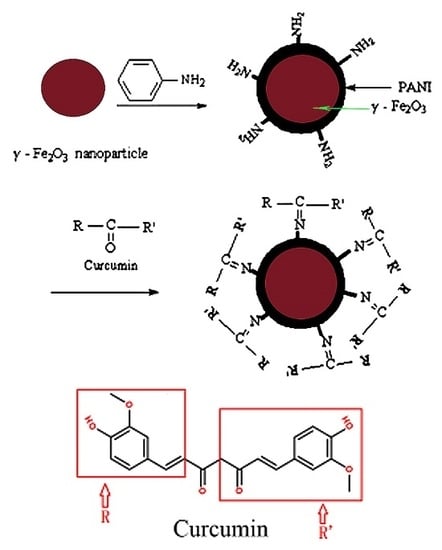
Preparation and Characteristics of γ-Fe2O3/Polyaniline-Curcumin Composites
Abstract
:1. Introduction
2. Materials
2.1. Preparation of γ-Fe2O3 Nanoparticles
2.2. Preparation of γ-Fe2O3/PANI
2.3. Preparation of a γ-Fe2O3/PANI-Curcumin Composite
3. Characterization of Samples
4. Results and Discussion
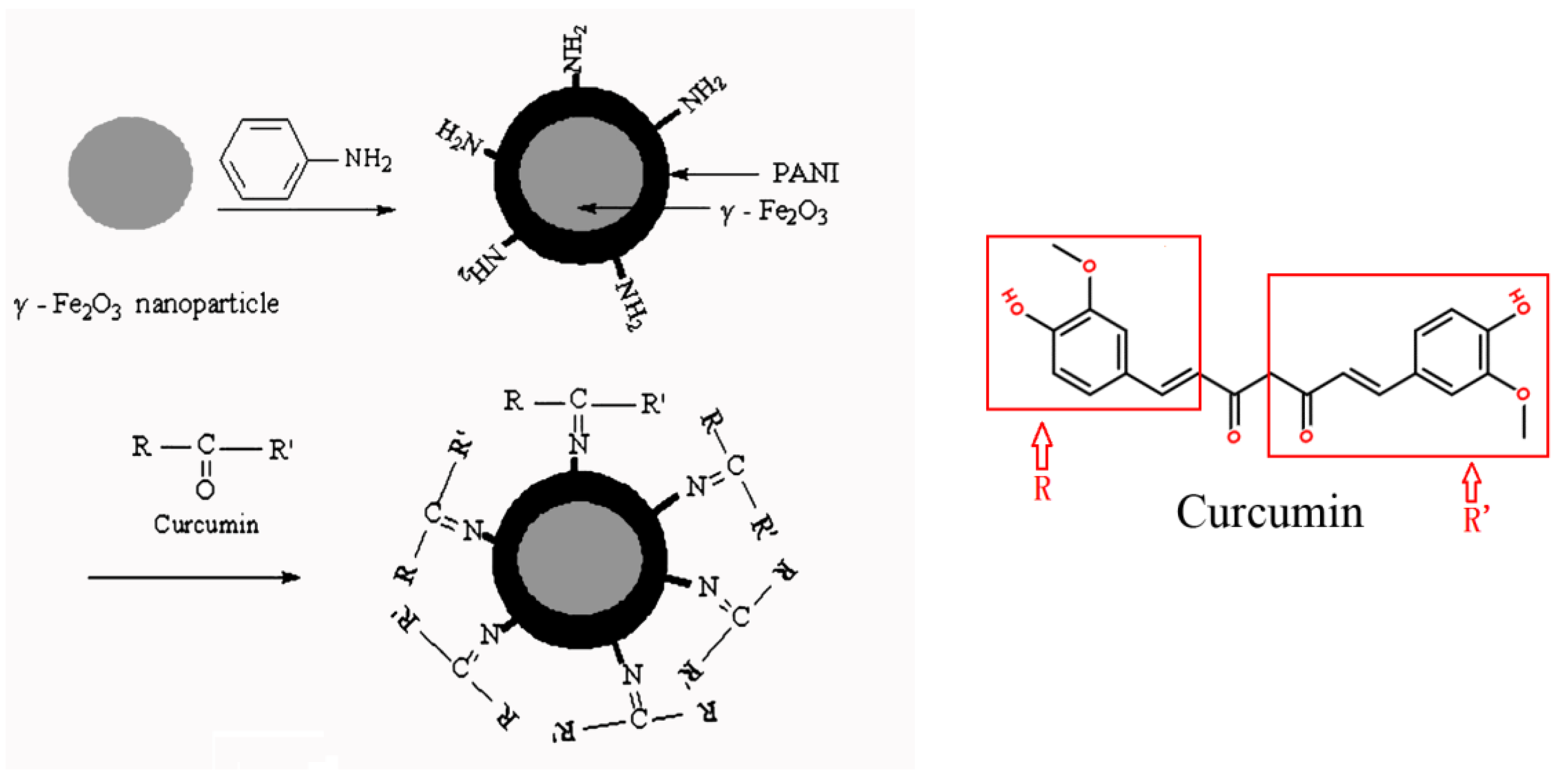
- Step 1:
- preparation of the skeleton material: we coated a layer of polyaniline on the prepared γ-Fe2O3 particles by a method of solution polymerization, forming a γ-Fe2O3/PANI composite with a core-shell structure.
