Effect of Hydrogen on Fatigue Life and Fracture Morphologies of TRIP-Aided Martensitic Steels with Added Nitrogen
Abstract
:1. Introduction
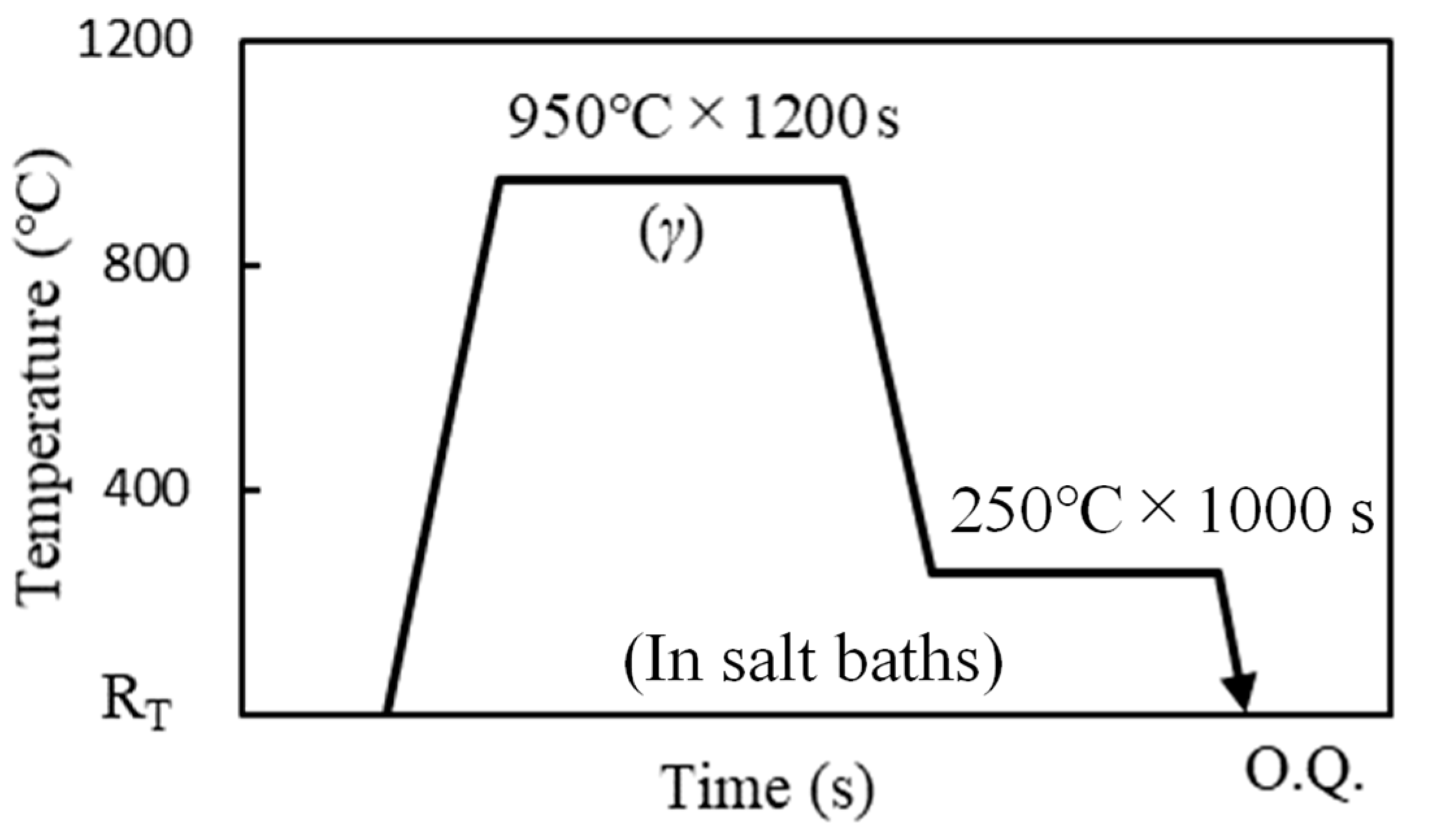
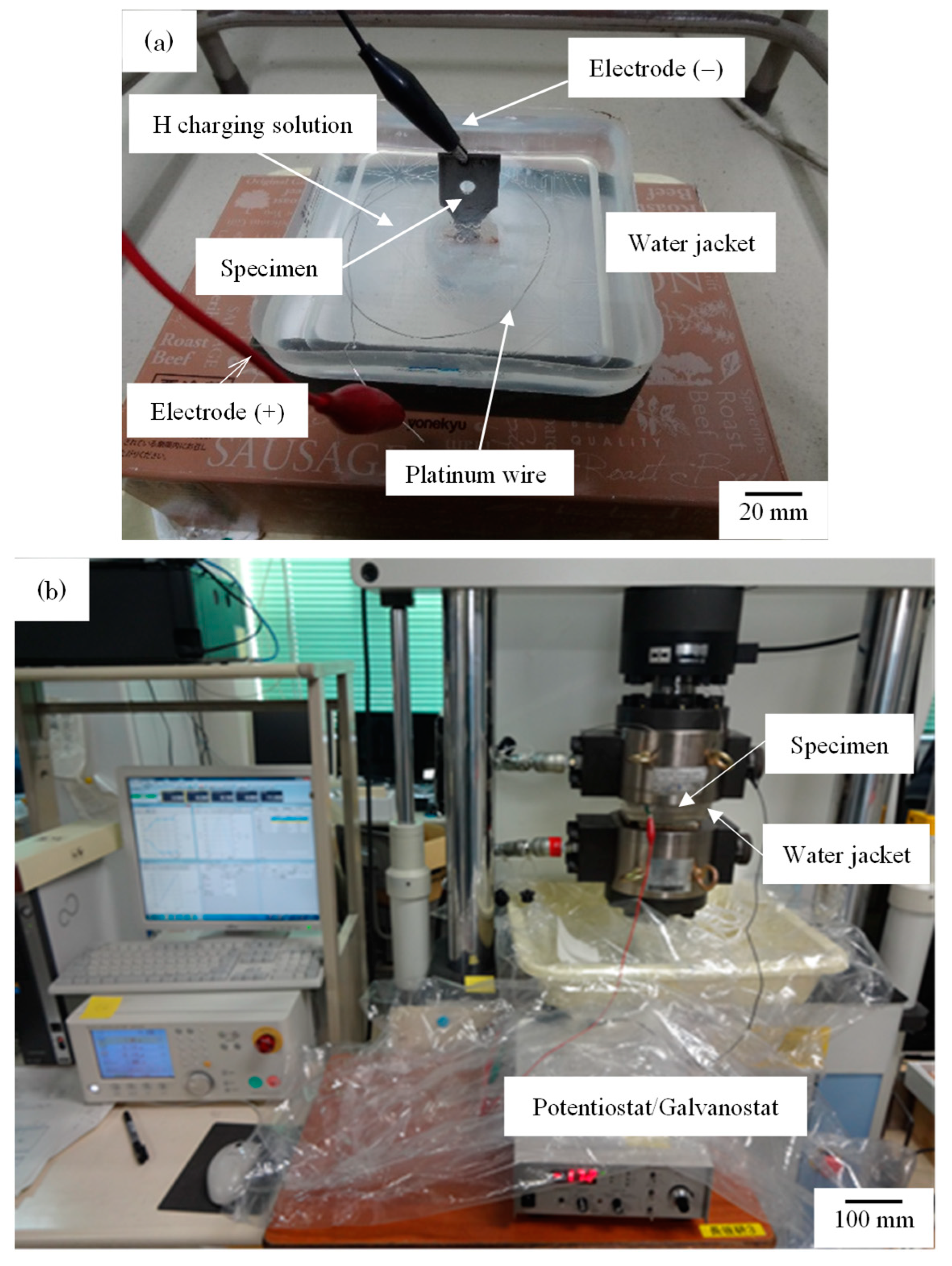
2. Materials and Methods
3. Results and Discussion
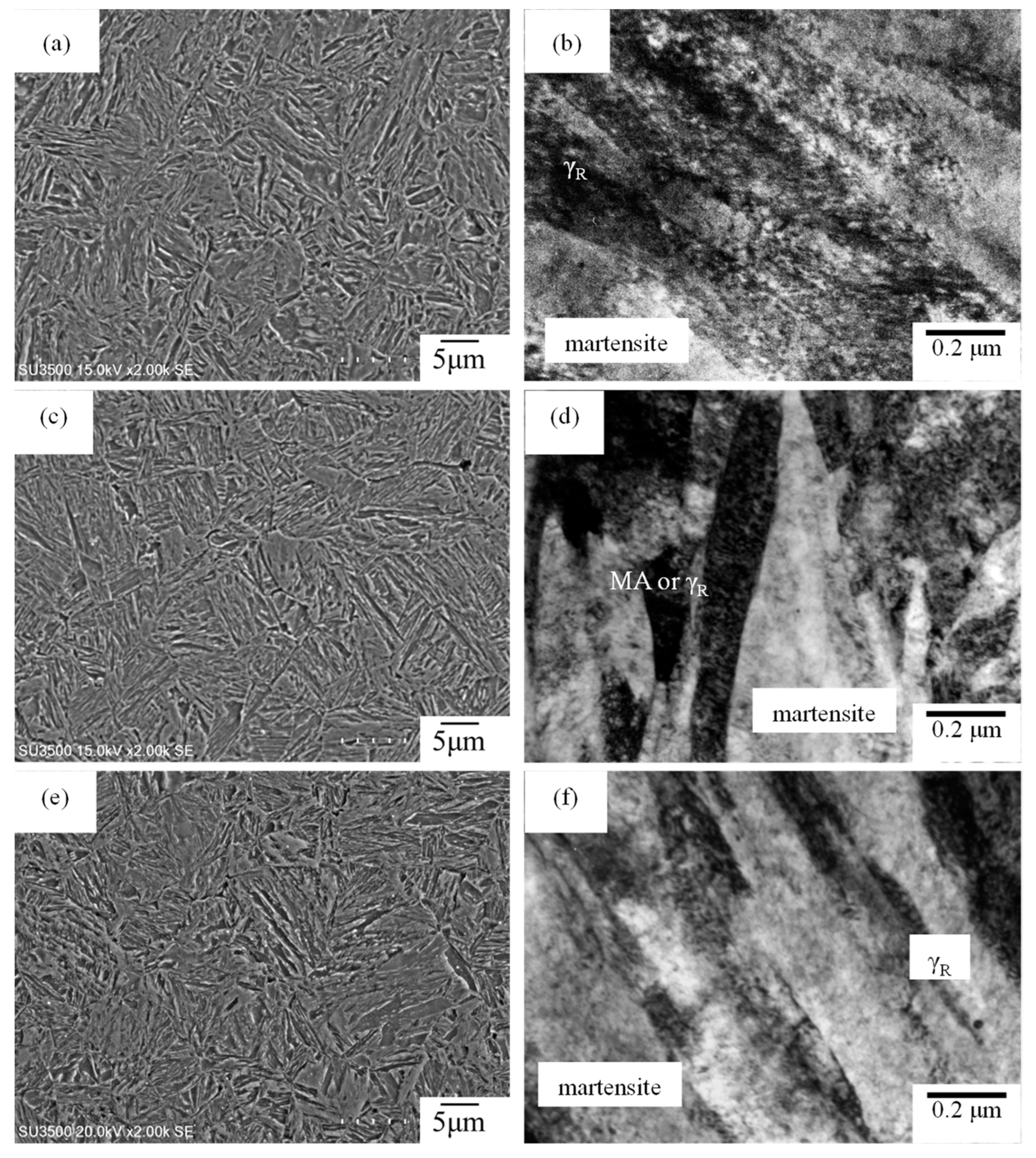
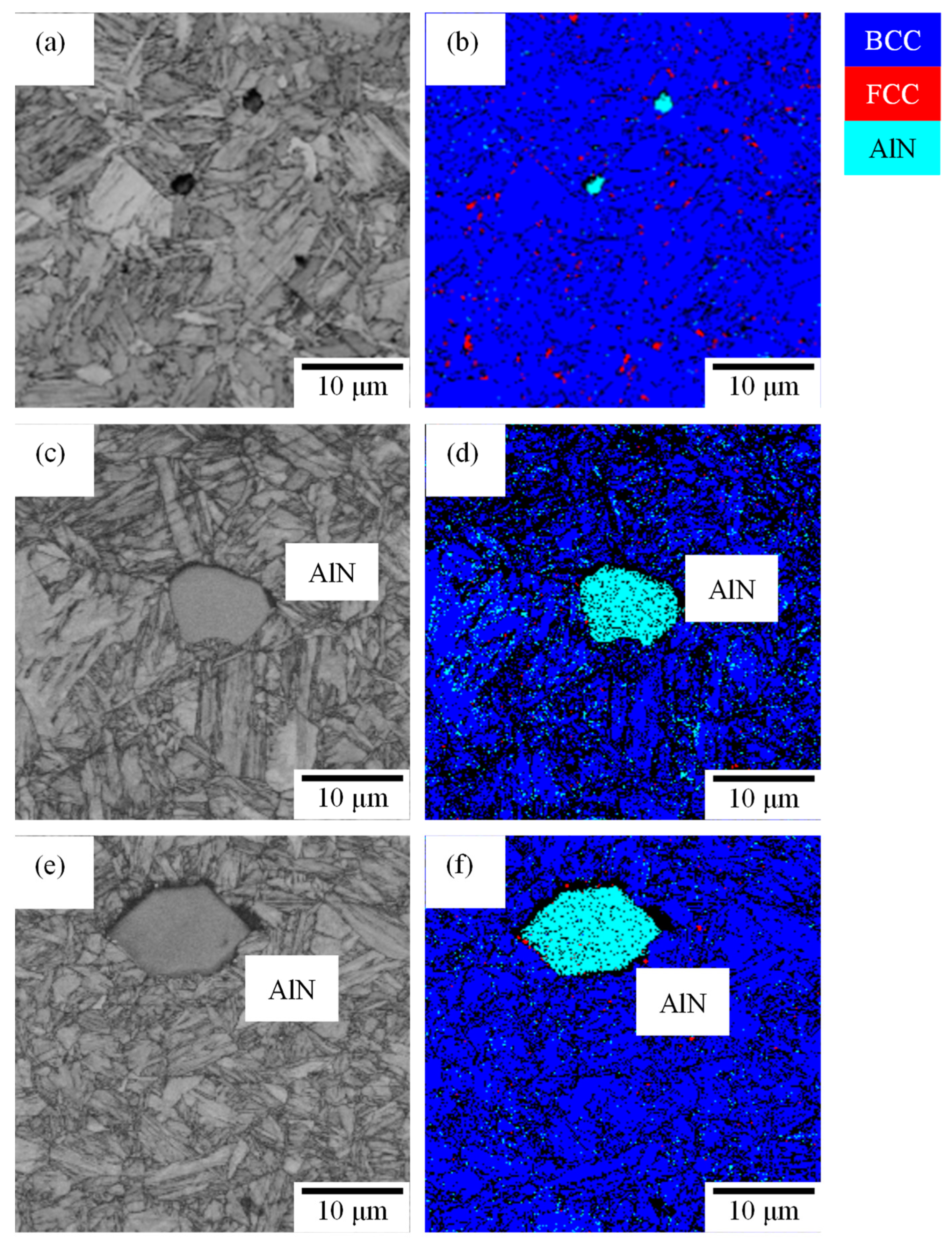
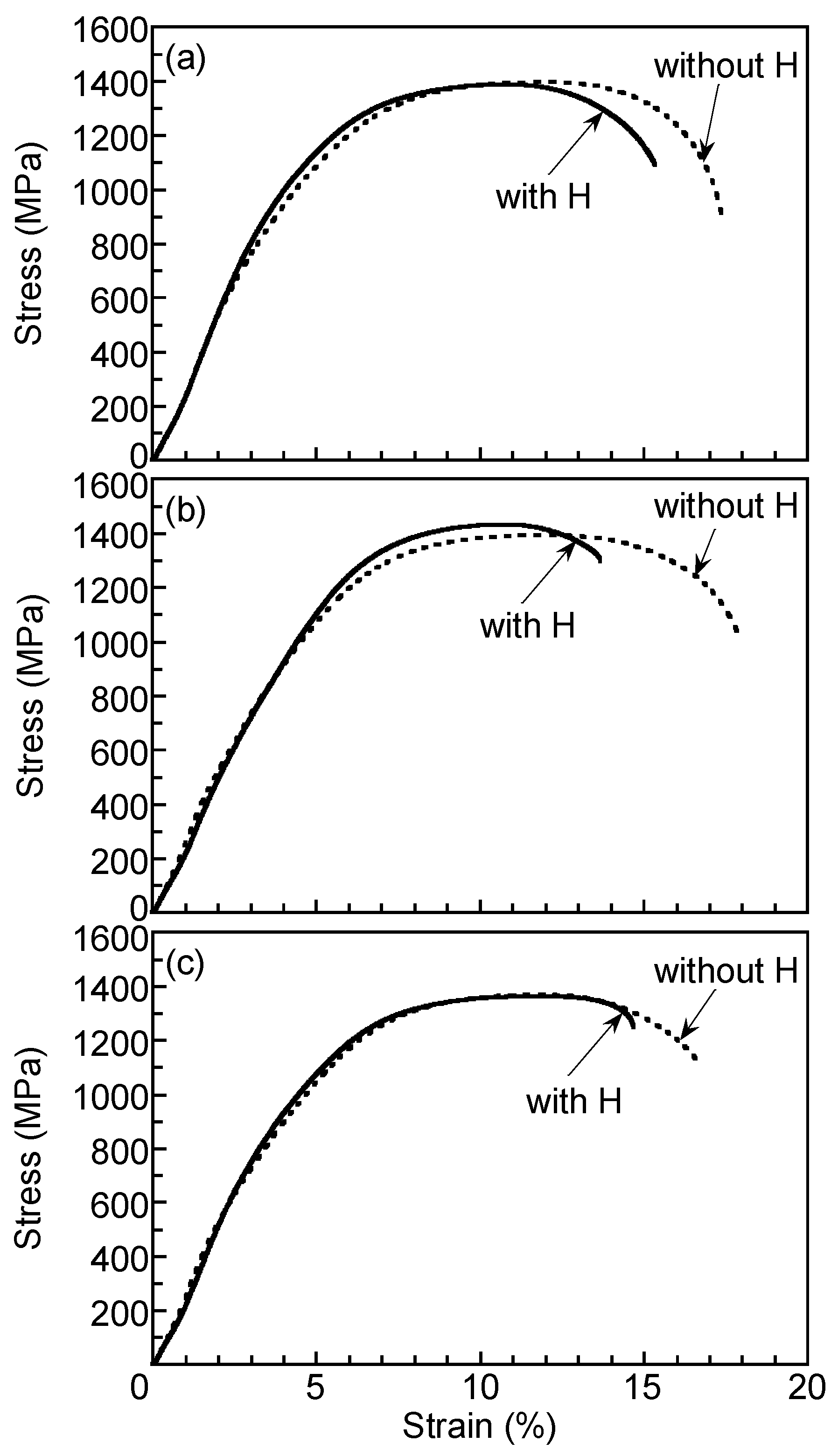
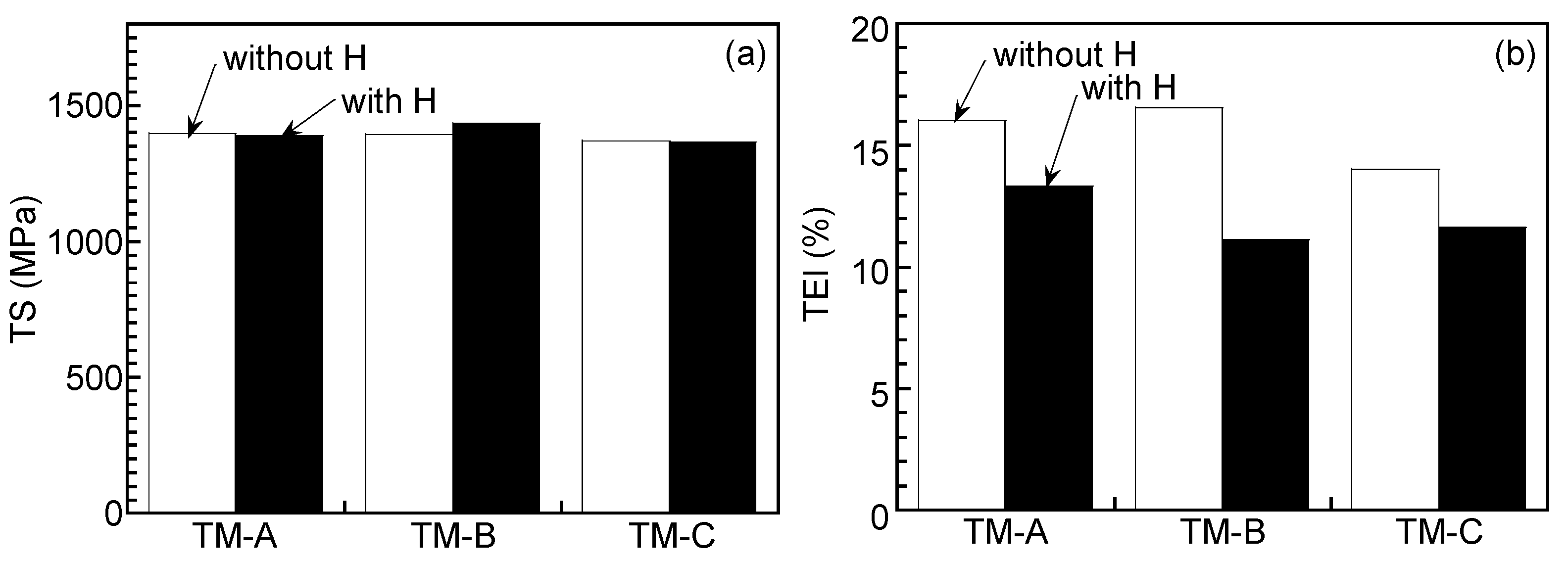
3.1. Microstructure and Tensile Properties
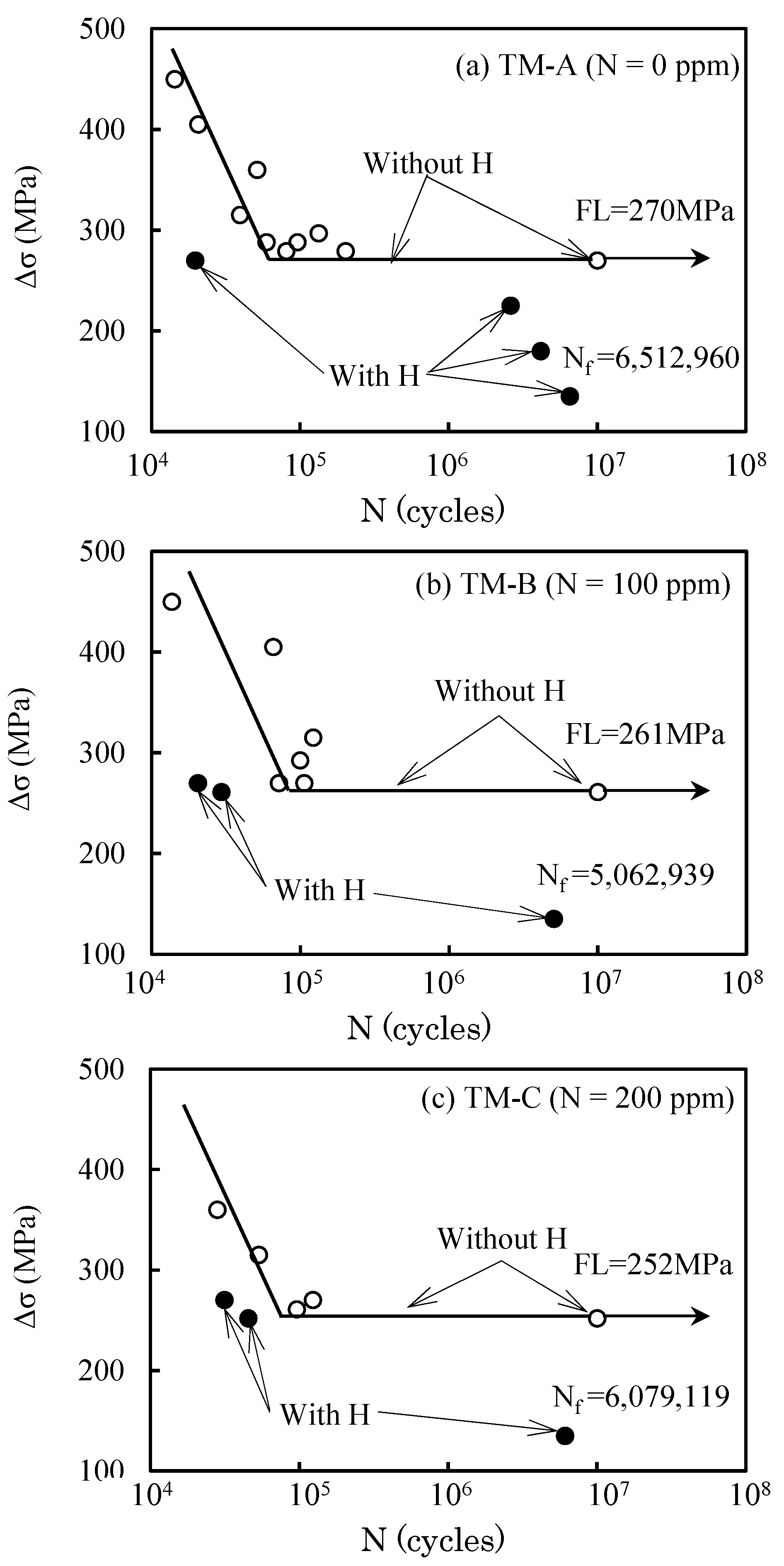
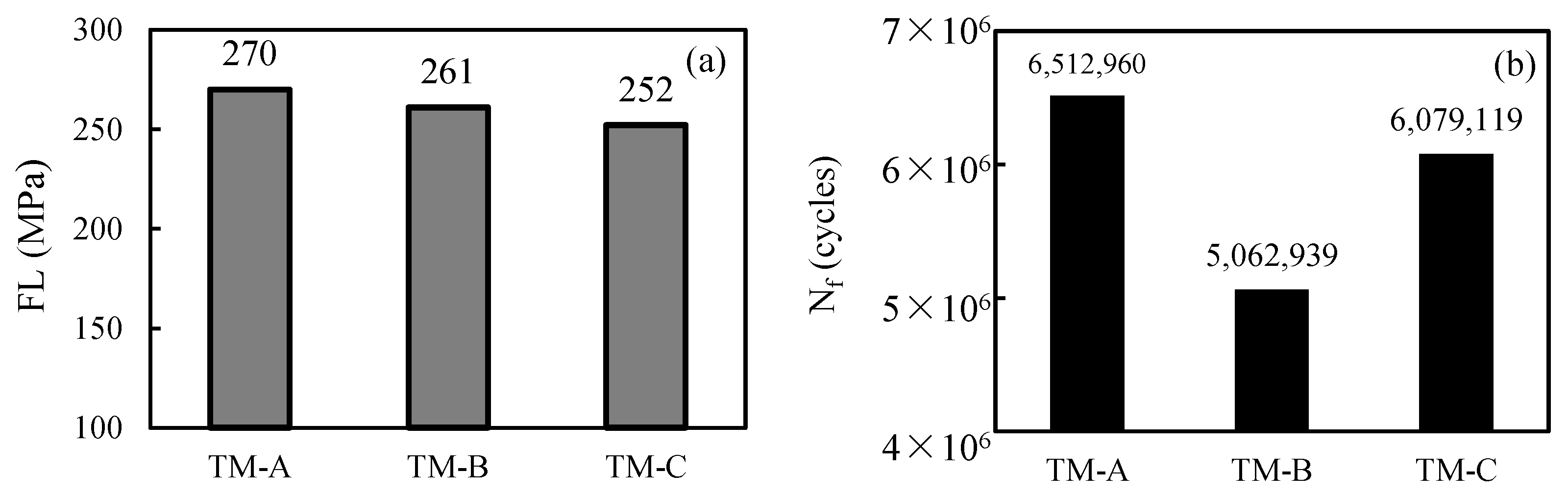
3.2. Fatigue Properties
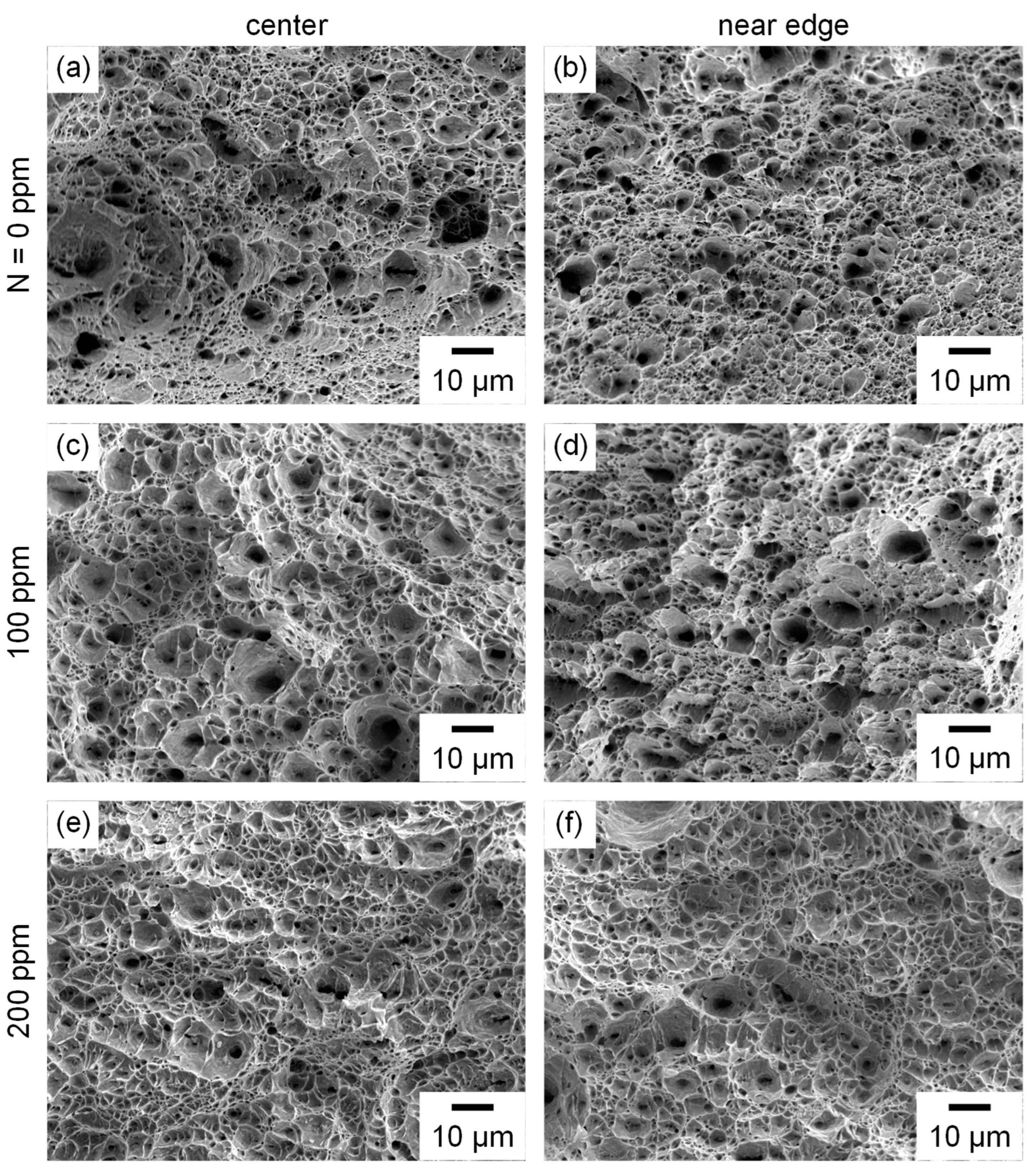
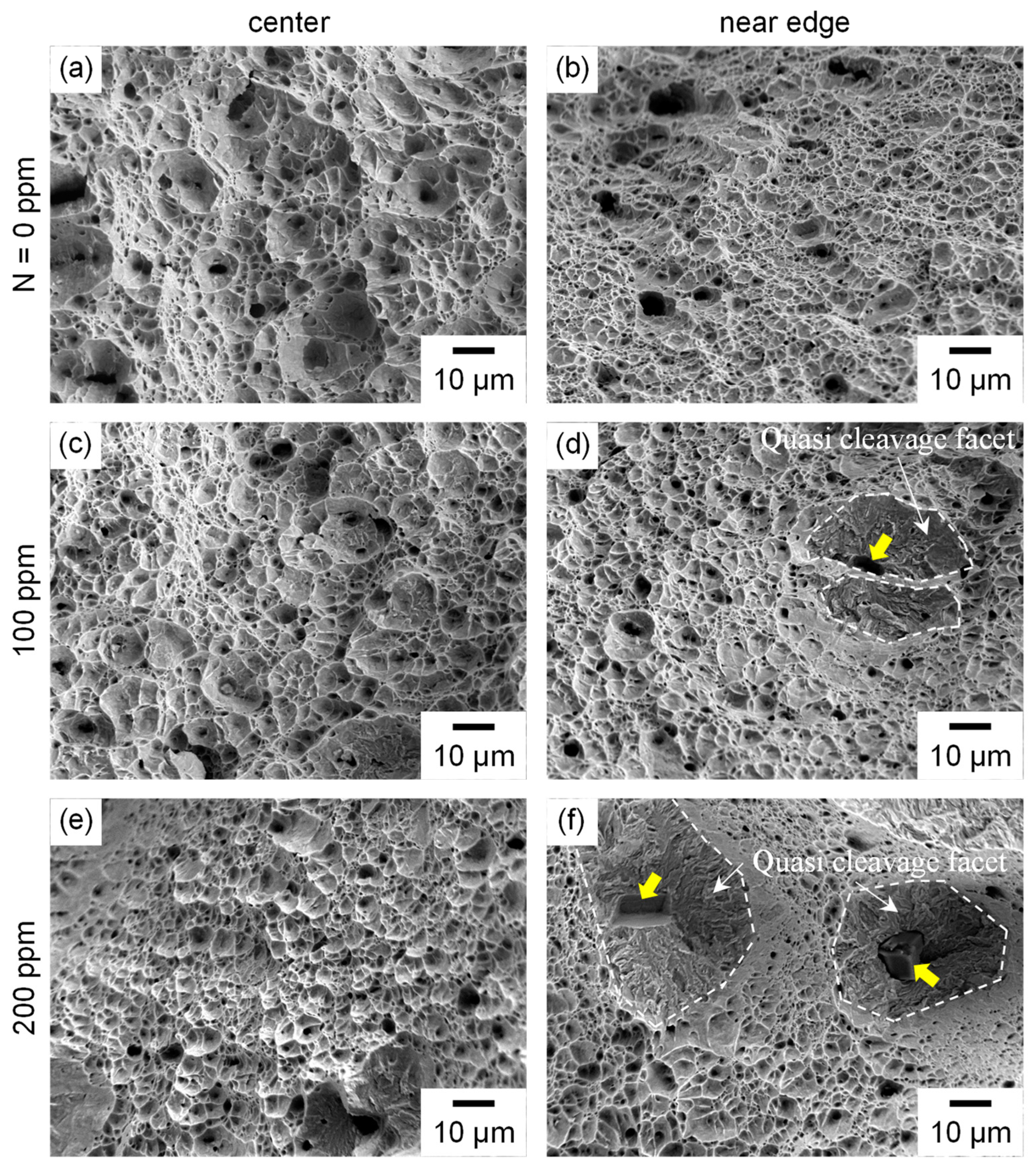
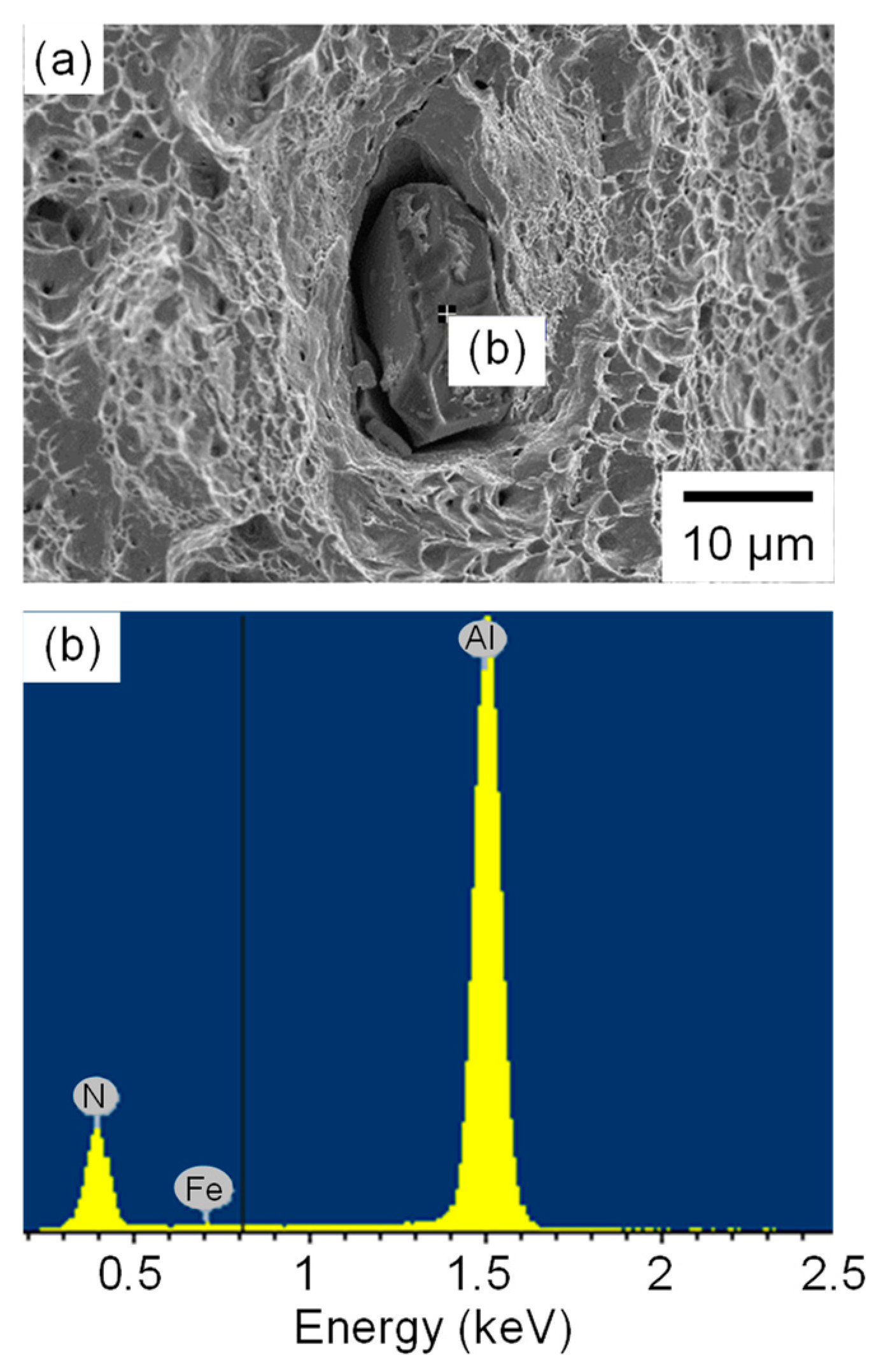
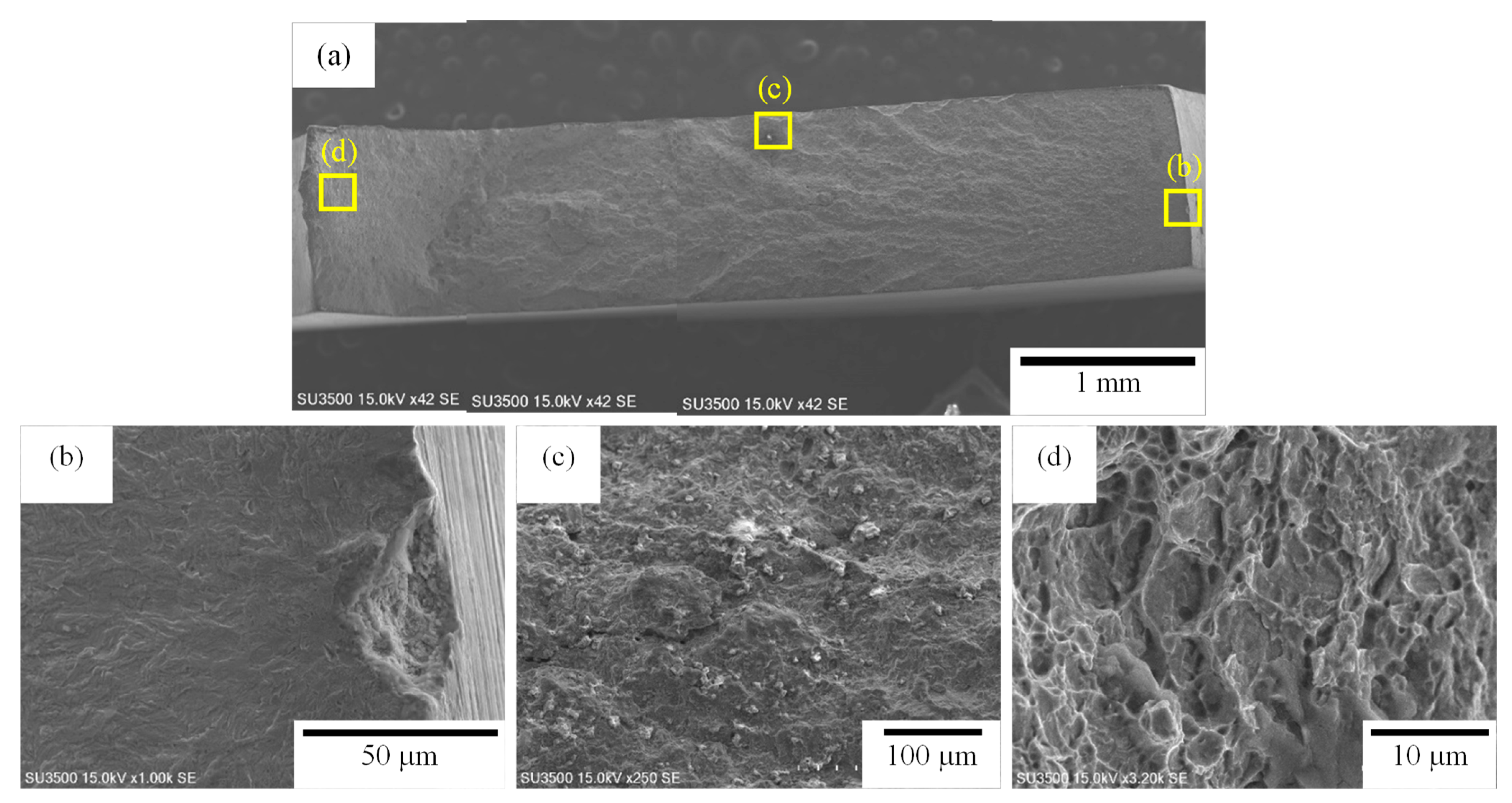
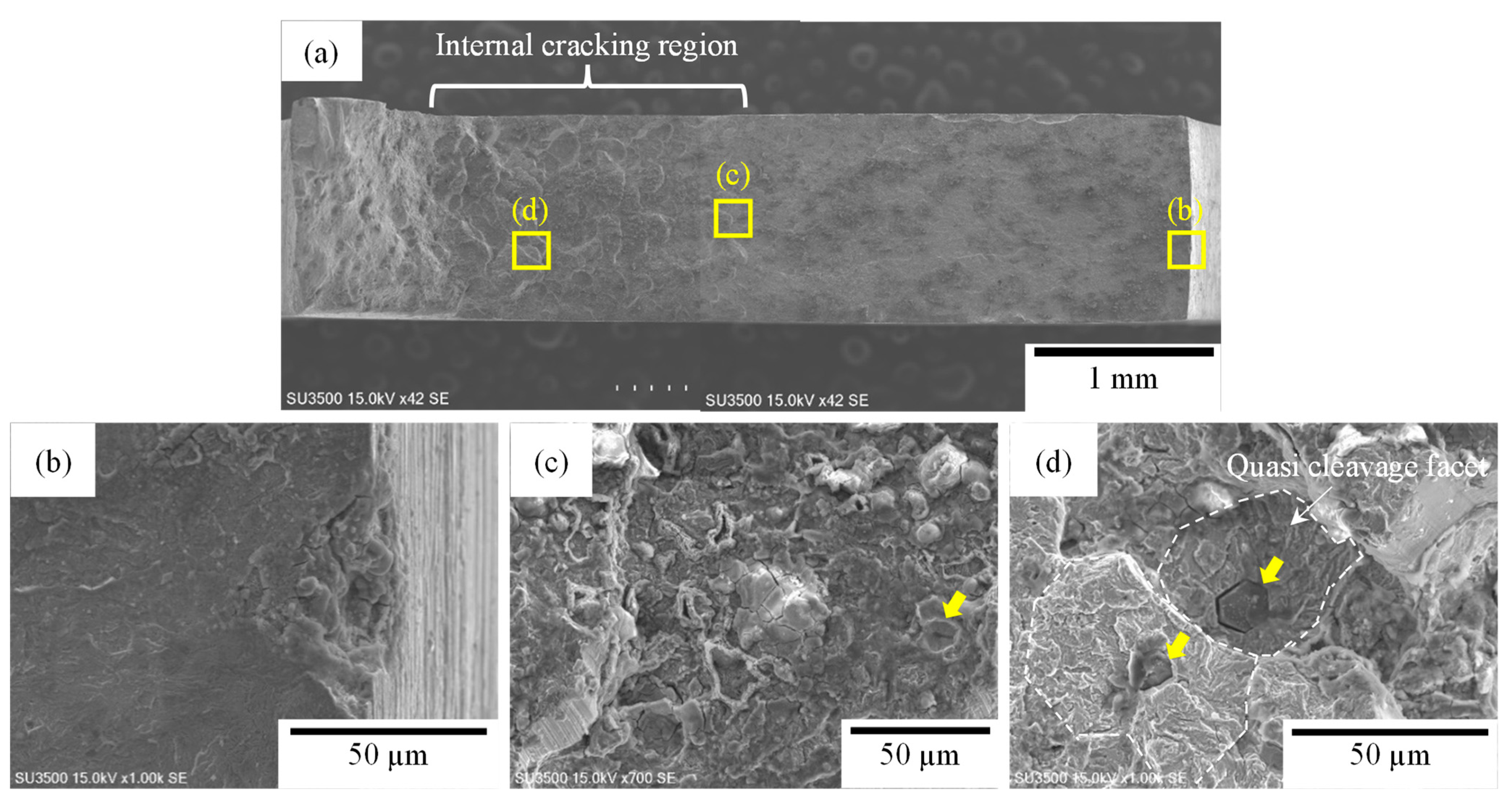
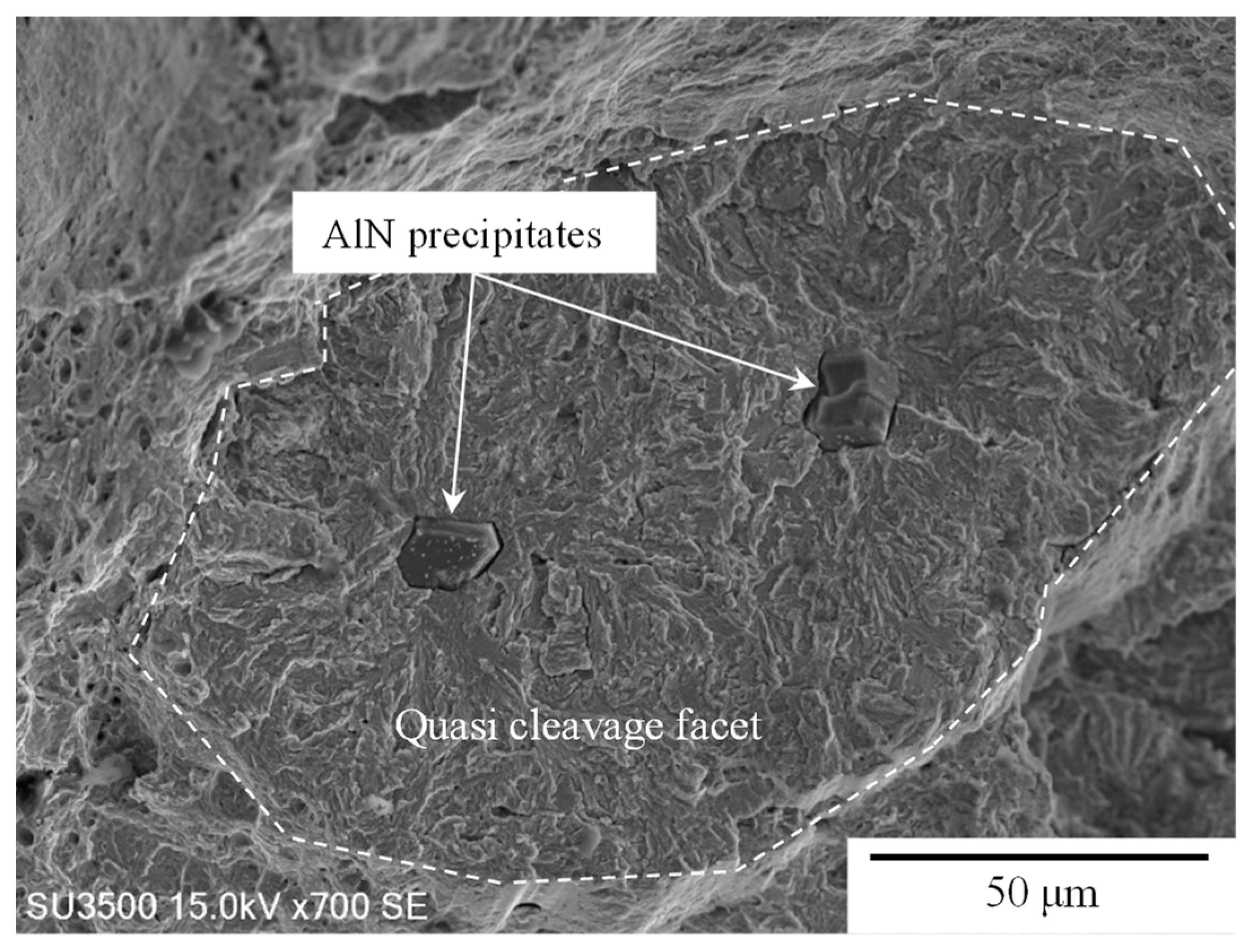
3.3. Fracture Surfaces
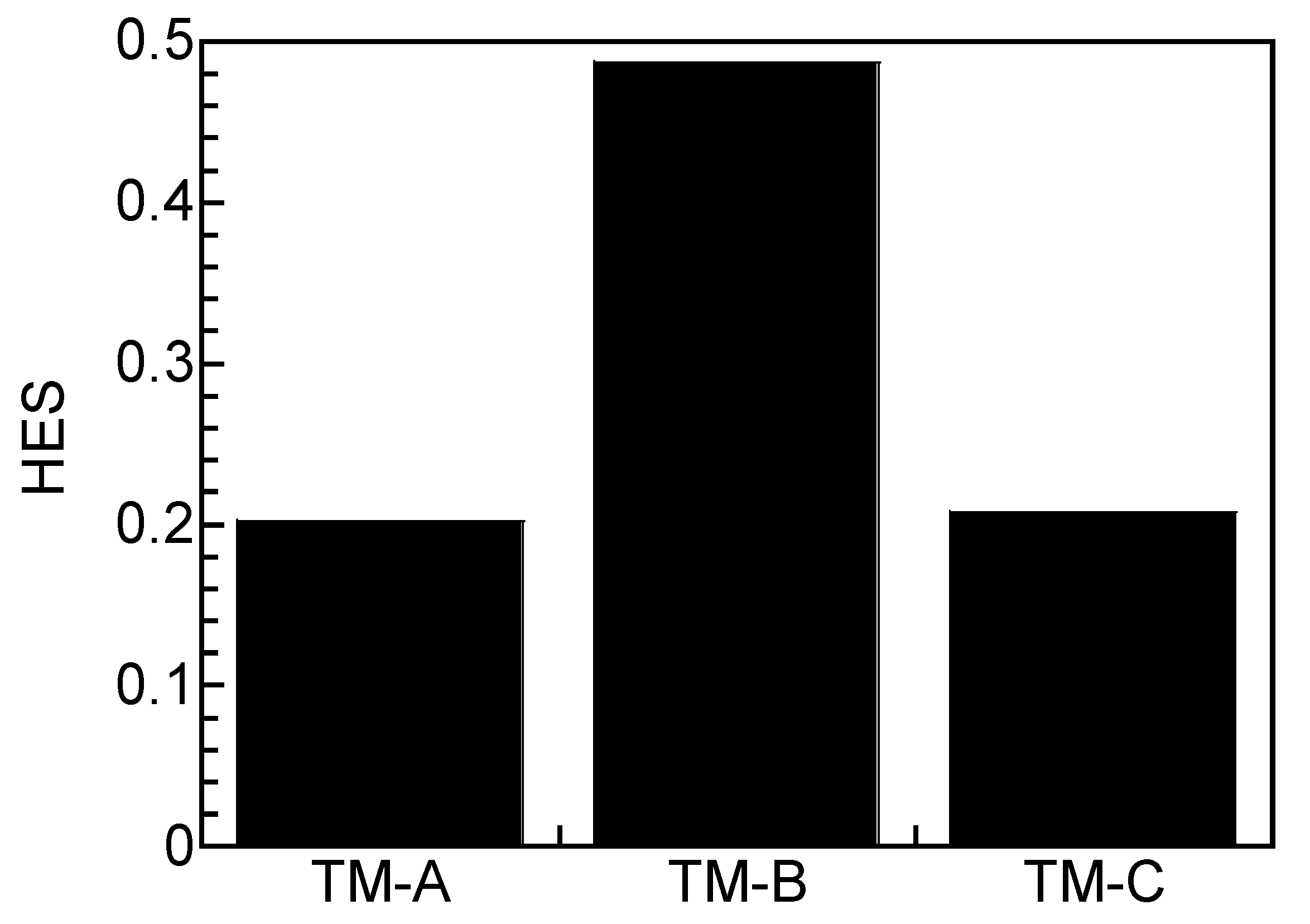
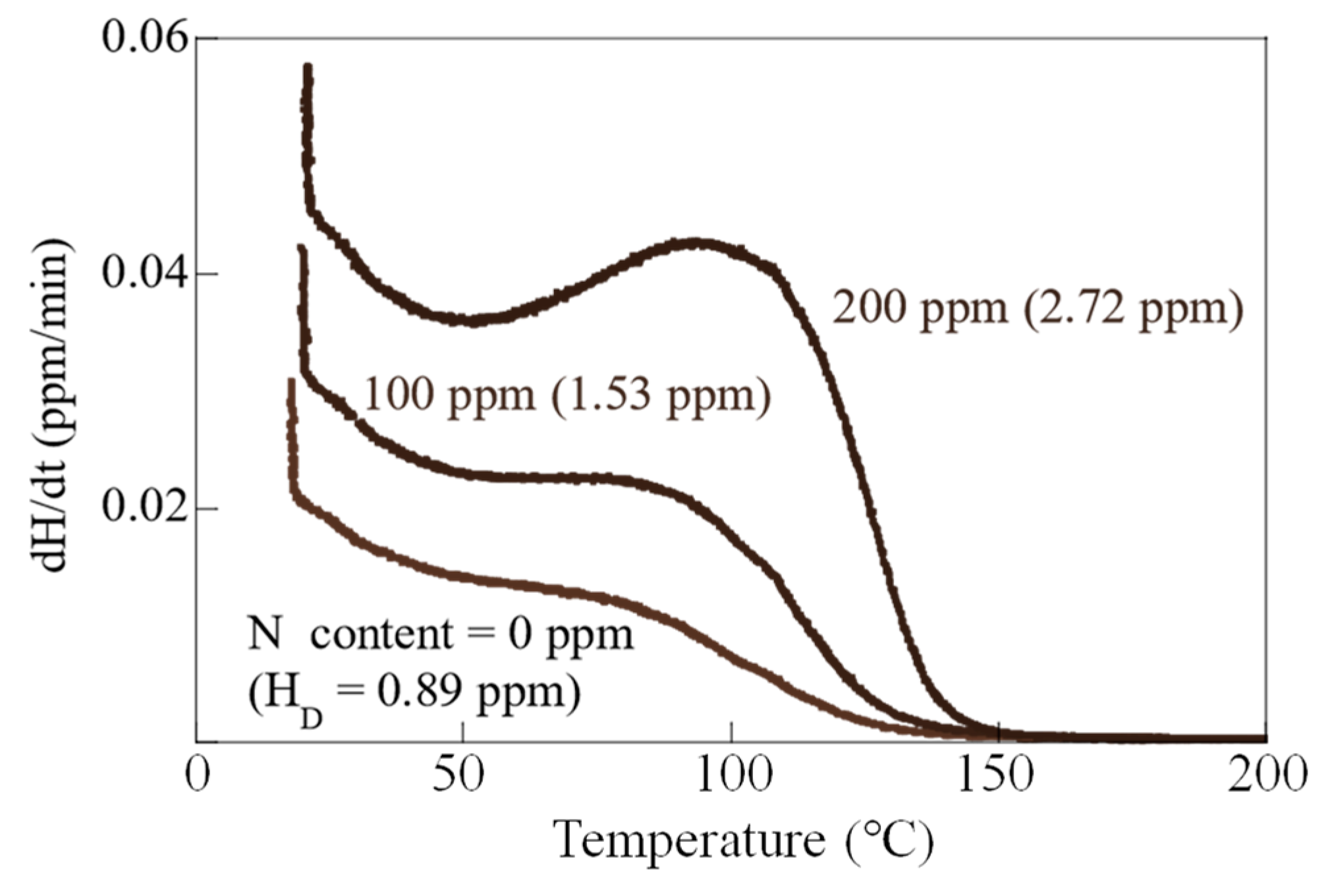
3.4. Hydrogen Analysis
3.5. Effect of Hydrogen on Fatigue Life
