The Effect of Increasing Sn Content on High-Temperature Mechanical Deformation of an Mg-3%Cu-1%Ca Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
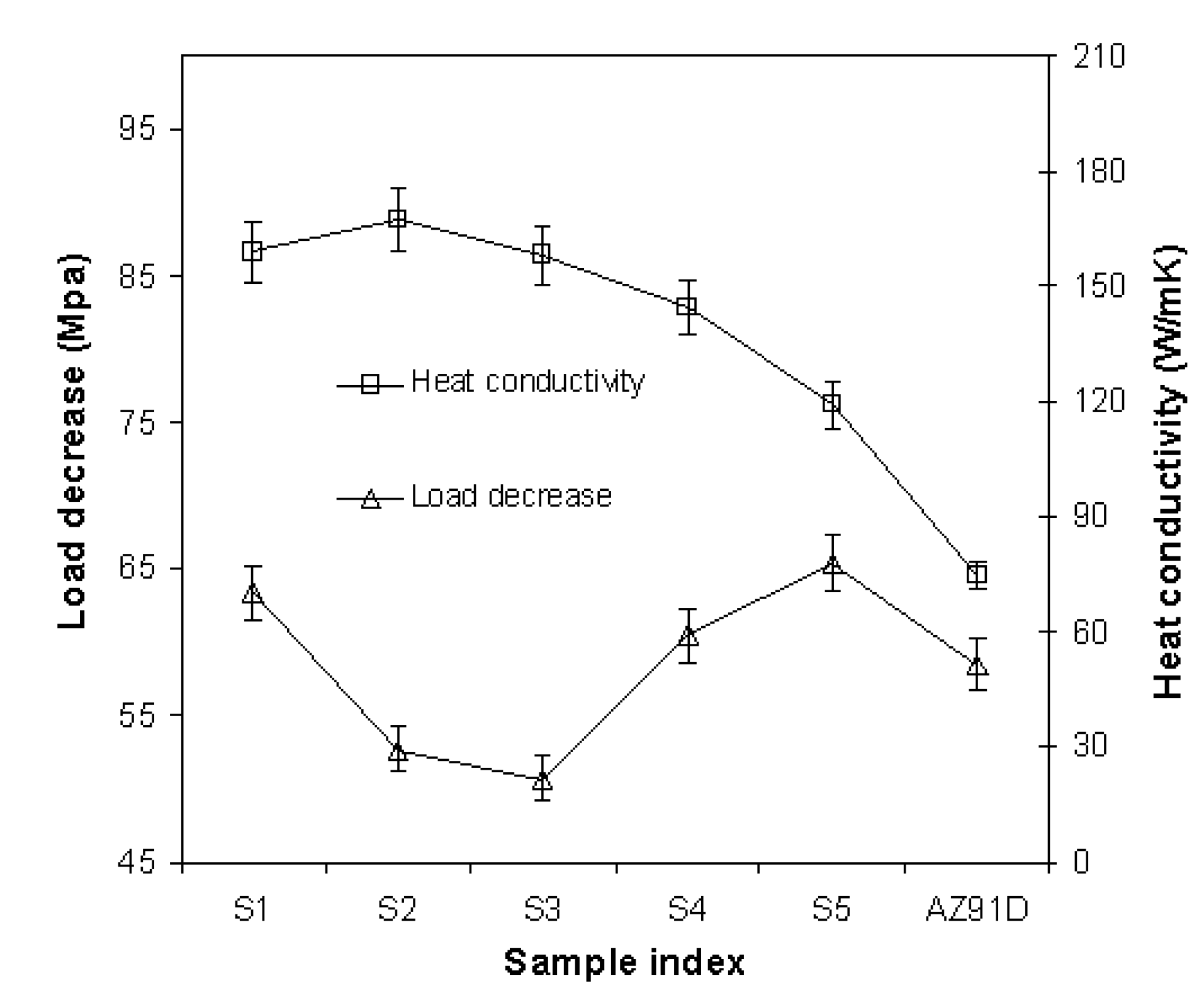
| Sample index | Composition (% w.t.) | Heat conductivity (W/mK) | Load decrease (MPa) |
|---|---|---|---|
| S1 | Mg3%Cu1%Ca | 155 | 65 |
| S2 | Mg3%Cu1%Ca0.1%Sn | 163 | 54 |
| S3 | Mg3%Cu1%Ca1%Sn | 154 | 52 |
| S4 | Mg3%Cu1%Ca2%Sn | 148 | 59 |
| S5 | Mg3%Cu1%Ca4%Sn | 122 | 67 |
| AZ91D | Mg9%Al1%Zn | 73 | 60 |

4. Conclusions
Conflicts of Interest
References
- Guo, K.W. A review of magnesium/magnesium alloys corrosion and its protection. Recent Pat. Corros. Sci. 2010, 2, 13–21. [Google Scholar] [CrossRef]
- Hakamada, M.; Furuta, T.; Chino, Y.; Chen, Y.; Kusuda, H.; Mabuchi, M. Life cycle inventory study on magnesium alloy substitution in vehicles. Energy 2007, 32, 1352–1360. [Google Scholar] [CrossRef]
- Antipas, G.S.E. A review of gas atomization and spray forming phenomenology. Powder Metall. 2013, 56, 317–330. [Google Scholar]
- Antipas, G.S.E. Spray forming of al alloys: Experiment and theory. Mater. Res. 2012, 15, 131–135. [Google Scholar] [CrossRef]
- Antipas, G.S.E.; Lekakou, C.; Tsakiropoulos, P. Microstructural characterisation of Al―Hf and Al―Li―Hf spray deposits. Mater. Charact. 2011, 62, 402–408. [Google Scholar] [CrossRef]
- Antipas, G.S.E. Modelling of the break up mechanism in gas atomization of liquid metals Part II. The gas flow model. Comput. Mater. Sci. 2009, 46, 955–959. [Google Scholar] [CrossRef]
- Antipas, G.S.E. Modelling of the break up mechanism in gas atomization of liquid metals. Part I: The surface wave formation model. Comput. Mater. Sci. 2006, 35, 416–422. [Google Scholar] [CrossRef]
- Antipas, G.S.E. Mathematical Modeling of Metal Spray Forming. University of Oxford: Oxford, UK, 1998. [Google Scholar]
- Zhou, D.W.; Liu, J.S.; Peng, P.; Chen, L.; Hu, Y.J. A first-principles study on the structural stability of Al2Ca Al4Ca and Mg2Ca phases. Mater. Lett. 2008, 62, 206–210. [Google Scholar] [CrossRef]
- Powell, B.R.; Rezhets, V.; Balogh, M.P.; Waldo, R.A. Microstructure and creep behavior in AE42 magnesium die-casting alloy. JOM 2002, 54, 34–38. [Google Scholar]
- Xiao, Z.H.; Luo, J.R.; Wu, S.S.; Li, D.N.; Mao, Y.W.; Song, X.J. Study on a semi-solid rheo-diecasting process with AZ91D alloy slurry. J. Mater. Eng. Perform. 2004, 13, 60–63. [Google Scholar] [CrossRef]