- Step 2:
- immobilization of curcumin: curcumin was immobilized into the outer layer of PANI, which was due to an interaction between a carbonyl group of curcumin and an amino group of polyaniline.
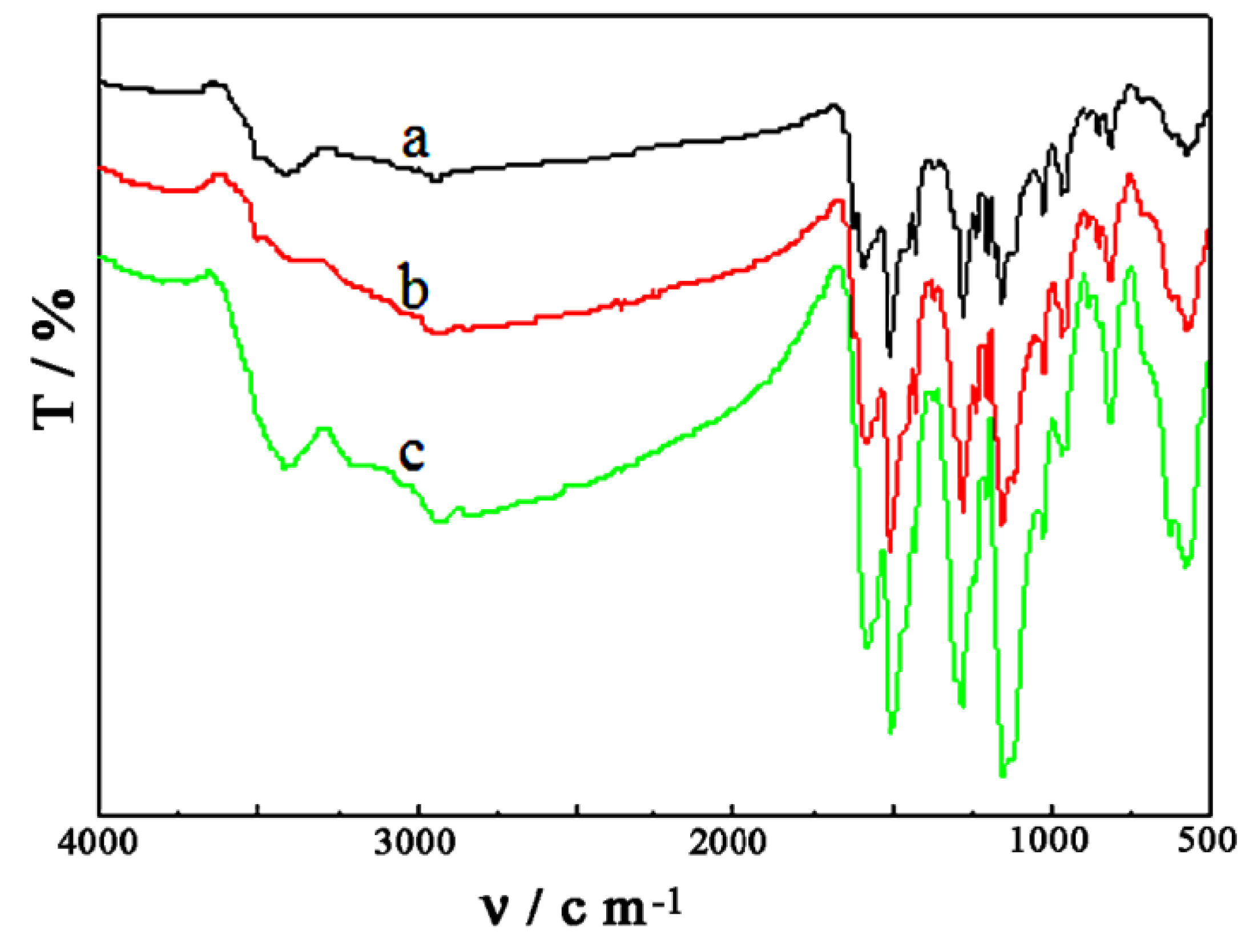
4.1. IR Spectra of Samples


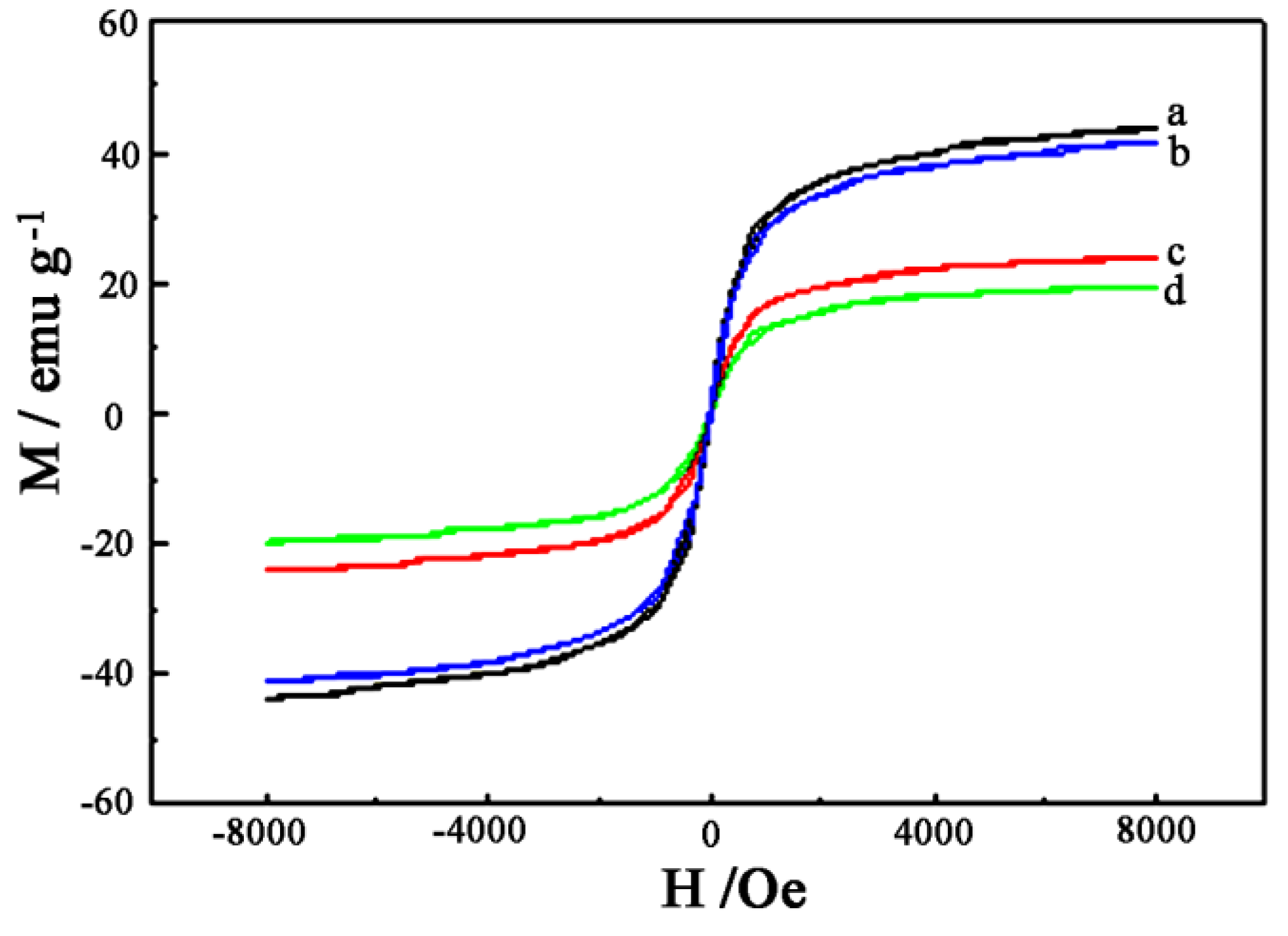
4.2. Magnetic Characterization of the Samples



4.3. TEM and Particle Size Distribution Images of Samples

4.4. Cyclic Voltammograms of Composite Particles

4.5. X-ray Diffraction of Samples

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galvin, M.E.; Wnek, G.E. Electrically conductive polymer composites: Polymerization of acetylene in polyethylene. Polymer 1982, 23, 795–797. [Google Scholar] [CrossRef]
- Pu, Z.; Zhou, X.; Yang, X.; Jia, K.; Liu, X. One step grafting of iron phthalocyanine containing flexible chains on Fe3O4 nanoparticles towards high performance polymer magnetic composites. J. Magn. Magn. Mater. 2015, 385, 368–376. [Google Scholar] [CrossRef]
- Held, G.A.; Grinstein, G.; Doyle, H.; Sun, S.; Murray, C.B. Competing interactions in dispersions of superparamagnetic nanoparticles. Phys. Rev. B 2001. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, Z.; Hu, P. Environmentally benign magnetic chitosan/Fe3O4 composites as reductant and stabilizer for anchoring Au NPs and their catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2014, 2, 13471–13478. [Google Scholar] [CrossRef]
- Urbanova, V.; Magro, M.; Gedanken, A.; Baratella, D.; Vianello, F.; Zboril, R. Nanocrystalline iron oxides, composites, and related materials as a platform for electrochemical, magnetic, and chemical biosensors. Chem. Mater. 2014, 26, 6653–6673. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Wu, J.; Xu, S.; Tian, R. Synthesis and evaluation of fluorescent magnetic composites as targeted drug delivery carriers. J. Mater. Eng. Perform. 2015, 24, 1237–1242. [Google Scholar] [CrossRef]