3.6. Effect of Nitrogen on Fatigue Life and Hydrogen Embrittlement Fracture Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zackay, V.F.; Parker, E.R.; Fahr, D.; Bush, R. The enhancement of ductility in high-strength steels. Trans. Am. Soc. Met. 1967, 60, 252–259. [Google Scholar]
- Matsuyama, S. Delayed Fracture of High Strength Steels. Tetsu-To-Hagané 1994, 80, 679–684. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mohanty, O.N.; Hashimoto, S.; Hojo, T.; Sugimoto, K. Strain-induced Transformation Behaviour of Retained Austenite and Tensile Properties of TRIP-aided Steels with Different Matrix Microstructure. ISIJ Int. 2006, 46, 316–324. [Google Scholar] [CrossRef]
- Hojo, T.; Chanvichitkul, K.; Waki, H.; Nishimura, F.; Akiyama, E. Hydrogen embrittlement properties of nitrogen added ultra-high-strength TRIP-aided martensitic steels evaluated by using conventional strain rate technique. Procedia Manuf. 2018, 15, 1581–1587. [Google Scholar] [CrossRef]
- Hojo, T.; Kikuchi, R.; Waki, H.; Nishimura, F.; Ukai, Y.; Akiyama, E. Effect of Strain Rate on the Hydrogen Embrittlement Property of Ultra High-strength Low Alloy TRIP-aided Steel. ISIJ Int. 2018, 58, 751–759. [Google Scholar] [CrossRef]