- Aljarrah, M.; Medraj, M.; Wang, X.; Essadiqi, E.; Muntasar, A.; Dénès, G. Experimental investigation of the MgAlCa system. J. Alloys Compd. 2007, 436, 131–141. [Google Scholar] [CrossRef]
- Pekguleryuz, M.O.; Baril, E. Creep resistant magnesium diecasting alloys based on alkaline earth elements. Mater. Trans. 2001, 42, 1258–1267. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Kadir, M.R.A.; Farahany, S.; Lotfabadi, A.F.; Suhasril, A.A. Effect of secondary phase on the corrosion behavior of Mg-Ca and Mg-Zn binary alloys. Adv. Sci. Lett. 2013, 19, 2553–2557. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable orthopedic magnesium-calcium (MgCa) alloys. Process. Corros. Perform. Mater. 2012, 5, 135–155. [Google Scholar]
- Avraham, S.; Katsman, A.; Bamberger, M. Strengthning Mechanisms in MG-AL-SN Based Alloys. In Proceedings of Magnesium International Conference, Haifa, Israel, 15–19 February 2009; pp. 471–475.
- Zajas, J.; Heiselberg, P. Determination of the local thermal conductivity of functionally graded materials by a laser flash method. Int. J. Heat Mass Transf. 2013, 60, 542–548. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yubuta, K.; Inoue, A. Phase stability of Cu2Mg and CuMg2 compounds against noncrystallizations analyzed with a plastic crystal model. Intermetallics 2008, 16, 1273–1278. [Google Scholar] [CrossRef]
- Chino, Y.; Kobata, M.; Iwasaki, H.; Mabuchi, M. Tensile properties from room temperature to 673 K of Mg-0.9 mass % Ca alloy containing lamella Mg2Ca. Mater. Trans. 2002, 43, 2643–2646. [Google Scholar] [CrossRef]
- Arróyave, R.; Liu, Z.-K. Intermetallics in the Mg-Ca-Sn ternary system: Structural, vibrational, and thermodynamic properties from first principles. Phys. Rev. B 2006, 74, 174118. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Antipas, G.S.E. The Effect of Increasing Sn Content on High-Temperature Mechanical Deformation of an Mg-3%Cu-1%Ca Alloy. Metals 2013, 3, 337-342. https://doi.org/10.3390/met3040337
Antipas GSE. The Effect of Increasing Sn Content on High-Temperature Mechanical Deformation of an Mg-3%Cu-1%Ca Alloy. Metals. 2013; 3(4):337-342. https://doi.org/10.3390/met3040337
Chicago/Turabian StyleAntipas, Georgios S.E. 2013. "The Effect of Increasing Sn Content on High-Temperature Mechanical Deformation of an Mg-3%Cu-1%Ca Alloy" Metals 3, no. 4: 337-342. https://doi.org/10.3390/met3040337
APA StyleAntipas, G. S. E. (2013). The Effect of Increasing Sn Content on High-Temperature Mechanical Deformation of an Mg-3%Cu-1%Ca Alloy. Metals, 3(4), 337-342. https://doi.org/10.3390/met3040337