- Fang, F.F.; Liu, Y.D.; Choi, H.J. Electrorheological and magnetorheological response of polypyrrole/magnetite nanocomposite particles. Colloid Polym. Sci. 2013, 291, 1781–1786. [Google Scholar] [CrossRef]
- Ma, Z.; Kan, J. Study of cylindrical Zn/PANI secondary batteries with the electrolyte containing alkylimidazolium ionic liquid. Synth. Met. 2013, 174, 58–62. [Google Scholar] [CrossRef]
- Wuang, S.C.; Neoh, K.G.; Kang, E.T.; Pack, D.W.; Leckband, D.E. HER-2-mediated endocytosis of magnetic nanospheres and the implications in cell targeting and particle magnetization. Biomaterials 2008, 29, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H. Functional polymer microspheres. Prog. Polym. Sci. 2000, 25, 1171–1210. [Google Scholar] [CrossRef]
- Gomez-Romero, P. Hybrid organic-inorganic materials—In search of synergic activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Xiao, H.M.; Zhang, W.D.; Wan, M.X.; Fu, S.Y. Novel electromagnetic functionalized γ-Fe2O3/polypyrrole composite nanostructures with high conductivity. J. Polym. Sci. Polym. Chem. 2009, 47, 4446–4453. [Google Scholar] [CrossRef]
- Deng, J.; Ding, X.; Zhang, W.; Peng, Y.; Wang, J.; Long, X.; Li, P.; Chan, A.S.C. Magnetic and conducting Fe3O4-cross-linked polyaniline nanoparticles with core-shell structure. Polymer 2002, 43, 2179–2184. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Kan, J. γ-Fe2O3/polyaniline-lonidamine prepared by doping/dedoping method. Mater. Chem. Phys. 2014, 145, 27–35. [Google Scholar] [CrossRef]
- Gao, R.; Li, J.; Han, S.; Wen, B.; Zhang, T.; Miao, H.; Zhang, Q. Magnetisation behaviour of mixtures of ferrofluids and paramagnetic fluids with same particle volume fractions. J. Exp. Nanosci. 2012, 7, 282–297. [Google Scholar] [CrossRef]