- Nagasaka, A.; Hojo, T.; Shibayama, Y.; Fujita, M.; Ohashi, T.; Miyasaka, M.; Akiyama, E. V-Bendability of Ultrahigh-Strength Low Alloy TRIP-Aided Steel Sheets with Bainitic Ferrite Matrix. ISIJ Int. 2022, 62, 247–256. [Google Scholar] [CrossRef]
- Hojo, T.; Kobayashi, J.; Sugimoto, K.; Takemoto, Y.; Nagasaka, A.; Koyama, M.; Akiyama, E. Effects of Matrix Structure and Nitrogen Content on Fatigue Properties of Ultrahigh-Strength Low Alloy TRIP-Aided Steels. ISIJ Int. 2021, 61, 591–598. [Google Scholar] [CrossRef]
- Hojo, T.; Kobayashi, J.; Sugimoto, K. Impact properties of low-alloy transformation-induced plasticity-steels with different matrix. Mater. Scie. Technol. 2016, 32, 1035–1042. [Google Scholar] [CrossRef]
- Bahrami, F.; Hendry, A. Microstructure and mechanical behaviour of nitrogen alloyed martensitic stainless steel. Mater. Sci. Technol. 2013, 11, 488–497. [Google Scholar] [CrossRef]
- Ono, A.A.; Alonso, N.; Tschiptsch, A.P. The Corrosion Resistance of Nitrogen Bearing Martensitic Stainless Steels. ISIJ Int. 1996, 36, 813–817. [Google Scholar] [CrossRef]
- Grabke, H.J. The Role of Nitrogen in the Corrosion of Iron and Steels. ISIJ Int. 1996, 36, 777–786. [Google Scholar] [CrossRef]
