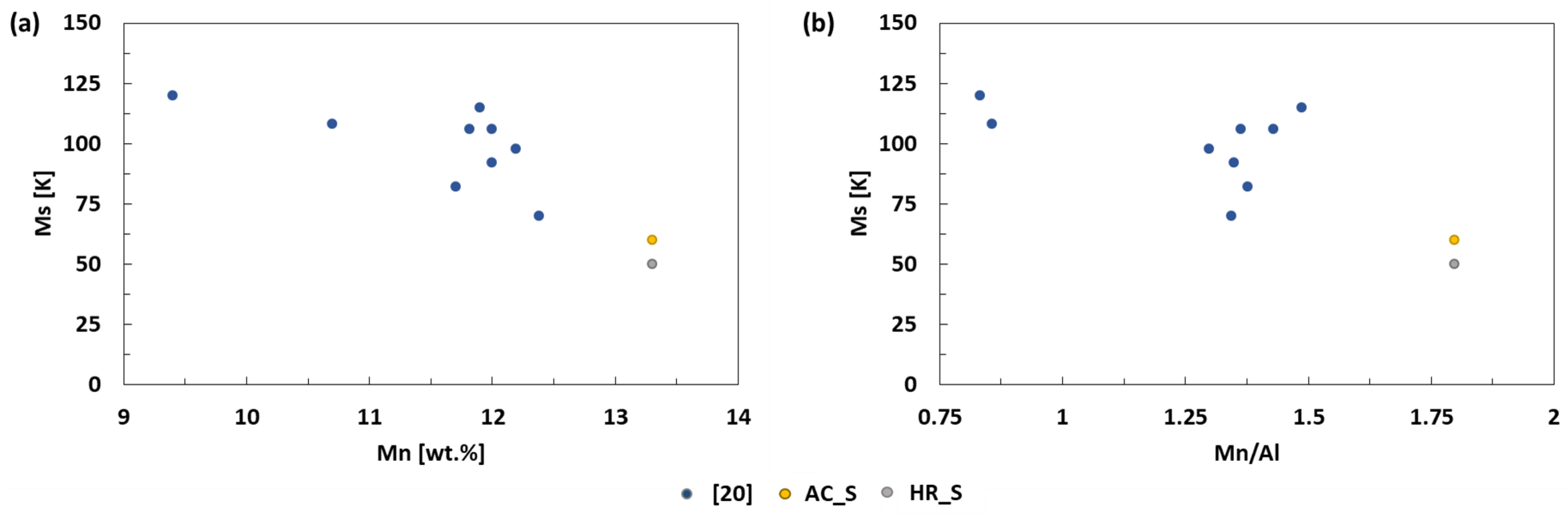
| Reference | Cu [wt.%] | Al [wt.%] | Mn [wt.%] | Ms [K] | Af [K] | Mn/Al | Tech. |
|---|---|---|---|---|---|---|---|
| [24] | 79.3 | 11.3 | 9.4 | 120 | ND | 0.832 | ND |
| [22] | 80.1 | 8 | 11.9 | 115 | 143 | 1.488 | AAS |
| [25] | 76.8 | 12.5 | 10.7 | 108 | 145 | 0.856 | ND |
| [22] | 79.6 | 8.4 | 12 | 106 | 137 | 1.429 | AAS |
| [26] | 79.5 | 8.7 | 11.8 | 106 | 122 | 1.363 | WDS |
| [26] | 78.4 | 9.4 | 12.2 | 98 | 116 | 1.299 | WDS |
| [22] | 79.1 | 8.9 | 12 | 92 | 123 | 1.348 | AAS |
| [11] | 79.8 | 8.5 | 11.7 | 82 | ND | 1.376 | ND |
| [26] | 78.4 | 9.2 | 12.4 | 70 | 91 | 1.345 | WDS |
| Sample | Post Processing Route |
|---|---|
| AC_S | As-cast and Solubilized at 1173 K for 1 h (wq) |
| HR | Hot-Rolled |
| HR_S | Hot-Rolled + Solubilized at 1173 K for 1 h (wq) |
| HR_S_373 | Hot-Rolled + Solubilized + Heat treated at 373 K for 30 min (wq) |
| HR_S_473 | Hot-Rolled + Solubilized + Heat treated at 473 K for 30 min (wq) |
| HR_S_673 | Hot-Rolled + Solubilized + Heat treated at 673 K for 30 min (wq) |
| wt.% | Cu | Al | Mn |
|---|---|---|---|
| nominal | 79 | 7.5 | 13.5 |
| measured | 79.3 (0.4) | 7.4 (0.3) | 13.3 (0.5) |
| Sample | Ms [K] | Mf [K] | As [K] | Af [K] |
|---|---|---|---|---|
| AC_S | 60 | 34 | 73 | 89 |
| HR | 42 | 20 | 48 | 80 |
| HR_S | 50 | 23 | 59 | 80 |
| HR_S_373 | 84 | 60 | 86 | 102 |
| HR_S_473 | 118 | 101 | 112 | 131 |
| HR_S_673 | 577 | 482 | 499 | 578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nespoli, A.; Passaretti, F.; Ninarello, D.; Pani, M.; Artini, C.; Ferro, F.; Fanciulli, C. A Study of a Cryogenic CuAlMn Shape Memory Alloy. Metals 2024, 14, 323. https://doi.org/10.3390/met14030323
Nespoli A, Passaretti F, Ninarello D, Pani M, Artini C, Ferro F, Fanciulli C. A Study of a Cryogenic CuAlMn Shape Memory Alloy. Metals. 2024; 14(3):323. https://doi.org/10.3390/met14030323
Chicago/Turabian StyleNespoli, Adelaide, Francesca Passaretti, Davide Ninarello, Marcella Pani, Cristina Artini, Francesca Ferro, and Carlo Fanciulli. 2024. "A Study of a Cryogenic CuAlMn Shape Memory Alloy" Metals 14, no. 3: 323. https://doi.org/10.3390/met14030323