- Shen, Q.; Lang, J.; Kan, J. γ-Fe2O3@Polyaniline-Chlorambucil synthesized by using doping/dedoping method of polyaniline. Asian J. Chem. 2014, 26, 6492–6498. [Google Scholar]
- Li, D.; Huang, J.X.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bidez, P.R.; Li, S.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Mattioli-Belmonte, M.; Giavaresi, G.; Biagini, G.; Virgili, L.; Giacomini, M.; Fini, M.; Giantomassi, F.; Natali, D.; Torricelli, P.; Giardino, R. Tailoring biomaterial compatibility: In vivo tissue response versus in vitro cell behavior. Int. J. Artif. Organs 2003, 26, 1077–1085. [Google Scholar] [PubMed]
- Layek, S.; Pandey, A.; Pandey, A.; Verma, H.C. Synthesis of γ-Fe2O3 nanoparticles with crystallographic and magnetic texture. Int. J. Eng. Sci. Technol. 2010, 2, 33–39. [Google Scholar]
- Pankhurst, Q.A.; Connolly, J.; Jonesa, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, 167–181. [Google Scholar] [CrossRef]
- Levy, M.; Wilhelm, C.; Siaugue, J.M.; Horner, O.; Bacri, J.C.; Gazeau, F. Magnetically induced hyperthermia: Size-dependent heating power of γ-Fe2O3 nanoparticles. J. Phys. Condens. Matter 2008, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Z.; Geng, Y.; Lam, J.W.Y.; Li, B.; Jing, X.; Wang, X.; Wang, F.; Pakhomov, A.B.; Zhang, X.X. Processible nanostructured materials with electrical conductivity and magnetic susceptibility: Preparation and properties of maghemite/polyaniline nanocomposite films. Chem. Mater. 1999, 11, 1581–1589. [Google Scholar] [CrossRef]
- Tang, B.Z.; Geng, Y.H.; Sun, Q.H.; Zhang, X.X.; Jing, X.B. Processible nanomaterials with high conductivity and magnetizability. Preparation and properties of maghemite/polyaniline nanocomposite films. Pure Appl. Chem. 2000, 72, 157–162. [Google Scholar] [CrossRef]