- Kim, K.S.; Kang, J.H.; Kim, S.J. Nitrogen effect on hydrogen diffusivity and hydrogen embrittlement behavior in austenitic stainless steels. Scr. Mater. 2020, 184, 70–73. [Google Scholar] [CrossRef]
- Luo, Y.; Li, W.; Jiang, L.; Zhong, N.; Jin, X. Hydrogen embrittlement and hydrogen diffusion behavior in interstitial nitrogen-alloyed austenitic steel. Int. J. Hydrog. Energy 2021, 46, 32710–32722. [Google Scholar] [CrossRef]
- Murakami, Y.; Endo, M. Effects of defects, inclusions and inhomogeneities on fatigue strength. Int. J. Fatigue 1994, 16, 163–182. [Google Scholar] [CrossRef]
- Matsunaga, H.; Yoshikawa, M.; Kondo, R.; Yamabe, J.; Matsuoka, S. Slow strain rate tensile and fatigue properties of Cr–Mo and carbon steels in a 115 MPa hydrogen gas atmosphere. Int. J. Hydrog. Energy 2015, 40, 5739–5748. [Google Scholar] [CrossRef]
- Macadre, A.; Yano, H.; Matsuoka, S.; Furtado, J. The effect of hydrogen on the fatigue life of Ni–Cr–Mo steel envisaged for use as a storage cylinder for a 70 MPa hydrogen station. Int. J. Fatigue 2011, 33, 1608–1619. [Google Scholar] [CrossRef]
- Aoki, Y.; Kawamoto, K.; Oda, Y.; Noguchi, H.; Higashida, K. Fatigue characteristics of a type 304 austenitic stainless steel in hydrogen gas environment. Int. J. Fract. 2005, 133, 277–288. [Google Scholar] [CrossRef]
- Nakamura, M.; Okazaki, S.; Matsunaga, H.; Matsuoka, S. SSRT and fatigue life properties of austenitic stainless steel weld metal 317L in high-pressure hydrogen gas. Trans. JSME 2018, 84, 17–00437. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y.; Matsuoka, S. Hydrogen Embrittlement Mechanism in Fatigue of Austenitic Stainless Steels. Metall. Mater. Trans. A 2008, 39, 1327–1339. [Google Scholar] [CrossRef]
- Matsuoka, S.; Tanaka, H.; Homma, N.; Murakami, Y. Influence of hydrogen and frequency on fatigue crack growth behavior of Cr-Mo steel. Int. J. Fract. 2010, 168, 101–112. [Google Scholar] [CrossRef]
- Fernández-Sousa, R.; Betegón, C.; Martínez-Pañeda, E. Analysis of the influence of microstructural traps on hydrogen assisted fatigue. Acta Mater. 2020, 199, 253–263. [Google Scholar] [CrossRef]