- Tang, R.; Liu, M.; Kan, J. Synthesis, characterisation, and conductive superparamagnetism studies of γ-Fe2O3/Polyaniline-Levodopa Composites. Polym. Renew. Resour. 2012, 3, 1–12. [Google Scholar]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J. Surface and internal spin canting in γ-Fe2O3 nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Cao, S.W.; Zhu, Y.J.; Ma, M.Y.; Li, L.; Zhang, L. Hierarchically nanostructured magnetic hollow spheres of Fe3O4 and γ-Fe2O3: Preparation and potential application in drug delivery. J. Phys. Chem. C 2008, 112, 1851–1856. [Google Scholar] [CrossRef]
- Vančik, H. Basic Organic Chemistry for the Life Sciences; Springer International Publishing: New York, NY, USA, 2014; p. 94. [Google Scholar]
- Bean, C.P.; Livingston, J.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Liu, J.; Wan, M.X. Composites of polypyrrole with conducting and ferromagnetic behaviors. J. Polym. Sci. Polym. Chem. 2000, 38, 2734–2739. [Google Scholar] [CrossRef]
- Olivier, J.C. Drug transport to brain with targeted nanoparticles. NeuroRx 2005, 2, 108–119. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhu, C.; Kan, J. Preparation and Characteristics of γ-Fe2O3/Polyaniline-Curcumin Composites. Metals 2015, 5, 2401-2412. https://doi.org/10.3390/met5042401
Li Y, Zhu C, Kan J. Preparation and Characteristics of γ-Fe2O3/Polyaniline-Curcumin Composites. Metals. 2015; 5(4):2401-2412. https://doi.org/10.3390/met5042401
Chicago/Turabian StyleLi, Yongli, Chunxia Zhu, and Jinqing Kan. 2015. "Preparation and Characteristics of γ-Fe2O3/Polyaniline-Curcumin Composites" Metals 5, no. 4: 2401-2412. https://doi.org/10.3390/met5042401