- Gao, G.; Liu, R.; Wang, K.; Gui, X.; Misra, R.D.K.; Bai, B. Role of retained austenite with different morphologies on sub-surface fatigue crack initiation in advanced bainitic steels. Scr. Mater. 2020, 184, 12–18. [Google Scholar] [CrossRef]
- Malitckii, E.; Yagodzinskyy, Y.; Vilaça, P. Role of retained austenite in hydrogen trapping and hydrogen-assisted fatigue fracture of high-strength steels. Mater. Sci. Eng. A 2019, 760, 68–75. [Google Scholar] [CrossRef]
- Tamura, I. Steel Material Study on the Strength; Nikkan-Kogyo Shinbun Ltd.: Tokyo, Japan, 1970. [Google Scholar]
- Maruyama, H. X-ray measurement of retained austenite volume fraction. J. Jpn. Soc. Heat Treat. 1977, 17, 198–204. [Google Scholar]
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Gu, J.; Chang, K.; Fang, H.S.; Bai, B. Delayed Fracture Properties of 1500 MPa Bainite/Martensite Dual-phase High Strength Steel and Its Hydrogen Traps. ISIJ Int. 2002, 42, 1560–1564. [Google Scholar] [CrossRef]
- Chan, S.L.I.; Lee, H.L.; Yang, J.R. Effect of retained austenite on the hydrogen content and effective diffusivity of martensitic structure. Metall. Trans. A 1991, 22, 2579–2586. [Google Scholar] [CrossRef]
- Nagao, A.; Hayashi, K.; Oi, K.; Mitao, S. Effect of Uniform Distribution of Fine Cementite on Hydrogen Embrittlement of Low Carbon Martensitic Steel Plates. ISIJ Int. 2012, 52, 213–221. [Google Scholar] [CrossRef]
- Sasaki, D.; Koyama, M.; Noguchi, H. Influence of Stress Re-distribution on Hydrogen-induced Fatigue Crack Propagation. ISIJ Int. 2019, 59, 1683–1690. [Google Scholar] [CrossRef]
- Matsuoka, S.; Yamabe, J.; Matsunaga, H. Criteria for determining hydrogen compatibility and the mechanisms for hydrogen-assisted surface crack growth in austenitic stainless steels. Eng. Fract. Mech. 2016, 153, 103–127. [Google Scholar] [CrossRef]
- Hojo, T.; Koyama, M.; Terao, N.; Tsuzaki, K.; Akiyama, E. Transformation-assisted hydrogen desorption during deformation in steels: Examples of α′- and ε-Martensite. Int. J. Hydrog. Energy 2019, 44, 30472–30477. [Google Scholar] [CrossRef]




| Steel | C | Si | Mn | Al | Nb | N | Fe | Ms (°C) |
|---|---|---|---|---|---|---|---|---|
| A | 0.200 | 1.00 | 1.50 | 0.48 | 0.049 | 0.0009 | Bal. | 434 |
| B | 0.187 | 1.01 | 1.50 | 0.48 | 0.050 | 0.0106 | Bal. | 438 |
| C | 0.176 | 1.04 | 1.50 | 0.53 | 0.052 | 0.0188 | Bal. | 444 |
| Steel | fγ0 | Cγ0 | TS | YS | TEl | UEl | FL |
|---|---|---|---|---|---|---|---|
| TM-A | 3.0 | 0.62 | 1396 | 1111 | 16.0 | 6.7 | 270 |
| TM-B | 3.0 | 0.63 | 1393 | 1102 | 16.5 | 6.6 | 261 |
| TM-C | 3.2 | 0.85 | 1369 | 1083 | 14.0 | 6.9 | 252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojo, T.; Nagasaka, A.; Kobayashi, J.; Shibayama, Y.; Akiyama, E. Effect of Hydrogen on Fatigue Life and Fracture Morphologies of TRIP-Aided Martensitic Steels with Added Nitrogen. Metals 2024, 14, 346. https://doi.org/10.3390/met14030346
Hojo T, Nagasaka A, Kobayashi J, Shibayama Y, Akiyama E. Effect of Hydrogen on Fatigue Life and Fracture Morphologies of TRIP-Aided Martensitic Steels with Added Nitrogen. Metals. 2024; 14(3):346. https://doi.org/10.3390/met14030346
Chicago/Turabian StyleHojo, Tomohiko, Akihiko Nagasaka, Junya Kobayashi, Yuki Shibayama, and Eiji Akiyama. 2024. "Effect of Hydrogen on Fatigue Life and Fracture Morphologies of TRIP-Aided Martensitic Steels with Added Nitrogen" Metals 14, no. 3: 346. https://doi.org/10.3390/met14030346






